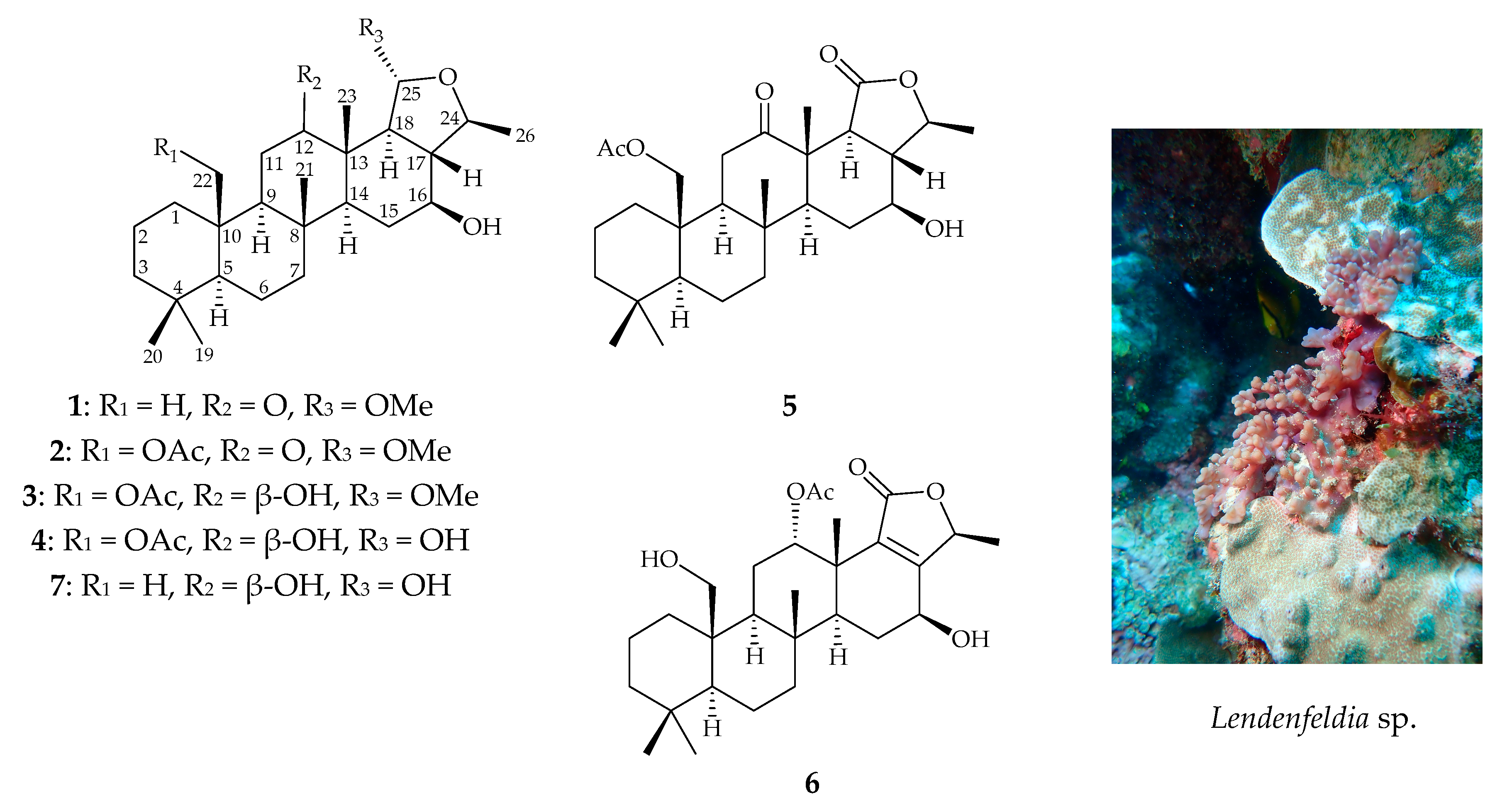
Sponge-Derived 24-Homoscalaranes as Potent Anti-Inflammatory Agents
Abstract
:1. Introduction
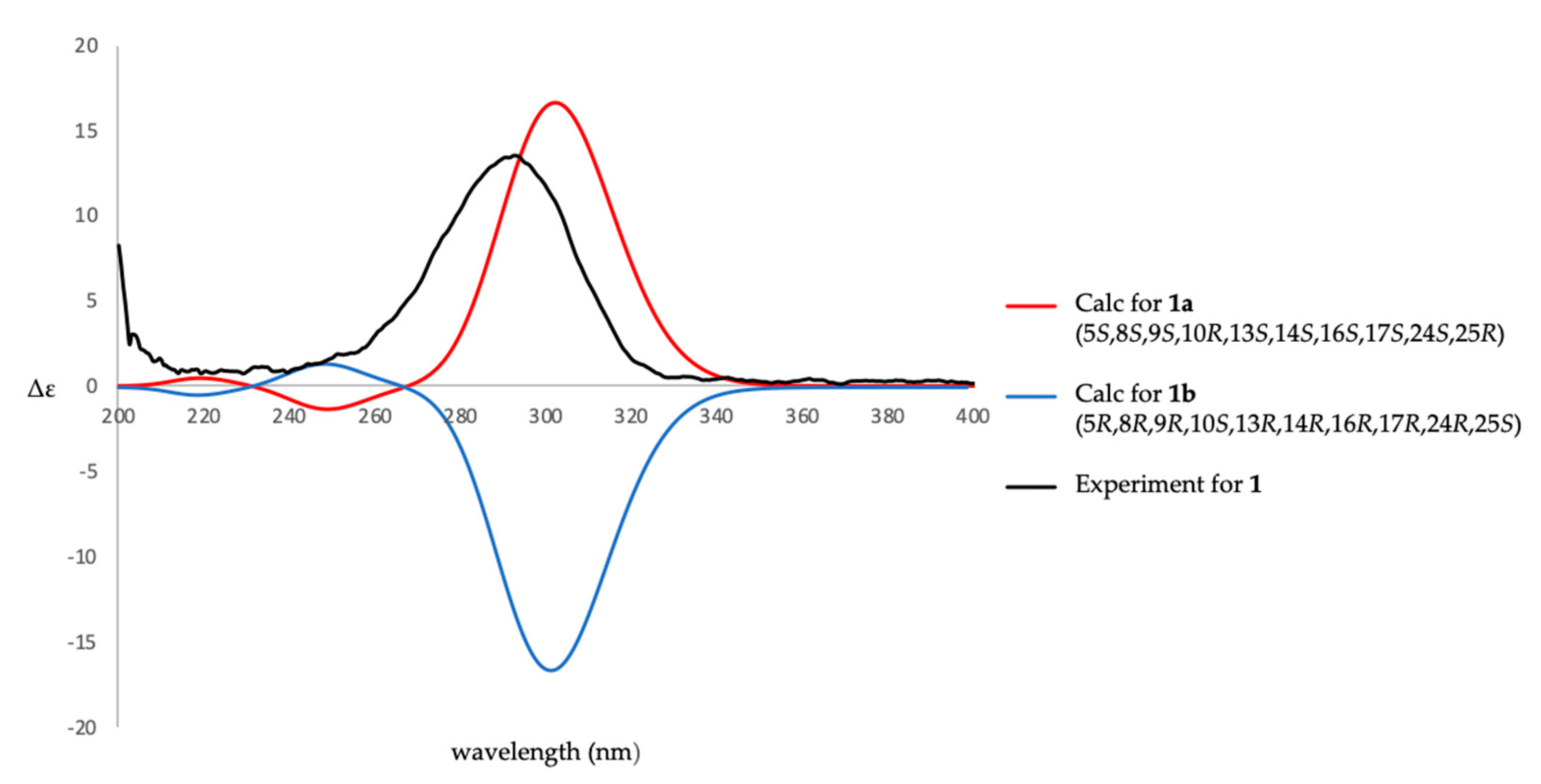
2. Results and Discussion
3. Material and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. ECD Calculations
3.5. Superoxide Anion Generation and Elastase Release by Human Neutrophils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kazlauskas, R.; Murphy, P.T.; Wells, R.J. Five new C26 tetracyclic terpenes from a sponge (Lendenfeldia sp.). Aust. J. Chem. 1982, 35, 51–59. [Google Scholar] [CrossRef]
- Alvi, K.A.; Crews, P. Homoscalarane sesterterpenes from Lendenfeldia frondosa. J. Nat. Prod. 1992, 55, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Stessman, C.C.; Ebel, R.; Corvino, A.J.; Crews, P. Employing dereplication and gradient 1D NMR methods to rapidly characterize sponge-derived sesterterpenes. J. Nat. Prod. 2002, 65, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Chill, L.; Rudi, A.; Aknin, M.; Loya, S.; Hizi, A.; Kashman, Y. New sesterterpenes from Madagascan Lendenfeldia sponges. Tetrahedron 2004, 60, 10619–10626. [Google Scholar] [CrossRef]
- Peng, B.-R.; Lai, K.-H.; Chen, Y.-Y.; Su, J.-H.; Huang, Y.M.; Chen, Y.-H.; Lu, M.-C.; Yu, S.S.-F.; Duh, C.-Y.; Sung, P.-J. Probing anti-proliferative 24-homoscalaranes from a sponge Lendenfeldia sp. Mar. Drugs 2020, 18, 76. [Google Scholar] [CrossRef] [Green Version]
- Oda, Y.; Zhang, Q.; Matsunaga, S.; Fujita, M.J.; Sakai, R. Two new mycosporine-like amino acids LC-343 and mycosporine-ethanolamine from the Micronesian marine sponge Lendenfeldia chondrodes. Chem. Lett. 2017, 46, 1272–1274. [Google Scholar] [CrossRef]
- Sera, Y.; Adachi, K.; Shizuri, Y. A new epidioxy sterol as an antifouling substance from a Palauan marine sponge, Lendenfeldia chondrodes. J. Nat. Prod. 1999, 62, 152–154. [Google Scholar] [CrossRef]
- Radwan, M.M.; Manly, S.P.; Ross, S.A. Two new sulfated sterols from the marine sponge Lendenfeldia dendyi. Nat. Prod. Commun. 2019, 2, 901–904. [Google Scholar] [CrossRef] [Green Version]
- Sakai, R.; Kamiya, H. 1-Deoxynojirimycin derivatives from the marine sponge Lendenfeldia chondrodes. J. Antibiot. 2006, 59, 507–511. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Liu, Y.; Zhou, Y.-D.; Nagle, D.G. Cytotoxic metabolites from an Indonesian sponge Lendenfeldia sp. J. Nat. Prod. 2007, 70, 1824–1826. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, R.; Mao, S.-C.; Morgan, J.B.; Jekabsons, M.B.; Zhou, Y.-D.; Nagle, D.G. Molecular-targeted antitumor agents. 19. Furospongolide from a marine Lendenfeldia sp. sponge inhibits hypoxia-inducible factor-1 activation in breast tumor cells. J. Nat. Prod. 2008, 71, 1854–1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radwan, M.M.; Wanas, A.S.; Fronczek, F.R.; Jacob, M.R.; Ross, S.A. Polybrominated diphenyl ethers from the marine organisms Lendenfeldia dendyi and Sinularia dura with anti-MRSa activity. Med. Chem. Res. 2015, 24, 3398–3404. [Google Scholar] [CrossRef]
- Lai, Y.-Y.; Chen, L.-C.; Wu, C.-F.; Lu, M.-C.; Wen, Z.-H.; Wu, T.-Y.; Fang, L.-S.; Wang, L.-H.; Wu, Y.-C.; Sung, P.-J. New cytotoxic 24-homoscalarane sesterterpenoids from the sponge Ircinia felix. Int. J. Mol. Sci. 2015, 16, 21950–21958. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-Y.; Lu, M.-C.; Wang, L.-H.; Chen, J.-J.; Fang, L.-S.; Wu, Y.-C.; Sung, P.-J. New scalarane sesterterpenoids from the Formosan sponge Ircinia felix. Mar. Drugs 2015, 13, 4296–4309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.-C.; Tseng, S.-W.; Liu, L.-L.; Chou, Y.; Ho, Y.-S.; Lu, M.-C.; Su, J.-H. Cytotoxic sesterterpenoids from a sponge Hippospongia sp. Mar Drugs 2012, 10, 987–997. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.-H.; Liu, Y.-C.; Su, J.-H.; El-Shazly, M.; Wu, C.-F.; Du, Y.-C.; Hsu, Y.-M.; Yang, J.-C.; Weng, M.-K.; Chou, C.-H.; et al. Antileukemic scalarane sesterterpenoids and meroditerpenoid from Carteriospongia (Phyllospongia) sp., induce apoptosis via dual inhibitory effects on topoisomerase II and Hsp90. Sci. Rep. 2016, 6, 36170. [Google Scholar] [CrossRef]
- González, M.A. Scalarane sesterterpenoids. Curr. Bioact. Compd. 2010, 6, 178–206. [Google Scholar] [CrossRef] [Green Version]
- Hahn, D.; Won, D.H.; Mun, B.; Kim, H.; Han, C.; Wang, W.; Chun, T.; Park, S.; Yoon, D.; Choi, H.; et al. Cytotoxic scalarane sesterterpenes from a Korean marine sponge Psammocinia sp. Bioorg. Med. Chem. Lett. 2003, 23, 2336–2339. [Google Scholar] [CrossRef]
- Alarif, W.M.; Al-Lihaibi, S.S.; Ghandourah, M.A.; Orif, M.I.; Basaif, S.A.; Ayyad, S.E.N. Cytotoxic scalarane-type sesterterpenes from the Saudi Red Sea sponge Hyrtios erectus. J. Asian Nat. Prod. Res. 2016, 18, 611–617. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, N.-H.; Lee, S.; Kim, Y.N.; Heo, J.D.; Jeong, E.J.; Rho, J.-R. Deacetylphylloketal, a new phylloketal derivative from a marine sponge, genus Phyllospongia, with potent anti-inflammatory activity in in vitro co-culture model of intestine. Mar. Drugs 2019, 17, 634. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Zeng, L.M.; Su, J.Y.; Pais, M.; Potier, P. Scalarane-type bishomosesterterpenes from the sponge Phyllospongia foliascens. J. Nat. Prod. 1992, 55, 1607–1613. [Google Scholar] [CrossRef]
- Sachs, C.W.; Christensen, R.H.; Pratt, P.C.; Lynn, W.S. Neutrophil elastase activity and superoxide production are diminished in neutrophils of alcoholics. Am. Rev. Respir. Dis. 1990, 141, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-C.; Yu, H.-P.; Chen, P.-J.; Yang, H.-W.; Chang, S.-H.; Tzeng, C.-C.; Cheng, W.-J.; Chen, Y.-R.; Chen, Y.-L.; Hwang, T.-L. A novel NOX2 inhibitor attenuates human neutrophil oxidative stress and ameliorates inflammatory arthritis in mice. Redox Biol. 2019, 26, 101273. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Ko, I.-L.; Lee, C.-L.; Hu, H.-C.; Chang, F.-R.; Wu, Y.-C.; Leu, Y.-L.; Wu, C.-C.; Lin, C.-Y.; Pan, C.-Y.; et al. Targeting allosteric site of AKT by 5,7-dimethoxy-1,4- phenanthrenequinone suppresses neutrophilic inflammation. EBioMedicine 2019, 40, 528–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
 ) and HMBC (
) and HMBC (  ) of 1–6.
) of 1–6.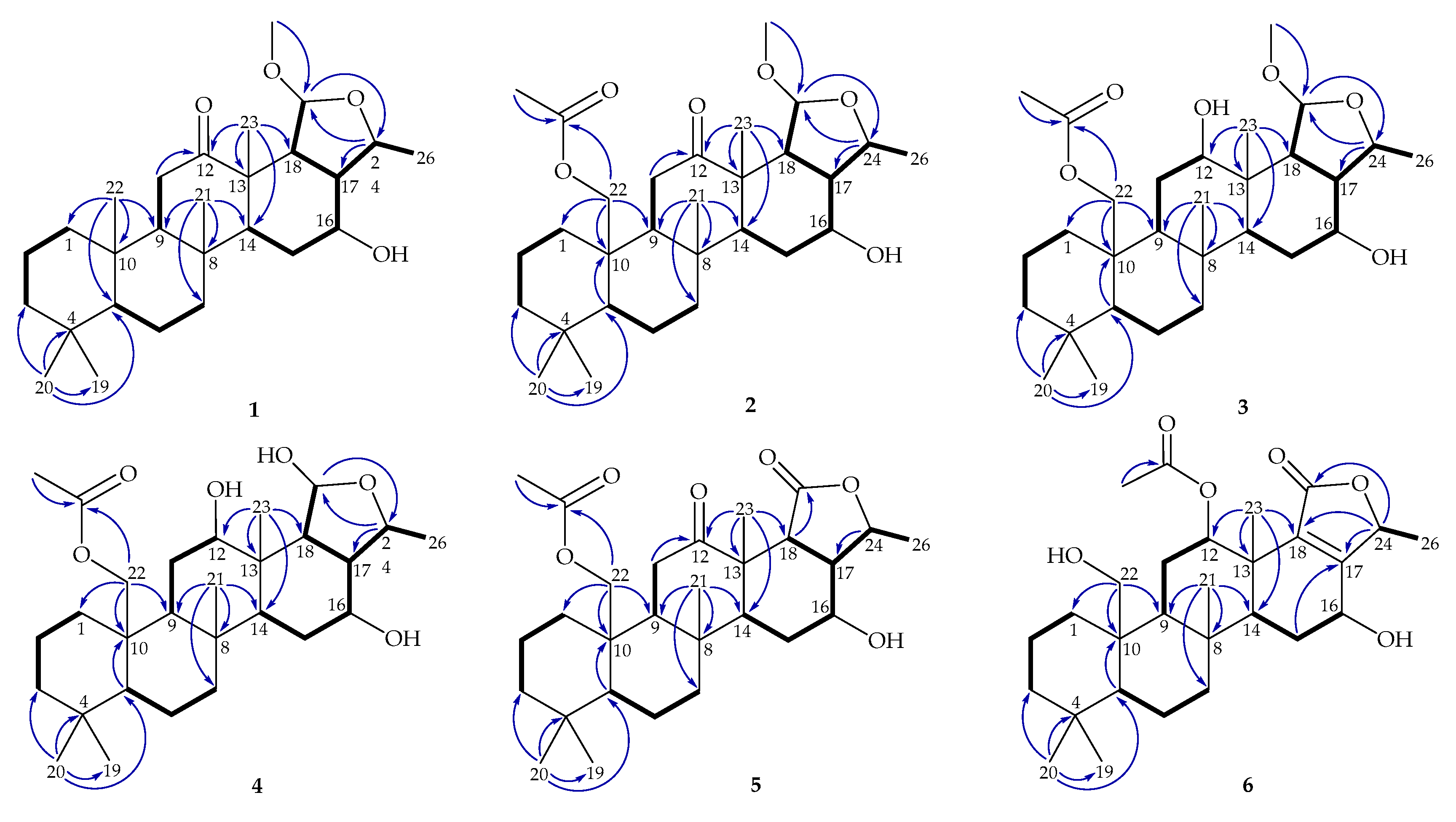
 ) of 1 and 2.
) of 1 and 2.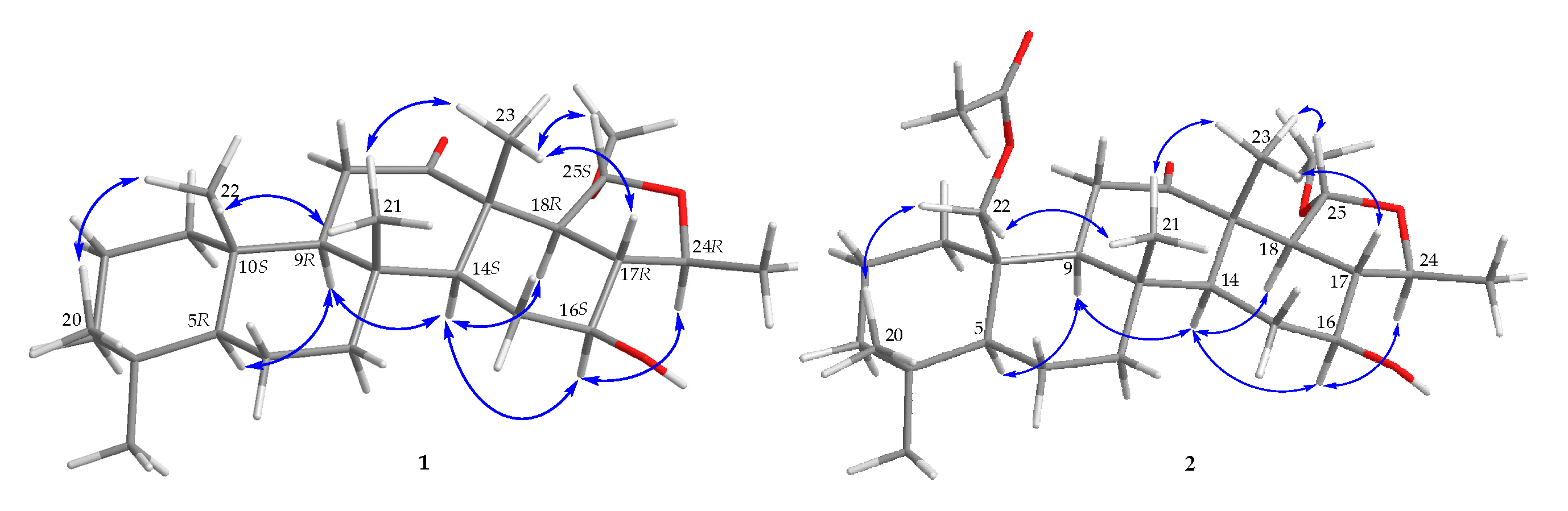
 ) of 3 and 4.
) of 3 and 4.
 ) of 5 and 6.
) of 5 and 6.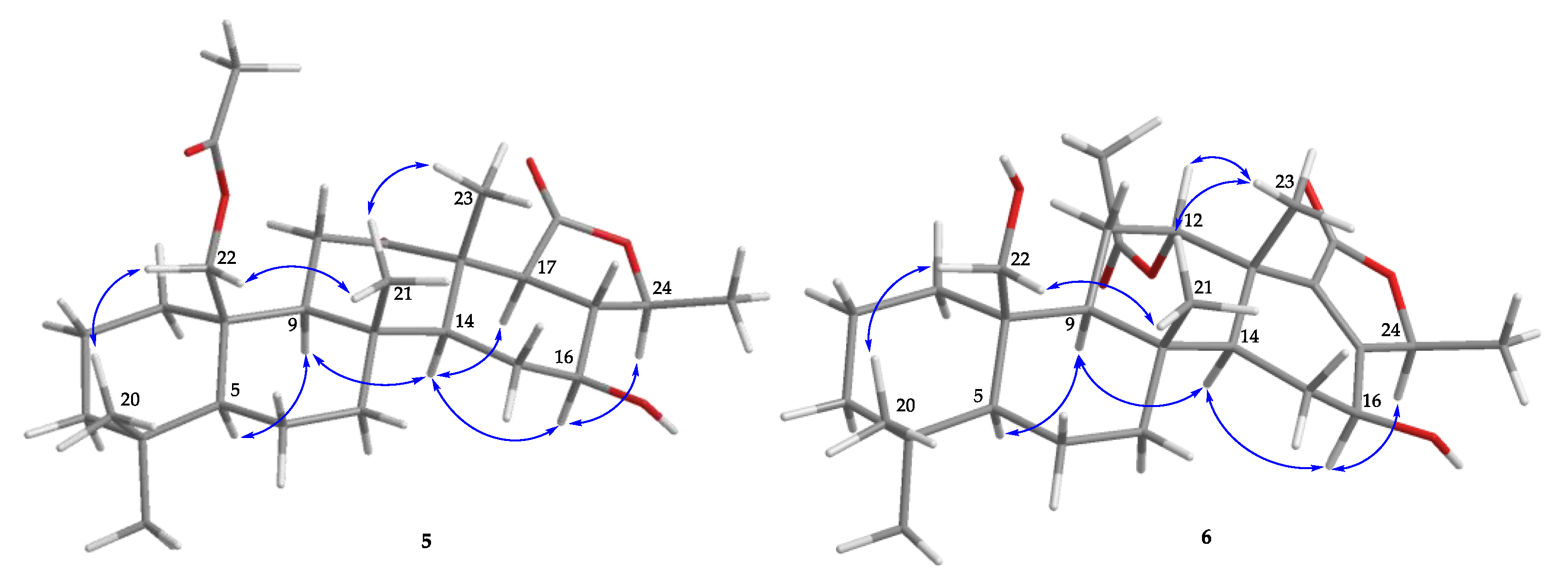
| 1 | 2 | |||
|---|---|---|---|---|
| C/H | δH (J in Hz) | δC Mult. | δH (J in Hz) | δC Mult. |
| 1 | 1.59 m; 0.80 m | 39.4, CH2 | 1.99 m; 0.77 m | 34.3, CH2 |
| 2 | 1.43 m; 1.58 m | 18.1, CH2 | 1.46 m; 1.58 m | 17.9, CH2 |
| 3 | 1.10 m; 1.36 m | 41.8, CH2 | 1.14 m; 1.43 m | 41.4, CH2 |
| 4 | 33.3, C | 33.0, C | ||
| 5 | 0.81 m | 56.6, CH | 0.97 m | 56.9, CH |
| 6 | 1.43 m | 18.4, CH2 | 1.46 m | 18.1, CH2 |
| 7 | 0.92 ddd (12.8, 12.8, 4.0) | 41.9, CH2 | 0.98 m; 1.90 m | 42.2, CH2 |
| 1.82 ddd (12.8, 2.8, 2.8) | ||||
| 8 | 37.8, C | 37.7, C | ||
| 9 | 1.15 m | 61.3, CH | 1.26 m | 61.1, CH |
| 10 | 38.1, C | 41.2, C | ||
| 11 | 2.66 dd (14.4, 13.6) | 35.1, CH2 | 2.99 dd (14.8, 13.6) | 37.9, CH2 |
| 2.24 dd (13.6, 2.0) | 2.43 dd (13.6, 2.4) | |||
| 12 | 215.3, C | 214.8, C | ||
| 13 | 51.8, C | 51.9, C | ||
| 14 | 1.13 m | 59.6, CH | 1.13 m | 59.7, CH |
| 15 | 1.52 m; 1.90 m | 30.8, CH2 | 1.56 m; 1.92 m | 31.0, CH2 |
| 16 | 3.43 ddd (10.4, 10.4, 4.4) | 74.8, CH | 3.43 ddd (10.0, 10.0, 4.4) | 74.6, CH |
| 17 | 1.89 m | 49.6, CH | 1.91 m | 49.5, CH |
| 18 | 1.62 m | 53.0, CH | 1.60 m | 52.8, CH |
| 19 | 0.82 s | 21.3, CH3 | 0.84 s | 21.8, CH3 |
| 20 | 0.85 s | 33.2, CH3 | 0.87 s | 33.7, CH3 |
| 21 | 1.07 s | 17.1, CH3 | 1.15 s | 16.5, CH3 |
| 22 | 0.86 s | 15.7, CH3 | 4.19 dd (12.4, 1.6); 4.65 d (12.4) | 64.6, CH2 |
| 23 | 1.36 s | 15.3, CH3 | 1.37 s | 15.4, CH3 |
| 24 | 3.84 qd (6.0, 2.4) | 78.7, CH | 3.83 qd (6.0, 2.0) | 78.8, CH |
| 25 | 5.35 d (4.0) | 104.8, CH | 5.33 d (4.4) | 104.7, CH |
| 26 | 1.40 d (6.0) | 23.5, CH3 | 1.40 d (6.0) | 23.5, CH3 |
| 22-OAc | 170.7, C | |||
| 2.04 s | 21.1, CH3 | |||
| 25-OMe | 3.30 s | 54.4, CH3 | 3.28 s | 54.4, CH3 |
| 3 | 4 | |||
|---|---|---|---|---|
| C/H | δH (J in Hz) | δC Mult. | δH (J in Hz) | δC Mult. |
| 1 | 2.04 m; 0.82 m | 34.5, CH2 | 2.03 m; 0.84 m | 34.6, CH2 |
| 2 | 1.45 m; 1.56 m | 18.4, CH2 | 1.45 m; 1.56 m | 18.4, CH2 |
| 3 | 1.18 m; 1.43 m | 41.5, CH2 | 1.17 br d (3.6); 1.42 m | 41.5, CH2 |
| 4 | 32.9, C | 33.0, C | ||
| 5 | 1.10 m | 56.6, CH | 1.09 m | 56.6, CH |
| 6 | 1.56 m | 18.1, CH2 | 1.56 m | 18.1, CH2 |
| 7 | 1.09 m; 1.78 ddd (12.8, 3.2, 3.2) | 42.0, CH2 | 1.08 m; 1.79 ddd (12.4, 3.2, 3.2) | 42.1, CH2 |
| 8 | 37.6, C | 37.6, C | ||
| 9 | 1.57 m | 52.0, CH | 1.58 m | 52.1, CH |
| 10 | 40.1, C | 40.2, C | ||
| 11 | 1.89 m; 1.29 m | 31.3, CH2 | 1.90 m; 1.29 m | 31.3, CH2 |
| 12 | 3.57 br s | 72.0, CH | 3.67 ddd (3.2, 3.2, 3.2) | 72.1, CH |
| 13 | 39.0, C | 39.0, C | ||
| 14 | 1.38 m | 52.2, CH | 1.40 m | 52.2, CH |
| 15 | 1.86–1.98 m | 25.9 CH2 | 1.91 m, 2.00 m | 26.1 CH2 |
| 16 | 3.55 ddd (10.0, 10.0, 4.8) | 72.9, CH | 3.58 ddd (10.0, 10.0, 4.8) | 73.0, CH |
| 17 | 1.57 m | 51.7, CH | 1.58 m | 52.1, CH |
| 18 | 1.92 m | 55.8, CH | 1.94 m | 56.7, CH |
| 19 | 0.82 s | 21.8, CH3 | 0.82 s | 21.8, CH3 |
| 20 | 0.86 s | 33.7, CH3 | 0.87 s | 33.7, CH3 |
| 21 | 0.90 s | 16.1, CH3 | 0.91 s | 16.1, CH3 |
| 22 | 4.17 d (11.6); 4.56 d (11.6) | 65.0, CH2 | 4.18 dd (12.0, 0.8); 4.57 d (12.0) | 65.0, CH2 |
| 23 | 0.93 s | 16.3, CH3 | 0.94 s | 16.2, CH3 |
| 24 | 3.98 qd (6.0, 3.2) | 79.5, CH | 4.09 qd (6.0, 3.2) | 79.7, CH |
| 25 | 4.85 d (6.4) | 103.9, CH | 5.35 dd (6.8, 3.2) | 96.5, CH |
| 26 | 1.37 d (6.0) | 20.3, CH3 | 1.36 d (6.0) | 20.5, CH3 |
| 22-OAc | 171.0, C | 171.0, C | ||
| 2.05 s | 21.2, CH3 | 2.06 s | 21.2, CH3 | |
| 25-OMe | 3.45 s | 56.6, CH3 | ||
| 5 | 6 | |||
|---|---|---|---|---|
| C/H | δH (J in Hz) a | δC Mult. b | δH (J in Hz) c | δC Mult. d |
| 1 | 1.99 m; 0.80 m | 34.4, CH2 | 2.10 m; 0.51 ddd (12.6, 12.6, 3.6) | 34.3, CH2 |
| 2 | 1.49 m; 1.64 m | 18.0, CH2 | 1.43 m; 1.56 m | 18.1, CH2 |
| 3 | 1.15 m; 1.45 m | 41.4, CH2 | 1.13 m; 1.44 m | 41.7, CH2 |
| 4 | 33.0, C | 33.0, C | ||
| 5 | 0.97 m | 57.0, CH | 0.98 dd (12.6, 2.4) | 57.0, CH |
| 6 | 1.50 m; 1.60 m | 18.1, CH2 | 1.42 m; 1.57 m | 17.9, CH2 |
| 7 | 0.99 m; 1.87 m | 41.9, CH2 | 1.12 m, 1.90 m | 42.0, CH2 |
| 8 | 38.5, C | 37.0, C | ||
| 9 | 1.28 br d (14.0) | 63.3, CH | 1.31 dd (7.2, 7.2) | 53.5, CH |
| 10 | 41.6, C | 41.8, C | ||
| 11 | 3.15 dd (14.0, 12.4); 2.51 dd (12.4, 2.4) | 37.6, CH2 | 1.87–1.95 m | 27.9, CH2 |
| 12 | 211.9, C | 5.51 t (3.0) | 73.8, CH | |
| 13 | 50.0, C | 39.0, C | ||
| 14 | 0.95 m | 59.3, CH | 1.79 dd (12.6, 2.4) | 46.2, CH |
| 15 | 1.50 m; 1.97 m | 31.1, CH2 | 2.21 m | 23.9 CH2 |
| 16 | 3.61 ddd (9.6, 9.6, 4.4) | 72.0, CH | 4.46 dd (5.4, 5.4) | 61.7, CH |
| 17 | 1.92 m | 51.4, CH | 161.3, C | |
| 18 | 2.58 d (14.4) | 49.7, CH | 135.7, C | |
| 19 | 0.84 s | 21.8, CH3 | 0.77 s | 21.8, CH3 |
| 20 | 0.87 s | 33.7, CH3 | 0.87 s | 33.8, CH3 |
| 21 | 1.18 s | 16.6, CH3 | 1.10 s | 16.3, CH3 |
| 22 | 4.17 dd (11.6, 1.6); 4.67 d (11.6) | 64.6, CH2 | 3.88 dd (11.4, 4.8); 4.04 d (11.4) | 62.8, CH2 |
| 23 | 1.38 s | 14.7, CH3 | 1.16 s | 19.4, CH3 |
| 24 | 4.30 qd (6.0, 2.4) | 79.5, CH | 5.07 q (6.6) | 76.5, CH |
| 25 | 172.3, C | 170.5, C | ||
| 26 | 1.53 d (6.0) | 20.1, CH3 | 1.41 d (6.6) | 18.3, CH3 |
| 12-OAc | 169.8, C | |||
| 1.98 s | 21.2, CH3 | |||
| 22-OAc | 170.7, C | |||
| 2.06 s | 21.1, CH3 | |||
| Compound | Superoxide Anions | Elastase Release | ||||
|---|---|---|---|---|---|---|
| IC50 (μM) a | Inh (Enh) b % | IC50 (μM) a | Inh % | |||
| 1 | (11.35 ± 3.65) | * | 1.74 ± 0.08 | 82.80 ± 3.91 | *** | |
| 2 | 6.17 ± 0.16 | 70.68 ± 3.86 | *** | 26.15 ± 3.40 | ** | |
| 3 | 6.81 ± 0.52 | 62.92 ± 2.58 | *** | 25.19 ± 4.01 | ** | |
| 4 | 7.13 ± 3.69 | 2.97 ± 1.63 | ||||
| 5 | 9.97 ± 4.38 | 6.81 ± 2.46 | ||||
| 6 | 4.52 ± 2.91 | 1.16 ± 0.89 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, B.-R.; Lai, K.-H.; Chang, Y.-C.; Chen, Y.-Y.; Su, J.-H.; Huang, Y.M.; Chen, P.-J.; Yu, S.S.-F.; Duh, C.-Y.; Sung, P.-J. Sponge-Derived 24-Homoscalaranes as Potent Anti-Inflammatory Agents. Mar. Drugs 2020, 18, 434. https://doi.org/10.3390/md18090434
Peng B-R, Lai K-H, Chang Y-C, Chen Y-Y, Su J-H, Huang YM, Chen P-J, Yu SS-F, Duh C-Y, Sung P-J. Sponge-Derived 24-Homoscalaranes as Potent Anti-Inflammatory Agents. Marine Drugs. 2020; 18(9):434. https://doi.org/10.3390/md18090434
Chicago/Turabian StylePeng, Bo-Rong, Kuei-Hung Lai, Yu-Chia Chang, You-Ying Chen, Jui-Hsin Su, Yusheng M. Huang, Po-Jen Chen, Steve Sheng-Fa Yu, Chang-Yih Duh, and Ping-Jyun Sung. 2020. "Sponge-Derived 24-Homoscalaranes as Potent Anti-Inflammatory Agents" Marine Drugs 18, no. 9: 434. https://doi.org/10.3390/md18090434
APA StylePeng, B. -R., Lai, K. -H., Chang, Y. -C., Chen, Y. -Y., Su, J. -H., Huang, Y. M., Chen, P. -J., Yu, S. S. -F., Duh, C. -Y., & Sung, P. -J. (2020). Sponge-Derived 24-Homoscalaranes as Potent Anti-Inflammatory Agents. Marine Drugs, 18(9), 434. https://doi.org/10.3390/md18090434






