Cyclodepsipeptides and Sesquiterpenes from Marine-Derived Fungus Trichothecium roseum and Their Biological Functions
Abstract
:1. Introduction
2. Results and Discussion
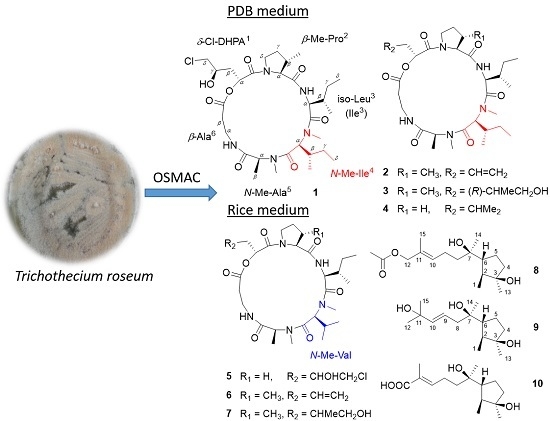
2.1. Structural Elucidation
2.2. Biological Evaluation
3. Experimental Section
3.1. General Procedures
3.2. Fungal Material
3.3. Fermentation and HPLC Analyses
3.4. Extraction and Isolation
3.5. Crystal Structure Determination
3.6. Cytotoxicity against Human Cancer Cell Lines
3.7. Brine Shrimp Lethality and Nematicidal Activity
3.8. Antifungal Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, P.J.; Challis, G.L. Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 2015, 13, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.A.; Teles, A.P.C.; Bracarense, A.A.P.; Gomes, D.C. Classical and epigenetic approaches to metabolite diversification in filamentous fungi. Phytochem. Rev. 2013, 12, 773–789. [Google Scholar] [CrossRef]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. Chembiochem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.Q.; Tang, H.Y.; Li, X.J.; Zhang, L.; Xiao, J.; Gao, Y.Q.; Zhang, A.L.; Gao, J.M. Potential allelopathic indole diketopiperazines produced by the plant endophytic Aspergillus fumigatus using the one strain−many compounds method. J. Agric. Food Chem. 2013, 61, 11447–11452. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Kusari, S.; Laatsch, H.; Golz, C.; Kusari, P.; Strohmann, C.; Kayser, O.; Spiteller, M. Antibacterial azaphilones from an endophytic fungus, Colletotrichum sp. BS4. J. Nat. Prod. 2016, 79, 704–710. [Google Scholar] [CrossRef]
- Liu, R.; Lin, Z.J.; Zhu, T.J.; Fang, Y.C.; Gu, Q.Q.; Zhu, W.M. Novel open-chain cytochalsins from the marine-derived fungus Spicaria elegans. J. Nat. Prod. 2008, 71, 1127–1132. [Google Scholar] [CrossRef]
- Lin, Z.J.; Zhu, T.J.; Wei, H.J.; Zhang, G.Q.; Wang, H.; Gu, Q.Q. Spicochalasin A and new aspochalasins from the marine-derived fungus Spicaria elegans. Eur. J. Org. Chem. 2009, 18, 3045–3051. [Google Scholar] [CrossRef]
- Wang, F.Z.; Wei, H.J.; Zhu, T.J.; Li, D.H.; Lin, Z.J.; Gu, Q.Q. Three new cytochalasins from the marine-derived fungus Spicaria elegans KLA03 by supplementing the cultures with L- and D-tryptophan. Chem. Biodivers. 2011, 8, 887–894. [Google Scholar] [CrossRef]
- Zhang, A.H.; Wang, X.Q.; Han, W.B.; Sun, Y.; Guo, Y.; Guo, Y.; Wu, Q.; Ge, H.M.; Song, Y.C.; Ng, S.W.; et al. Discovery of a new class of immunosuppressants from Trichothecium roseum co-inspired by cross-kingdom similarity in innate immunity and pharmacophore motif. Chem. Asian J. 2013, 8, 3101–3107. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kang, Q.J.; Lu, Y.Z.; Bai, L.Q.; Wang, C.S. Unveiling the biosynthetic puzzle of destruxins in Metarhizium species. Proc. Natl. Acad. Sci. USA 2012, 109, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Tzeng, Y.M. Development and applications of destruxins: A review. Biotechnol. Adv. 2012, 30, 1242–1254. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Takeuchi, H.; Ishida, Y.; Yashiroda, Y.; Yoshida, M.; Takagi, M.; Shi-ya, K.; Doi, T. Synthesis, structure determination, and biological evaluation of Destruxin E. Org. Lett. 2010, 12, 3792–3795. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Chou, C.K.; Sun, C.M.; Yeh, S.F. Suppressive effects of destruxin B on hepatitis B virus surface antigen gene expression in human hepatoma cells. Antivir. Res. 1997, 34, 137–144. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Chumala, P.B.; Wei, J.; Islam, M.S.; Hauck, D.W. The phytopathogenic fungus Alternaria brassicicola: Phytotoxin production and phytoalexin elicitation. Phyotochemistry 2009, 70, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, J.L.; Liu, J.M.; Chen, R.D.; Xie, K.B.; Chen, D.W.; Feng, K.P.; Zhang, D.; Dai, J.G. Neural anti-inflammatory sesquiterpenoids from the endophytic fungus Trichoderma sp. Xy24. J. Asian Nat. Prod. Res. 2017, 19, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Langhanki, J.; Rudolph, K.; Erkel, G.; Opatz, T. Total synthesis and biological evaluation of the natural product (−)-cyclonerodiol, a new inhibitor of IL-4 signaling. Org. Biomol. Chem. 2014, 12, 9707–9715. [Google Scholar] [CrossRef]
- Wu, S.H.; Zhao, L.X.; Chen, Y.W.; Huang, R.; Miao, C.P.; Wang, J. Sesquiterpenoids from the endophytic fungus Trichoderma sp. PR-35 of Paeonia delavayi. Chem. Biodivers. 2011, 8, 1717–1723. [Google Scholar] [CrossRef]
- Du, F.Y.; Li, X.M.; Zhang, P.; Li, C.S.; Cui, C.M.; Wang, B.G. Cyclodepsipeptides and other O-containing heterocyclic metabolites from Beauveria felina EN-135, a marine-derived entomopathogenic fungus. Mar. Drugs 2014, 12, 2816–2826. [Google Scholar] [CrossRef]
- Du, F.Y.; Zhang, P.; Li, X.M.; Li, C.S.; Cui, C.M.; Wang, B.G. Cyclohexadepsipeptides of the isaridin class from the marine-derived fungus Beauveria felina EN-135. J. Nat. Prod. 2014, 77, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Du, F.Y.; Li, X.; Li, X.M.; Zhu, L.W.; Wang, B.G. Indolediketopiperazine alkaloids from Eurotium cristatum EN-220, an endophytic fungus isolated from the marine alga Sargassum thunbergii. Mar. Drugs 2017, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Laurent, D.; Goasdoue, N.; Kohler, F.; Pellegrin, F.; Platzer, N. Characterization of cyclonerodiol isolated from corn infested by Fusarium moniliforme Sheld.: One- and two-dimensional 1H and 13C NMR Study. Magn. Reson. Chem. 1990, 28, 662–664. [Google Scholar] [CrossRef]
- Liang, X.R.; Miao, F.P.; Song, Y.P.; Liu, X.H.; Ji, N.Y. Citrinovirin with a new norditerpene skeleton from the marine algicolous fungus Trichoderma citrinoviride. Bioorg. Med. Chem. Lett. 2016, 26, 5029–5031. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.; Hanson, J.R.; Nyfeler, R. The biosynthesis of the sesquiterpenoids, cyclonerodiol and cyclonerotriol. J. Chem. Soc. Chem. Commun. 1975, 20, 814–815. [Google Scholar] [CrossRef]
- Evans, R.; Hanson, J.R.; Nyfeler, R. Studies in terpenoid biosynthesis. Part XVII. Biosynthesis of the sesquiterpenoids cyclonerodiol and cyclonerotriol. J. Chem. Soc. Perkin Trans. 1 1976, 11, 1214–1217. [Google Scholar] [CrossRef]
- Cane, D.E.; Iyengar, R.; Shiao, M. Cyclonerodiol biosynthesis and the stereochemistry of the conversion of farnesyl to nerolidyl pyrophosphate. J. Am. Chem. Soc. 1978, 100, 7122–7125. [Google Scholar] [CrossRef]
- Gupta, S.; Roberts, D.W.; Renwick, J.A.A. Insecticidal cyclodepsipeptides from Metarhizium anisopliae. J. Chem. Soc. Perkin Trans. 1 1989, 2347–2357. [Google Scholar] [CrossRef]
- Tsunoo, A.; Kamijo, M.; Taketomo, N.; Sato, Y.; Ajisaka, K. Roseocardin, a novel cardiotonic cyclodepsipeptide from Trichothecium roseum TT103. J. Antibiot. 1997, 50, 1007–1013. [Google Scholar] [CrossRef]
- Morais-Urano, R.P.; Chagas, A.C.S.; Berlinck, R.G.S. Acaricidal action of destruxins produced by a marine-derived Beauveria felina on the bovine tick Rhipicephalus (Boophilus) microplus. Exp. Parasitol. 2012, 132, 362–366. [Google Scholar] [CrossRef]
- Xie, L.R.; Li, D.Y.; Li, Z.L.; Hua, H.M.; Wang, P.L.; Wu, X. A new cyclonerol derivative from a marine-derived fungus Ascotricha sp. ZJ-M-5. Nat. Prod. Res. 2013, 27, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, Q.; Gao, Y.Q.; Tang, J.J.; Zhang, A.L.; Gao, J.M. Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J. Agric. Food Chem. 2014, 62, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiao, J.; Gao, Y.Q.; Tang, J.J.; Zhang, A.L.; Gao, J.M. Chaetoglobosins from Chaetomium globosum, an endophytic fungus in Ginkgo biloba, and their phytotoxic and cytotoxic activities. J. Agric. Food Chem. 2014, 62, 3734–3741. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.M.; Liang, X.A.; Zhang, H.C.; Liu, R. Cytotoxic and antibiotic cyclic pentapeptide from an endophytic Aspergillus tamarii of Ficus carica. J. Agric. Food Chem. 2016, 64, 3789–3793. [Google Scholar] [CrossRef] [PubMed]
- Dornetshuber-Fleiss, R.; Heffeter, P.; Mohr, T.; Hazemi, P.; Kryeziu, K.; Seger, C.; Berger, W.; Lemmens-Gruber, R. Destruxins: Fungal-derived cyclohexadepsipeptides with multifaceted anticancer and antiangiogenic activities. Biochem. Pharmacol. 2013, 86, 361–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.Q.; Zhou, H.; Han, J.; He, Y.Q.; Lu, L.P. Evaluate insecticidal activity using Artemia salina L. Agrochemicals 2011, 50, 261–263. [Google Scholar]
- Yao, C.C. Development and Application of Artemia nauplii Screening Method for Insecticidal Activities of Bamboo Extracts. Master’s Thesis, Anhui Agricultural University, Hefei, China, June 2009. [Google Scholar]
- Zhang, D.L.; Wang, H.Y.; Ji, X.X.; Wang, K.Y.; Wang, D.; Kang, Q. Effect of abamectin on the cereal cyst nematode (CCN, Heterodera avenae) and wheat yield. Plant Dis. 2017, 101, 973–976. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Software for Empirical Absorption Correction; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXTL, Structure Determination Software Programs; Bruker Analytical X-ray System Inc.: Madison, WI, USA, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97 and SHELXS-97, Program for X-ray Crystal Structure Solution and Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Zhang, X.M.; Li, G.H.; Ma, J.; Zeng, Y.; Ma, W.G.; Zhao, P.J. Endophytic fungus Trichothecium roseum LZ93 antagonizing pathogenic fungi in vitro and its secondary metabolites. J. Microbiol. 2010, 48, 784–790. [Google Scholar] [CrossRef]





| No. | δC (type) | δH (mult., J in Hz) | No. | δC (type) | δH (mult., J in Hz) |
|---|---|---|---|---|---|
| δ-Cl-DHPA1 | N-Me-Ile4 | ||||
| CO | 170.4, C | CO | 172.1, C | ||
| α | 70.7, CH | 5.21, t (7.0) | α | 56.9, CH | 5.09, d (11.0) |
| β | 34.9, CH2 | 2.18, m | β | 33.4, CH | 2.06, m |
| γ | 67.2, CH | 3.82, m | β-Me | 14.8, CH3 | 0.90, overlapped |
| δ | 48.3, CH2 | 3.61, d (5.0) | γ | 25.3, CH2 | 1.50, m; 1.07, m |
| β-Me-Pro2 | δ | 10.0, CH3 | 0.94, overlapped | ||
| CO | 172.4, C | N-Me | 30.1, CH3 | 3.21, s | |
| α | 67.5, CH | 4.08, m | N-Me-Ala5 | ||
| β | 37.5, CH | 2.55, m | CO | 170.8, C | |
| γ | 30.6, CH2 | 2.10, m; 1.77, br s | α | 55.4, CH | 5.32, q (6.5) |
| δ | 45.4, CH2 | 4.06, m; 3.95, m | β | 14.3, CH3 | 1.32, overlapped |
| β-Me | 17.9, CH3 | 1.16, d (6.8) | N-Me | 27.5, CH3 | 2.71, s |
| Ile3 | β-Ala6 | ||||
| CO | 173.6, C | CO | 173.6, C | ||
| α | 53.4, CH | 4.76, overlapped | α | 33.8, CH2 | 2.69, m; 2.58, m |
| β | 37.0, CH | 1.94, m | β | 33.4, CH2 | 3.80, m; 3.15, m |
| β-Me | 14.3, CH3 | 0.86, overlapped | NH | 8.56, br. s | |
| γ | 24.6, CH2 | 1.49, m;1.33, overlapped | |||
| δ | 9.7, CH3 | 0.87, overlapped | |||
| NH | 7.03, d (7.8) |
| Compound 8 | Compound 9 | ||||
|---|---|---|---|---|---|
| No. | δC (type) | δH (mult., J in Hz) | No. | δC (type) | δH (mult., J in Hz) |
| 1 | 14.0, CH3 | 1.05 (6.6) | 1 | 14.5, CH3 | 1.05 (6.8) |
| 2 | 44.1, CH | 1.57, m | 2 | 44.3, CH | 1.61, m |
| 3 | 80.7, C | 3 | 81.4, C | ||
| 4 | 40.0, CH2 | 1.63, m; 1.55, m | 4 | 40.4, CH2 | 1.68, m; 1.53, m |
| 5 | 23.8, CH2 | 1.85, m; 1.60, m | 5 | 24.4, CH2 | 1.86, m; 1.61, m |
| 6 | 54.1, CH | 1.87, m | 6 | 54.2, CH | 1.86, m |
| 7 | 74.1, C | 7 | 74.5, C | ||
| 8 | 40.0, CH2 | 1.52, t (8.4) | 8 | 43.4, CH2 | 2.20, m |
| 9 | 22.0, CH2 | 2.13, m | 9 | 122.1, CH | 5.70, overlapped |
| 10 | 129.6, CH | 5.47, t (7.0) | 10 | 142.3, CH | 5.70, overlapped |
| 11 | 129.9, C | 11 | 70.8, C | ||
| 12 | 69.9, CH2 | 4.45, s | 12 | 29.9, CH3 | 1.33, s |
| 13 | 24.7, CH3 | 1.26, s | 13 | 26.1, CH3 | 1.25, s |
| 14 | 23.2, CH3 | 1.17, s | 14 | 25.2, CH3 | 1.13, s |
| 15 | 12.5, CH3 | 1.67, s | 15 | 29.9, CH3 | 1.33, s |
| COCH3 | 19.4, CH3 | 2.08, s | |||
| C=O | 171.5, C | ||||
| Compound | MCF-7 | SW480 | HL-60 | A-549 | SMMC-7721 |
|---|---|---|---|---|---|
| 1 | 0.079 ± 0.004 | 0.107 ± 0.015 | 0.149 ± 0.007 | >40 | >40 |
| Cisplatin | 19.44 ± 1.57 | 20.80 ± 1.04 | 3.72 ± 0.09 | 16.97 ± 0.69 | 12.35 ± 0.52 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Positive Control | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| brine shrimp lethality | 0.48 | 0.74 | >50 | 3.22 | 2.47 | 2.81 | >50 | n.a. | n.a. | n.a. | 8.4 a |
| nematicidal activity | 94.9 | 143.6 | >500 | 221.8 | 207.7 | 293.4 | >500 | n.a. | n.a. | n.a. | 23.1 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.-M.; Ju, G.-L.; Xiao, L.; Zhang, X.-F.; Du, F.-Y. Cyclodepsipeptides and Sesquiterpenes from Marine-Derived Fungus Trichothecium roseum and Their Biological Functions. Mar. Drugs 2018, 16, 519. https://doi.org/10.3390/md16120519
Zhou Y-M, Ju G-L, Xiao L, Zhang X-F, Du F-Y. Cyclodepsipeptides and Sesquiterpenes from Marine-Derived Fungus Trichothecium roseum and Their Biological Functions. Marine Drugs. 2018; 16(12):519. https://doi.org/10.3390/md16120519
Chicago/Turabian StyleZhou, Yuan-Ming, Guang-Lin Ju, Lin Xiao, Xiang-Fei Zhang, and Feng-Yu Du. 2018. "Cyclodepsipeptides and Sesquiterpenes from Marine-Derived Fungus Trichothecium roseum and Their Biological Functions" Marine Drugs 16, no. 12: 519. https://doi.org/10.3390/md16120519
APA StyleZhou, Y. -M., Ju, G. -L., Xiao, L., Zhang, X. -F., & Du, F. -Y. (2018). Cyclodepsipeptides and Sesquiterpenes from Marine-Derived Fungus Trichothecium roseum and Their Biological Functions. Marine Drugs, 16(12), 519. https://doi.org/10.3390/md16120519