Evaluation of Intestinal Absorption of Dietary Halocynthiaxanthin, a Carotenoid from the Sea Squirt Halocynthia roretzi
Abstract
:1. Introduction
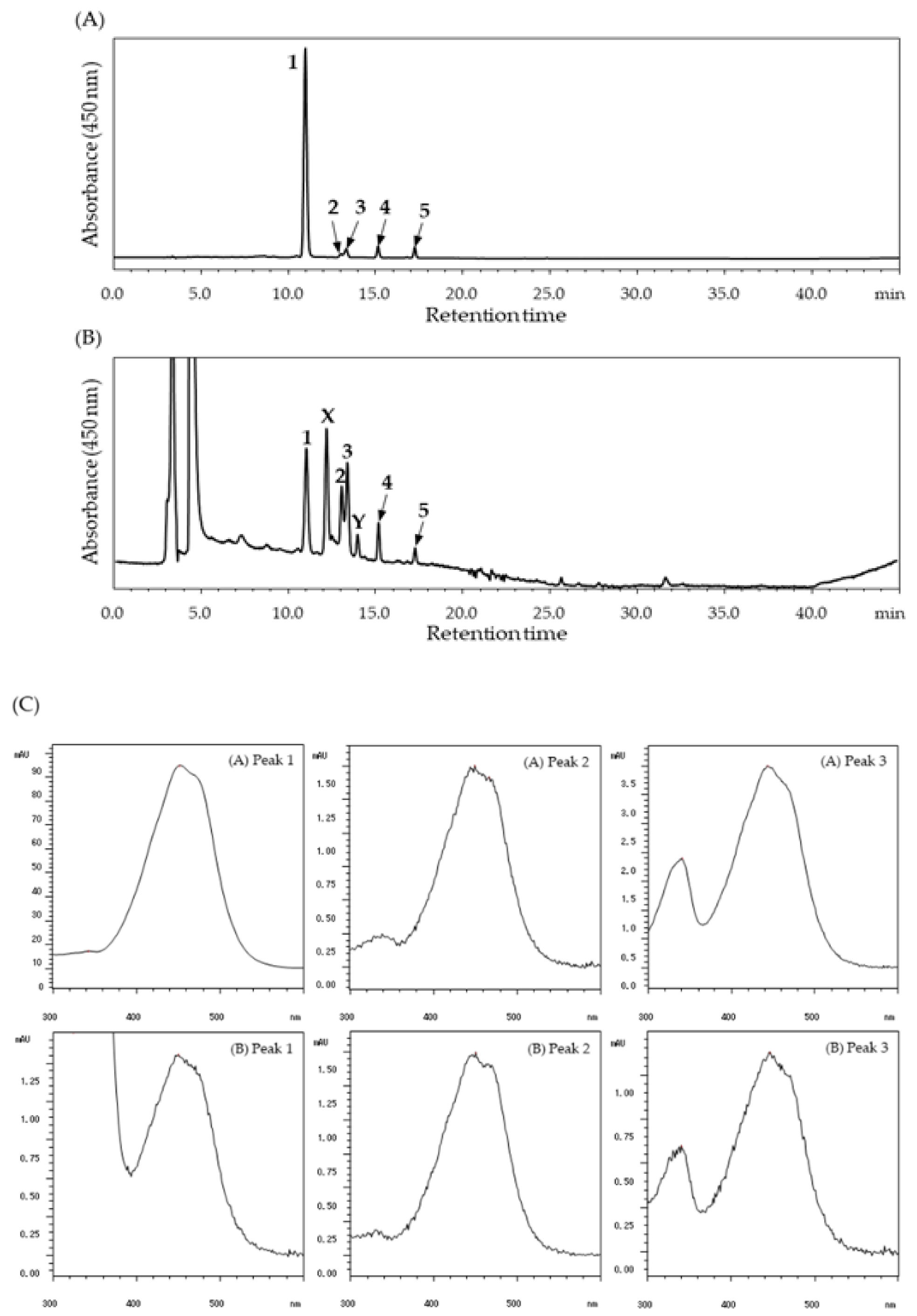
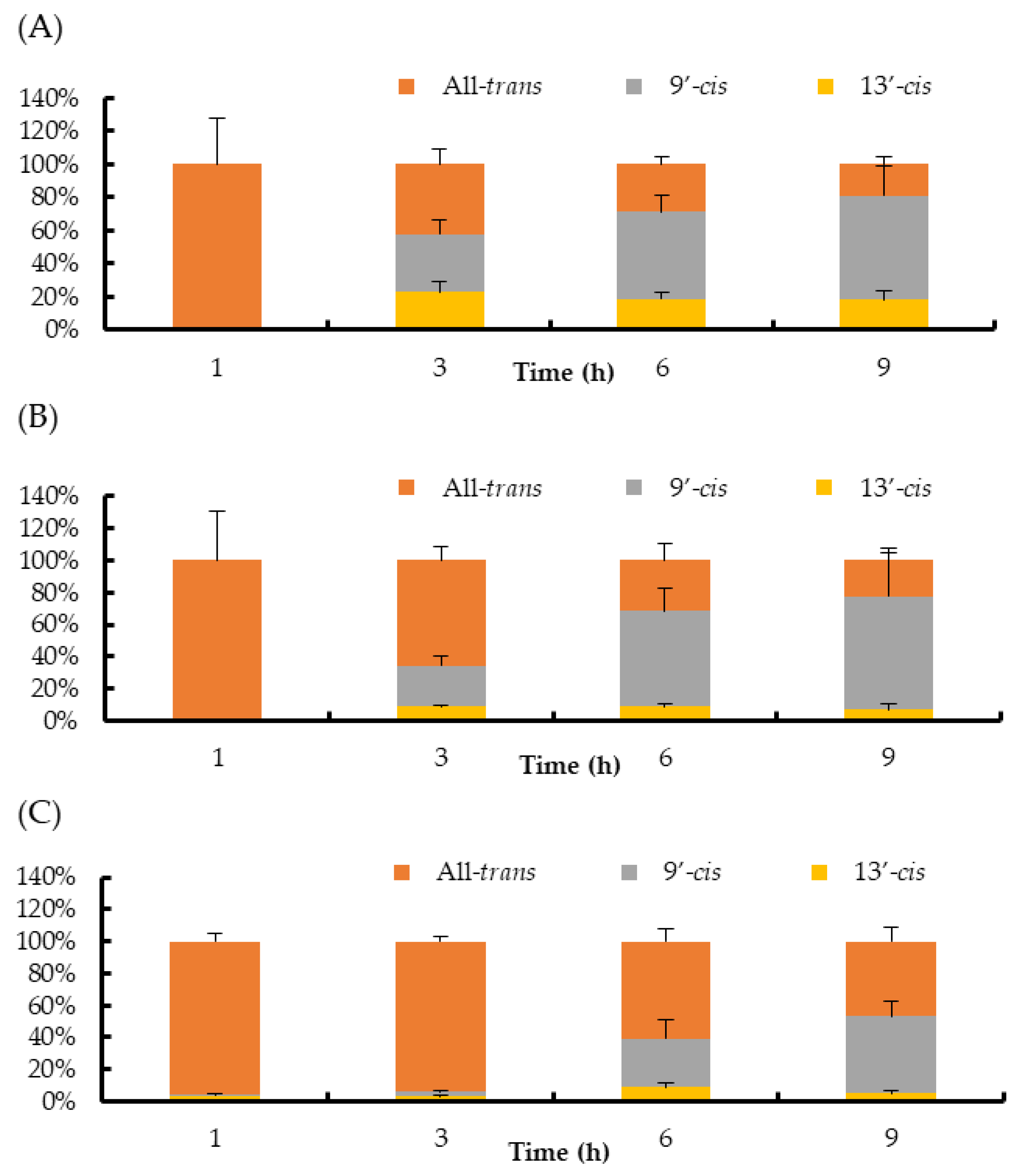
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of Halocynthiaxanthin
4.2. Animal Studies
4.3. Lipid Extraction
4.4. HPLC Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids Handbook; Birkhäuser: Basel, Switzerland, 2004. [Google Scholar]
- Maoka, T. Recent progress in structural studies of carotenoids in animals and plants. Arch. Biochem. 2009, 48, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2019, 74, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, K.; Ohgami, K.; Ilieva, I.; Jin, X.; Koyama, Y.; Miyashita, K.; Yoshida, K.; Kase, S.; Ohno, S. Effects of fucoxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res. 2005, 81, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Ganesan, P.; Li, Z.; Manabe, Y.; Hirata, T. Siphonaxanthin, a green algal carotenoid, as a novel functional compound. Mar. Drugs. 2014, 12, 3660–3668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, P.; Noda, K.; Manabe, Y.; Ohkubo, T.; Tanaka, Y.; Maoka, T.; Sugawara, T.; Hirata, T. Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta. 2011, 1810, 497–503. [Google Scholar] [CrossRef]
- Ganesan, P.; Matsubara, K.; Ohkubo, T.; Tanaka, Y.; Noda, K.; Sugawara, T.; Hirata, T. Anti-angiogenic effect of siphonaxanthin from green alga, Codium fragile. Phytomedicine 2010, 17, 1140–1144. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, P.; Matsubara, K.; Sugawara, T.; Hirata, T. Marine algal carotenoids inhibit angiogenesis by down-regulating FGF-2-mediated intracellular signals in vascular endothelial cells. Mol. Cell. Biochem. 2013, 380, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; Noda, K.; Fujita, E.; Manabe, Y.; Hirata, T.; Sugawara, T. The green algal carotenoid siphonaxanthin inhibits adipogenesis in 3T3-L1 preadipocytes and the accumulation of lipids in white adipose tissue of KK-Ay mice. J. Nutr. 2015, 145, 490–498. [Google Scholar] [CrossRef] [Green Version]
- Manabe, Y.; Hirata, T.; Sugawara, T. Suppressive effects of carotenoids on the antigen-induced degranulation in RBL-2H3 rat basophilic leukemia cells. J. Oleo. Sci. 2014, 63, 291–294. [Google Scholar] [CrossRef] [Green Version]
- Matsuno, T.; Ookubo, M. A new carotenoid, halocynthiaxanthin from the sea squirt, Halocynthia roretzi. Tetrahedron Lett. 1981, 22, 4659–4660. [Google Scholar] [CrossRef]
- Matsuno, T.; Ookubo, M.; Nishizara, T.; Shimizu, I. Carotenoids of sea squirts. I. New marine carotenoids, halocynthiaxanthin and mytiloxanthinone from Halocynthia roretzi. Chem. Pharm. Bull. 1984, 32, 4309–4315. [Google Scholar] [CrossRef] [Green Version]
- Palanisamy, S.K.; Trisciuoglio, D.; Zwergel, C.; Del Bufalo, D.; Mai, A. Metabolite profiling of ascidian Styela plicata using LC–MS with multivariate statistical analysis and their antitumor activity. J. Enzyme Inhib. Med. 2017, 32, 614–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuno, T.; Ookubo, M. Carotenoids of sea squirts: II. Comparative biochemical studies of carotenoids in sea squirts. Comp. Biochem. Physiol. 1985, 81, 137–141. [Google Scholar]
- Maoka, T. Carotenoids in Marine Animals. Mar. Drugs. 2011, 9, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, H.; Tsushima, M.; Matsuno, T.; Tanaka, Y.; Okuzumi, J.; Murakoshi, M.; Satomi, Y.; Takayasu, J.; Tokuda, H.; Nishino, A.; et al. Anti-neoplastic effect of halocynthiaxanthin, a metabolite of fucoxanthin. Anticancer Drugs. 1992, 3, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Konishi, I.; Hosokawa, M.; Sashima, T.; Kobayashi, H.; Miyashita, K. Halocynthiaxanthin and fucoxanthinol isolated from Halocynthia roretzi induce apoptosis in human leukemia, breast and colon cancer cells. Comp. Biochem. Physiol. 2006, 142, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, M.; Maoka, T.; Katsuyama, M.; Kozuka, M.; Matsuno, T.; Tokuda, H.; Nishino, H.; Iwashima, A. Inhibitory effect of natural carotenoids on Epstein-Barr virus activation activity of a tumor promoter in Raji cells. A screening study for anti-tumor promoters. Biol. Pharm. Bull. 1995, 18, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Murakami, A.; Nakashima, M.; Koshiba, T.; Maoka, T.; Nishino, H.; Yano, M.; Sumida, T.; Kim, O.; Koshimizu, K.; Ohigashi, H. Modifying effects of carotenoids on superoxide and nitric oxide generation from stimulated leucocytes. Cancer Lett. 2000, 149, 115–123. [Google Scholar] [CrossRef]
- Manabe, Y.; Hirata, T.; Sugawara, T. Inhibitory effect of carotenoids on ligand-induced lipid raft translocation of immunoreceptors. J. Oleo Sci. 2019, 68, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, L.; Nagao, A. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food. Res. 2007, 51, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, A.; Kim, S.J.; Kobori, M.; Miyashita, K.; Nagao, A. Acyclo-retinoic acid induces apoptosis in human prostate cancer cells. Anticancer Res. 2002, 22, 689–695. [Google Scholar] [PubMed]
- Asai, A.; Sugawara, T.; Ono, H.; Nagao, A. Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and HepG2 cells: Formation and cytotoxicity of fucoxanthin metabolites. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Xu, J.; Yang, L.; Gu, C.; Xue, C. Thermal stability and oral absorbability of astaxanthin esters from Haematococcus pluvialis in Balb/c mice. J. Sci. Food. Agric. 2019, 99, 3662–3671. [Google Scholar] [CrossRef]
- Baskaran, V.; Sugawara, T.; Nagao, A. Phospholipids affect intestinal absorption of carotenoids in mice. Lipids 2003, 38, 705–711. [Google Scholar] [CrossRef]
- Clinton, S.K. Lycopene: Chemistry, biology, and implications for human health and disease. Nutr. Rev. 1998, 56, 35–51. [Google Scholar] [CrossRef]
- Honest, K.; Wei Zhang, H.; Zhang, L. Lycopene: Isomerization effects on bioavailability and bioactivity properties. Food Rev. Int. 2011, 27, 248–258. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Sashima, T.; Hosokawa, M.; Miyashita, K. Comparative evaluation of growth inhibitory effect of stereoisomers of fucoxanthin in human cancer cell lines. J. Funct. Foods 2009, 1, 88–97. [Google Scholar] [CrossRef]
- Liu, X.; Osawa, T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem. Biophys. Res. Commun. 2007, 357, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Böhm, V.; Puspitasari-Nienaber, N.; Ferruzzi, M.; Schwartz, S. Trolox equivalent antioxidant capacity of different geometrical isomers of α-carotene, β-carotene, lycopene, and zeaxanthin. J. Agric. Food Chem. 2002, 50, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, J.; Luo, X.; Manabe, Y.; Hirata, T.; Sugawara, T. Absorption and Tissue Distribution of Siphonaxanthin from Green Algae. Mar. Drugs 2020, 18, 291. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, C.; Manabe, Y.; Tomonaga, N.; Wada, T.; Maoka, T.; Sugawara, T. Evaluation of Intestinal Absorption of Dietary Halocynthiaxanthin, a Carotenoid from the Sea Squirt Halocynthia roretzi. Mar. Drugs 2020, 18, 588. https://doi.org/10.3390/md18120588
Ikeda C, Manabe Y, Tomonaga N, Wada T, Maoka T, Sugawara T. Evaluation of Intestinal Absorption of Dietary Halocynthiaxanthin, a Carotenoid from the Sea Squirt Halocynthia roretzi. Marine Drugs. 2020; 18(12):588. https://doi.org/10.3390/md18120588
Chicago/Turabian StyleIkeda, Chiaki, Yuki Manabe, Nami Tomonaga, Tatsuya Wada, Takashi Maoka, and Tatsuya Sugawara. 2020. "Evaluation of Intestinal Absorption of Dietary Halocynthiaxanthin, a Carotenoid from the Sea Squirt Halocynthia roretzi" Marine Drugs 18, no. 12: 588. https://doi.org/10.3390/md18120588
APA StyleIkeda, C., Manabe, Y., Tomonaga, N., Wada, T., Maoka, T., & Sugawara, T. (2020). Evaluation of Intestinal Absorption of Dietary Halocynthiaxanthin, a Carotenoid from the Sea Squirt Halocynthia roretzi. Marine Drugs, 18(12), 588. https://doi.org/10.3390/md18120588




