Marine Bromophenol Bis(2,3,6-Tribromo-4,5-Dihydroxybenzyl)ether Inhibits Angiogenesis in Human Umbilical Vein Endothelial Cells and Reduces Vasculogenic Mimicry in Human Lung Cancer A549 Cells
Abstract
:1. Introduction
2. Results
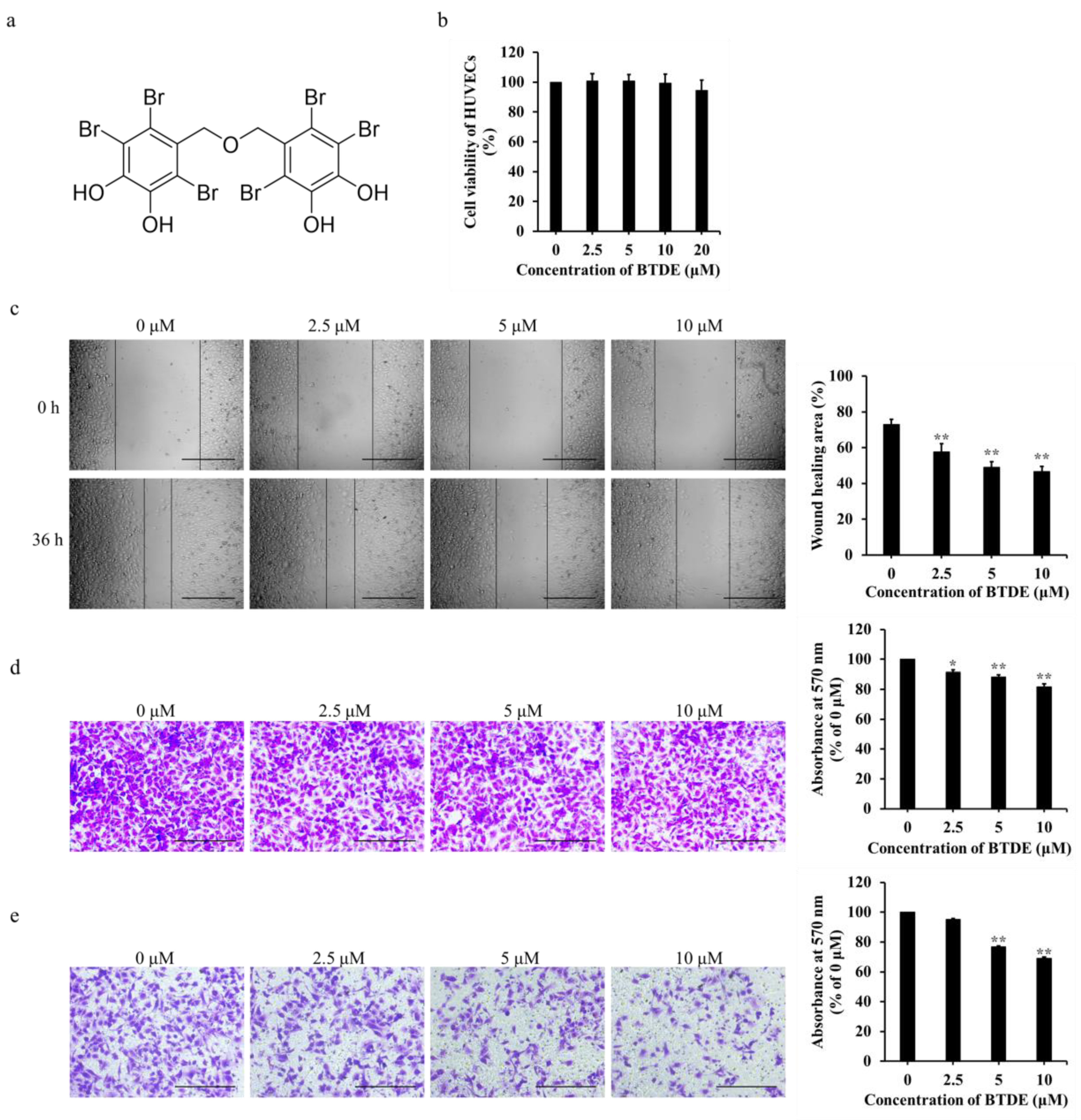
2.1. BTDE Inhibits the Migration and Invasion of HUVECs
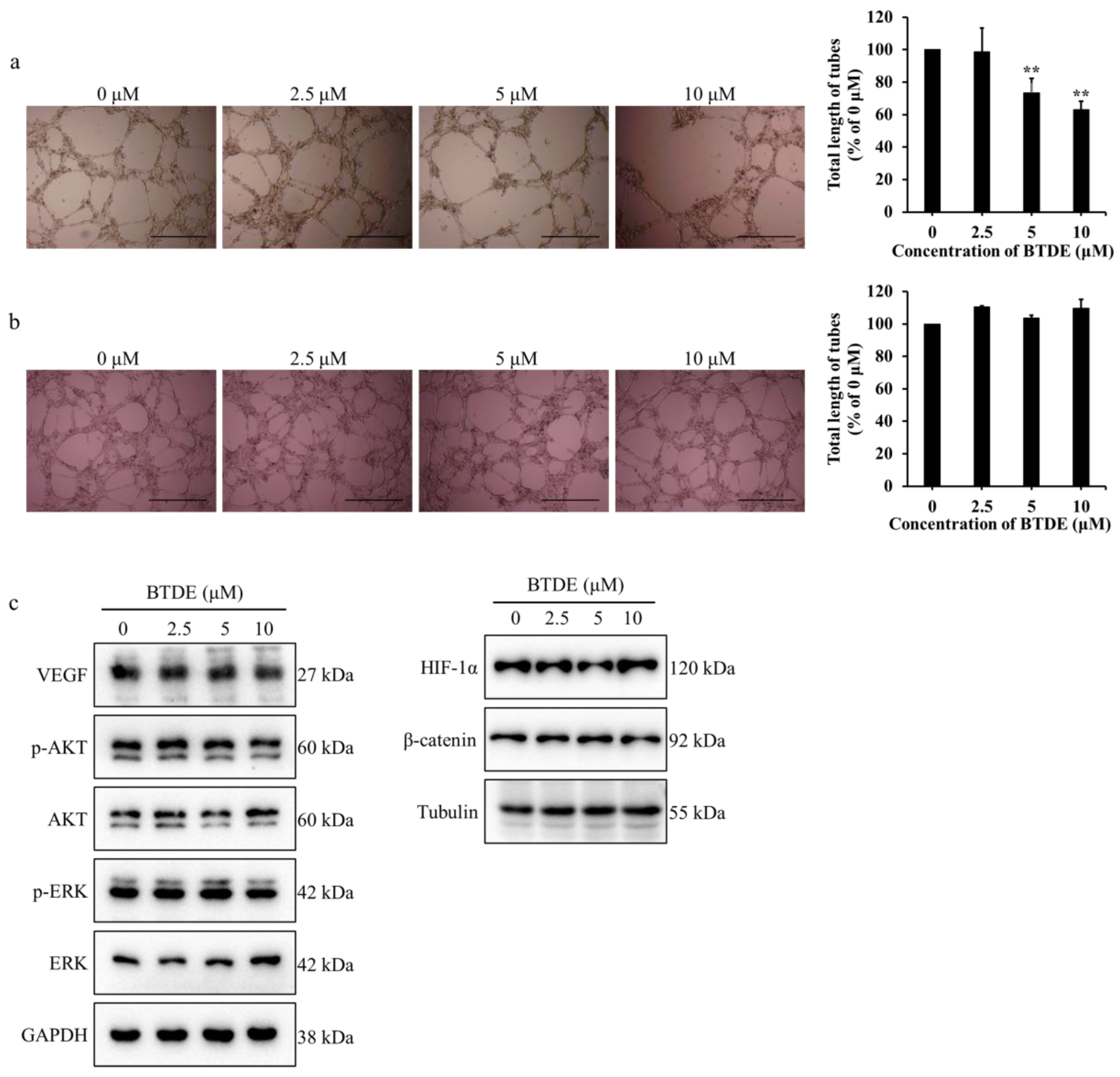
2.2. BTDE Reduces HUVECs Tube Formation and MMP9 Activity
2.3. BTDE Blocks Intersegmental Vessel Formation in Zebrafish Embryos
2.4. BTDE Decreases the Migration and Invasion of A549
2.5. BTDE Decreases the Vasculogenic Mimicry of A549 Cells
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Cell Lines and Cell Culture
4.3. Cell Viability Assay
4.4. Scratch-Wound Cell Migration Assay
4.5. Transwell Migration and Invasion Assay
4.6. HUVECs Tube Formation and A549 Vasculogenic Mimicry Assay
4.7. Zebrafish Embryo Assay
4.8. Gelatin Zymography Assay
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ellis, L.M.; Liu, W.; Ahmad, S.A.; Fan, F.; Jung, Y.D.; Shaheen, R.M.; Reinmuth, N. Overview of angiogenesis: Biologic implications for antiangiogenic therapy. Semin. Oncol. 2001, 28, 94–104. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Chan, L.; Daruwalla, J.; Christophi, C. Selective targeting of the tumour vasculature. ANZ J. Surg. 2008, 78, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Deplanque, G.; Harris, A.L. Anti-angiogenic agents: Clinical trial design and therapies in development. Eur. J. Cancer 2000, 36, 1713–1724. [Google Scholar] [CrossRef]
- Taraboletti, G.; Margosio, B. Antiangiogenic and antivascular therapy for cancer. Curr. Opin. Pharmacol. 2001, 1, 378–384. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yang, S.C.; Hao, J.; Yuan, X.; Luo, W.; Jiang, L.; Hu, Y.; Fu, Z.; Zhang, Y.; Zou, C. Endostar attenuates melanoma tumor growth via its interruption of b-FGF mediated angiogenesis. Cancer Lett. 2015, 359, 148–154. [Google Scholar] [CrossRef]
- Loges, S.; Schmidt, T.; Carmeliet, P. Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes Cancer 2010, 1, 12–25. [Google Scholar] [CrossRef]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Peter, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular channel formation by human melanoma cells in vivo and in vitro: Vasculogenic mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef] [Green Version]
- Hendrix, M.; Seftor, E.A.; Hess, A.R.; Seftor, R. Molecular plasticity of human melanoma cells. Oncogene 2003, 22, 3070–3075. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhu, D.M.; Zhou, X.G.; Yin, N.; Zhang, Y. HIF-2α promotes the formation of vasculogenic mimicry in pancreatic cancer by regulating the binding of Twist1 to the VE-cadherin promoter. Oncotarget 2017, 8, 47801–47815. [Google Scholar] [CrossRef]
- Van Beurden, A.; Schmitz, R.F.; Van Dijk, C.M.; Baeten, C.I.M. Periodic acid Schiff loops and blood lakes associated with metastasis in cutaneous melanoma. Melanoma Res. 2012, 22, 424. [Google Scholar] [CrossRef]
- Liu, T.J.; Sun, B.C.; Zhao, X.L.; Zhao, X.M.; Sun, T.; Gu, Q.; Yao, Z.; Dong, X.Y.; Zhao, N.; Liu, N. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene 2013, 32, 544–553. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Yu, L.; Wang, D.; Zhou, L.; Cheng, Z.; Chai, D.; Ma, L.; Tao, Y. Aberrant expression of CD133 in non-small cell lung cancer and its relationship to vasculogenic mimicry. BMC Cancer 2012, 12, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, J.; Zhou, H.; Fan, G.; Li, Q. Molecular mechanisms and anticancer therapeutic strategies in vasculogenic mimicry. J. Cancer 2019, 10, 6327–6340. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Liu, Q.; Wu, J.; Wang, Y.; Lian, Y. Galectin-1 promotes vasculogenic mimicry in gastric cancer by upregulating EMT signaling. J. Cancer 2019, 10, 6286–6297. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.C.; Metcalf, R.L.; Trapani, F.; Mohan, S.; Antonello, J.; Abbott, B.; Leong, H.S.; Chester, C.; Simms, N.; Polanski, R. Vasculogenic mimicry in small cell lung cancer. Nat. Commun. 2016, 7, 13322. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Guerrero, A.; Rodríguez, A.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine pharmacology in 2016–2017: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2021, 19, 49. [Google Scholar]
- Dyshlovoy, S. Recent updates on marine cancer-preventive compounds. Mar. Drugs 2021, 19, 558. [Google Scholar] [CrossRef]
- Yue, Z.; Jie, Z.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar]
- Newman, D.; Cragg, G. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.; Dong, S.; Hansen, P.E.; Stagos, D.; Lin, X.; Liu, M. Progress of bromophenols in marine algae from 2011 to 2020: Structure, bioactivities, and applications. Mar. Drugs 2020, 18, 411. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, L.J.; Bo, J.; Wu, N.; Shi, D. Anti-angiogenic properties of BDDPM, a bromophenol from marine red alga rhodomela confervoides, with multi receptor tyrosine kinase inhibition effects. Int. J. Mol. Sci. 2015, 16, 13548–13560. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Liu, G.; Qiu, L.; Lin, X.; Liu, M. Marine bromophenol bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, represses angiogenesis in HUVEC cells and in zebrafish embryos via inhibiting the VEGF signal systems. Biomed. Pharmacother. 2015, 75, 58–66. [Google Scholar] [CrossRef]
- Kurata, K.; Amiya, T. Bis(2,3,6-tribromo-4,5-dihydroxybenzyl) ether from the red alga, Symphyocladia latiuscula. Phytochemistry 1980, 19, 141–142. [Google Scholar] [CrossRef]
- Duan, X.J.; Li, X.M.; Wang, B.G. Highly brominated mono- and bis-phenols from the marine red alga Symphyocladia latiuscula with radical-scavenging activity. J. Nat. Prod. 2007, 70, 1210–1213. [Google Scholar] [CrossRef]
- Dong, H.; Liu, M.; Wang, L.; Liu, Y.; Lu, X.; Stagos, D.; Lin, X.; Liu, M. Bromophenol bis (2,3,6-tribromo-4,5-dihydroxybenzyl) ether protects HaCaT skin cells from oxidative damage via Nrf2-mediated pathways. Antioxidants 2021, 10, 1436. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Park, H.J.; Jung, H.A.; Choi, J.S. Anti-diabetic activity of 2,3,6-tribromo-4,5-dihydroxybenzyl derivatives from Symphyocladia latiuscula through PTP1B downregulation and α-glucosidase inhibition. Mar. Drugs 2019, 17, 166. [Google Scholar] [CrossRef] [Green Version]
- Paudel, P.; Park, S.; Seong, S.; Jung, H.; Choi, J. Symphyocladia latiuscula bromophenols from target human monoamine oxidase and dopaminergic receptors for the management of neurodegenerative diseases. J. Agric. Food Chem. 2020, 68, 2426–2436. [Google Scholar] [CrossRef]
- Paudel, P.; Wagle, A.; Seong, S.H.; Park, H.J.; Jung, H.A.; Choi, J.S. A new tyrosinase inhibitor from the red alga Symphyocladia latiuscula (harvey) yamada (rhodomelaceae). Mar. Drugs 2019, 17, 295. [Google Scholar] [CrossRef] [Green Version]
- Mikami, D.; Kurihara, H.; Ono, M.; Kim, S.M.; Takahashi, K. Inhibition of algal bromophenols and their related phenols against glucose 6-phosphate dehydrogenase. Fitoterapia 2016, 108, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobierajska, K.; Ciszewski, W.M.; Sacewicz-Hofman, I.; Niewiarowska, J. Endothelial cells in the tumor microenvironment. Adv. Exp. Med. Biol. 2020, 1234, 71–86. [Google Scholar] [PubMed]
- Kubota, Y. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J. Cell Biol. 1988, 107, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef]
- McCawley, L.J.; Matrisian, L.M. Matrix metalloproteinases: They’re not just for matrix anymore! Curr. Opin. Cell Biol. 2001, 13, 534–540. [Google Scholar] [CrossRef]
- Miriam; Martini; Maria; Chiara; De; Santis; Laura; Braccini; Federico; Gulluni. PI3K/AKT signaling pathway and cancer: An updated review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef]
- Ruchika, S.; Tahera, Z.; Huang, H.; Zhang, J.; Parul, G.; Soledad, F.; Colleen, K.J.; Landreth, G.E.; Gustavo, L.; Ostrowski, M.C. Erk1 and Erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLoS ONE 2009, 4, e8283. [Google Scholar]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef]
- Bai, X.; Zhi, X.; Zhang, Q.; Liang, F.; Chen, W.; Liang, C.; Hu, Q.; Sun, X.; Zhuang, Z.; Liang, T. Inhibition of protein phosphatase 2A sensitizes pancreatic cancer to chemotherapy by increasing drug perfusion via HIF-1α-VEGF mediated angiogenesis. Cancer Lett. 2014, 355, 281–287. [Google Scholar] [CrossRef]
- Cross, L.M.; Cook, M.A.; Lin, S.; Chen, J.N.; Rubinstein, A.L. Rapid analysis of angiogenesis drugs in a live fluorescent zebrafish assay. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 911–912. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.; Chen, J.N.; Garrity, D.M.; Fishman, M.C. Patterning of angiogenesis in the zebrafish embryo. Development 2002, 129, 973–982. [Google Scholar] [CrossRef]
- Guo, C.L.; Wang, L.J.; Yue, Z.; Liu, H.; Li, X.Q.; Bo, J.; Jiao, L.; Guo, S.J.; Wu, N.; Shi, D.Y. A novel bromophenol derivative BOS-102 induces cell cycle arrest and apoptosis in human A549 lung cancer cells via ROS-mediated PI3K/Akt and the MAPK signaling pathway. Mar. Drugs 2018, 16, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopoulos, N.; Martin, J.; Qin, R.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, H.; Zhao, J.; Zhou, L.; Wen, H.; Deng, W.; Li, C.; Ji, Q.; Liu, X.; Feng, Y.; Chai, N.; et al. Tanshinone IIA inhibits beta-catenin/VEGF-mediated angiogenesis by targeting TGF-beta 1 in normoxic and HIF-1 alpha in hypoxic microenvironments in human colorectal cancer. Cancer Lett. 2017, 403, 86–97. [Google Scholar] [CrossRef]
- Vasudev, N.S.; Reynolds, A.R. Anti-angiogenic therapy for cancer: Current progress, unresolved questions and future directions. Angiogenesis 2014, 17, 495–497. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Shang, B.; Zhang, G.; Miele, L.; Zhou, Q. Tumor cell-mediated neovascularization and lymphangiogenesis contrive tumor progression and cancer metastasis. BBA Rev. Cancer 2013, 1836, 273–286. [Google Scholar] [CrossRef]
- Yeo, C.; Lee, H.J.; Lee, E.O. Serum promotes vasculogenic mimicry through the EphA2/VE-cadherin/AKT path-way in PC-3 human prostate cancer cells. Life Sci. 2019, 15, 267–273. [Google Scholar] [CrossRef]
- Masckauchan, T.N.H.; Agalliu, D.; Vorontchikhina, M.; Ahn, A.; Parmalee, N.L.; Li, C.M.; Khoo, A.; Tycko, B.; Brown, A.M.C.; Kitajewski, J. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol. Biol. Cell 2006, 17, 5163–5172. [Google Scholar] [CrossRef]
- Yang, D.H.; Yoon, J.Y.; Lee, S.H.; Bryja, V.; Andersson, E.R.; Arenas, E.; Kwon, Y.G.; Choi, K.Y. Wnt5a is required for endothelial differentiation of embryonic stem cells and vascularization via pathways involving both Wnt/beta-catenin and protein kinase Calpha. Circ. Res. 2009, 104, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Tosetti, F.; Ferrari, N.; De Flora, S.; Albini, A. Angioprevention: Angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J. 2002, 16, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; D’Agostini, F.; Giunciuglio, D.; Paglieri, I.; Balansky, R.; De Flora, S. Inhibition of invasion, gelatinase activity, tumor take and metastasis of malignant cells byN-acetylcysteine. Int. J. Cancer 1995, 61, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Fassina, G.F.; Morini, M.; Aluigi, M.G.; Albini, A. N-acetylcysteine inhibits endothelial cell invasion and angiogenesis. Lab. Investig. J. Tech. Methods Pathol. 1999, 79, 1151–1159. [Google Scholar]



| BTDE (μM) | Number of Embryos | Number of Deformities | Deformity Rate (%) | Number of Mortalities | Mortality Rate (%) |
|---|---|---|---|---|---|
| 0 | 71 | 2 | 2.8 | 0 | 0 |
| 2.5 | 70 | 1 | 1.4 | 0 | 0 |
| 5 | 71 | 1 | 1.4 | 1 | 1.4 |
| 10 | 70 | 0 | 0 | 2 | 2.8 |
| 20 | 73 | 1 | 1.4 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Chen, Z.; Wang, L.; Liu, Y.; Stagos, D.; Lin, X.; Liu, M. Marine Bromophenol Bis(2,3,6-Tribromo-4,5-Dihydroxybenzyl)ether Inhibits Angiogenesis in Human Umbilical Vein Endothelial Cells and Reduces Vasculogenic Mimicry in Human Lung Cancer A549 Cells. Mar. Drugs 2021, 19, 641. https://doi.org/10.3390/md19110641
Dong S, Chen Z, Wang L, Liu Y, Stagos D, Lin X, Liu M. Marine Bromophenol Bis(2,3,6-Tribromo-4,5-Dihydroxybenzyl)ether Inhibits Angiogenesis in Human Umbilical Vein Endothelial Cells and Reduces Vasculogenic Mimicry in Human Lung Cancer A549 Cells. Marine Drugs. 2021; 19(11):641. https://doi.org/10.3390/md19110641
Chicago/Turabian StyleDong, Songtao, Zhongyuan Chen, Li Wang, Yankai Liu, Dimitrios Stagos, Xiukun Lin, and Ming Liu. 2021. "Marine Bromophenol Bis(2,3,6-Tribromo-4,5-Dihydroxybenzyl)ether Inhibits Angiogenesis in Human Umbilical Vein Endothelial Cells and Reduces Vasculogenic Mimicry in Human Lung Cancer A549 Cells" Marine Drugs 19, no. 11: 641. https://doi.org/10.3390/md19110641
APA StyleDong, S., Chen, Z., Wang, L., Liu, Y., Stagos, D., Lin, X., & Liu, M. (2021). Marine Bromophenol Bis(2,3,6-Tribromo-4,5-Dihydroxybenzyl)ether Inhibits Angiogenesis in Human Umbilical Vein Endothelial Cells and Reduces Vasculogenic Mimicry in Human Lung Cancer A549 Cells. Marine Drugs, 19(11), 641. https://doi.org/10.3390/md19110641






