Direct Extraction of Lipids, β-Carotene, and Polyphenolic Compounds from Wet Microalga Dunaliella salina by Liquefied Dimethyl Ether
Abstract
:1. Introduction
2. Results and Discussion
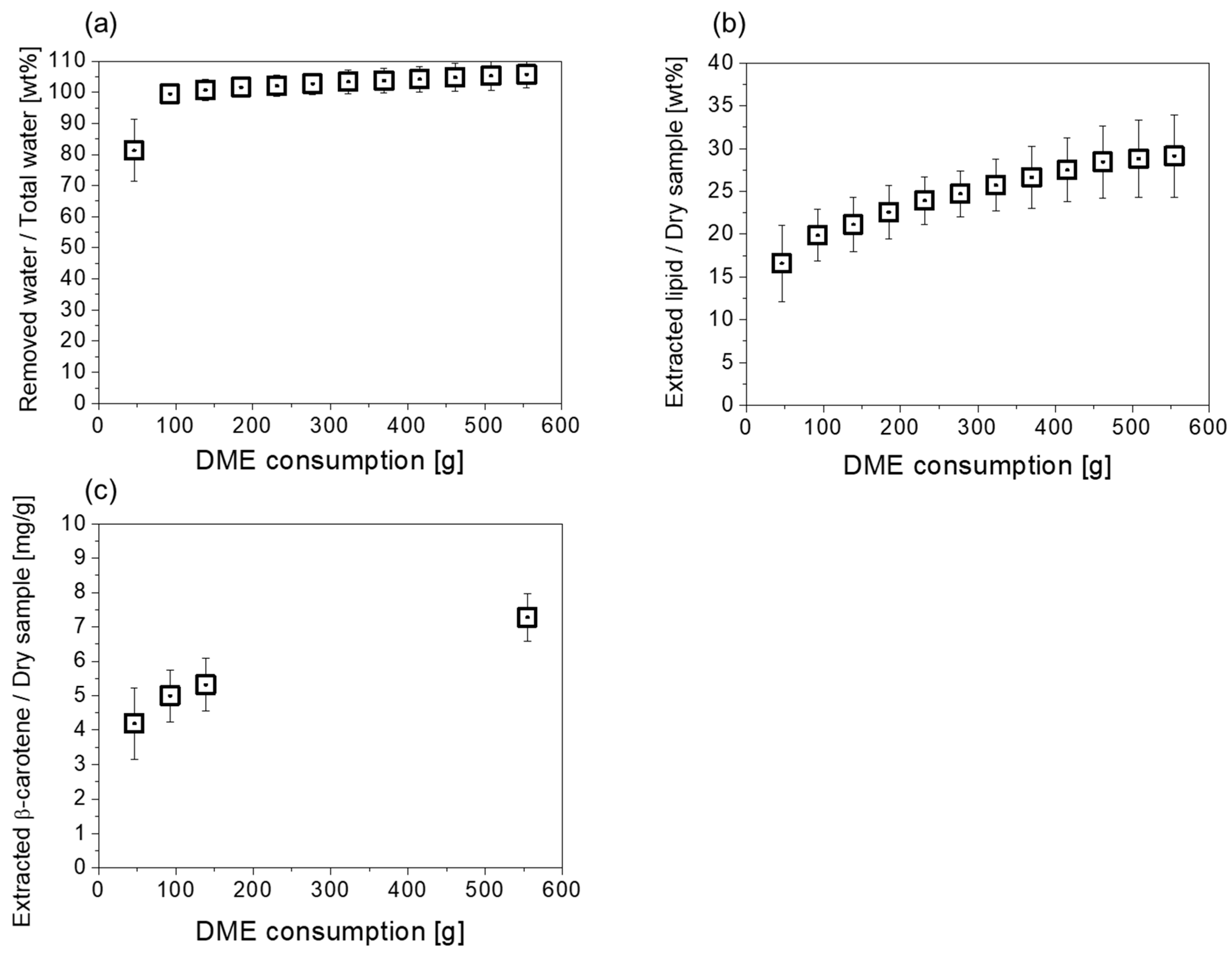
2.1. Basic Extraction Behavior
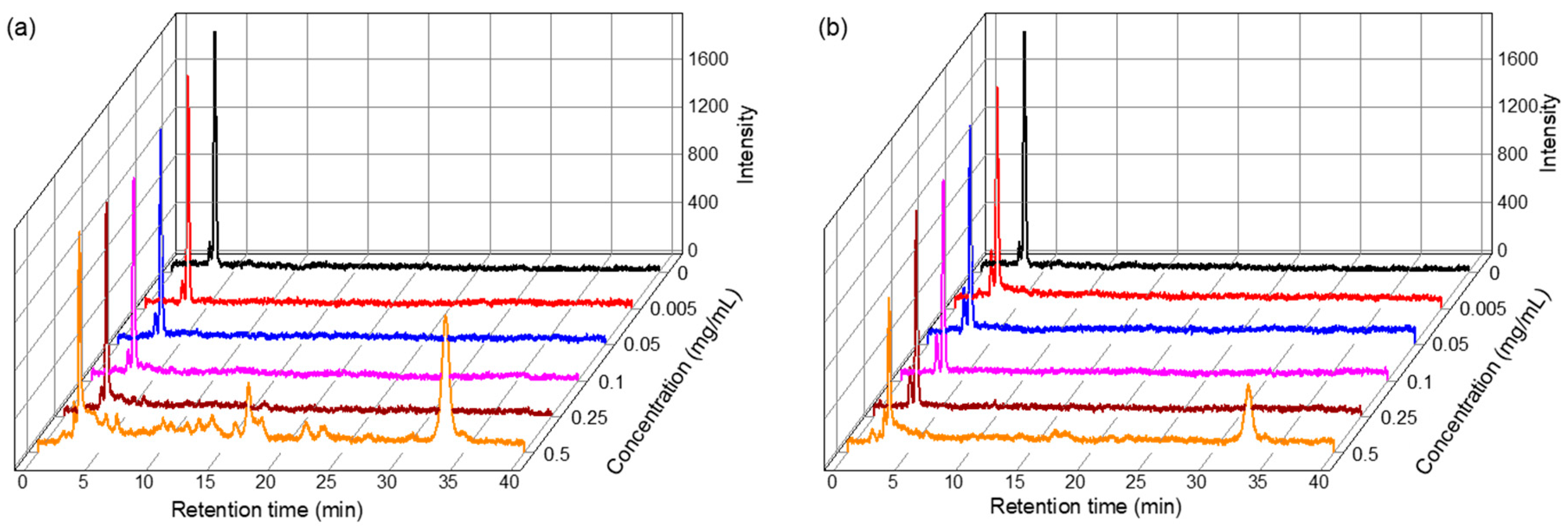
2.2. Polyphenolic Compound Characterizations
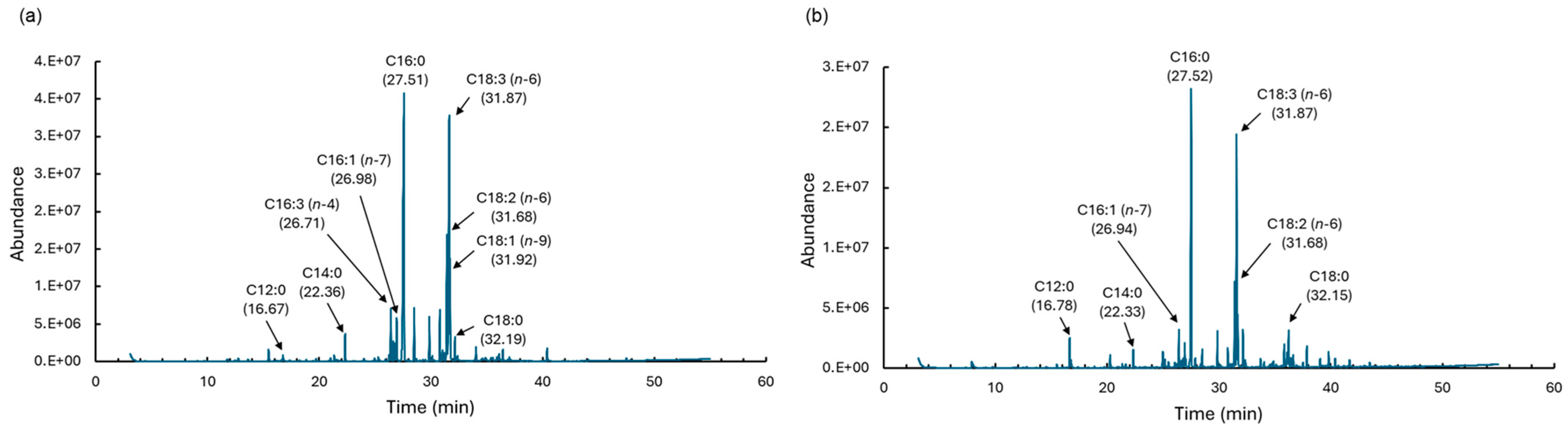
2.3. Lipid Characterizations
3. Materials and Methods
3.1. Materials
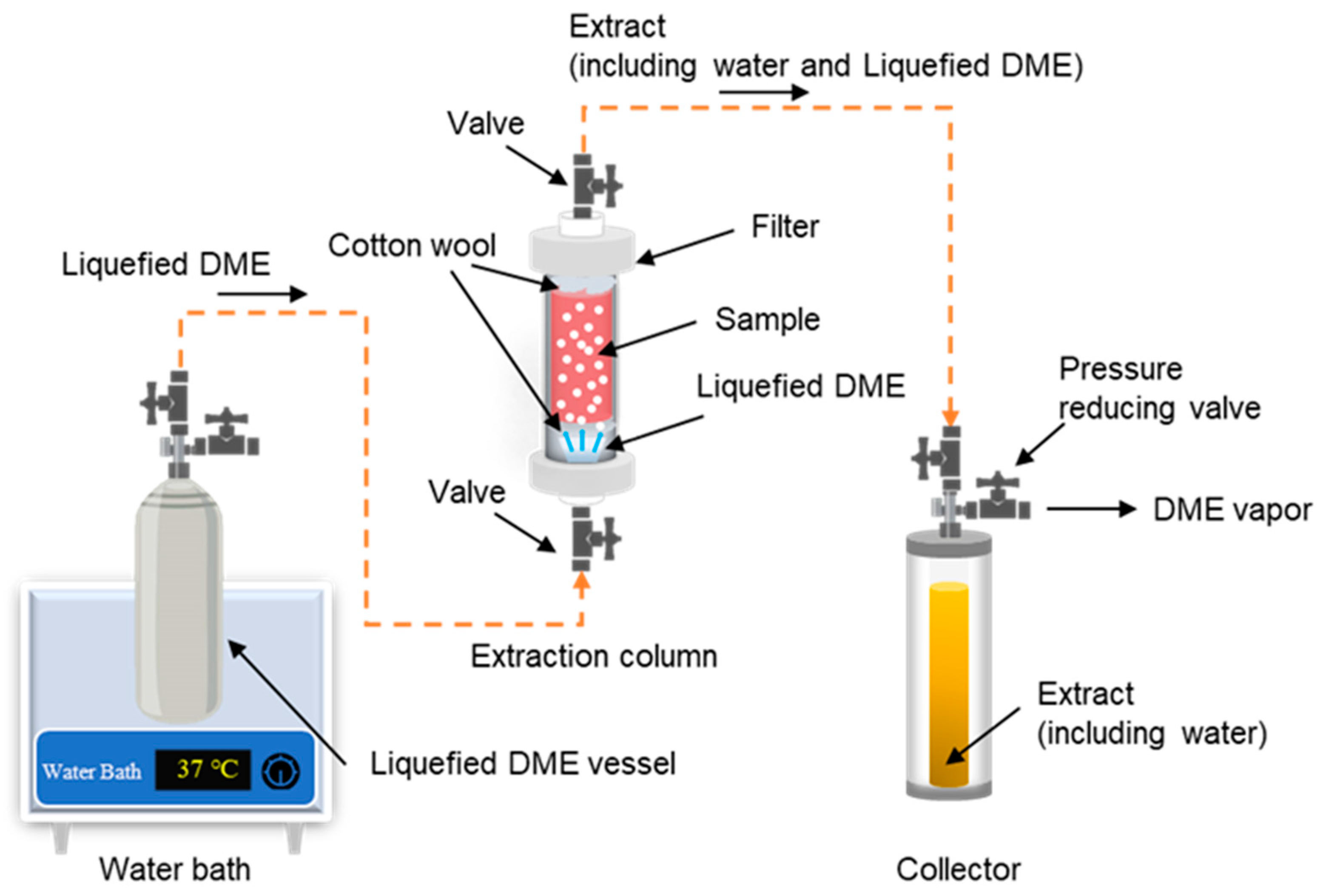
3.2. Liquefied DME extraction
3.3. Chloroform–Methanol Extraction
3.4. Analytical Methods
3.4.1. β-Carotene Determination
3.4.2. FT-IR Characterization
3.4.3. TPC Assay
3.4.4. DPPH Radical Scavenging Activity Assay
3.4.5. Elemental Analysis
3.4.6. FE-SEM Characterization
3.4.7. Fatty Acids Analysis
3.4.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lafarga, T.; Clemente, I.; Garcia-Vaquero, M. Carotenoids from microalgae. In Carotenoids: Properties, Processing and Applications; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 149–187. [Google Scholar]
- Machmudah, S.; Diono, W.; Kanda, H.; Goto, M. Supercritical fluids extraction of valuable compounds from algae: Future perspectives and challenges. Eng. J. 2018, 22, 13–30. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Gonçalves, J.; Conde, T.A.; Couto, D.; Melo, T.; Maia, I.B.; Pereira, H.; Silva, J.; Domingues, M.R.; Nunes, C. Chrysotila pseudoroscoffensis as a source of high-value polar lipids with antioxidant activity: A lipidomic approach. Algal Res. 2022, 66, 102756. [Google Scholar] [CrossRef]
- Couto, D.; Conde, T.A.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, R.; Domingues, P. The chemodiversity of polar lipidomes of microalgae from different taxa. Algal Res. 2023, 70, 103006. [Google Scholar] [CrossRef]
- Goto, M.; Kanda, H.; Wahyudiono; Machmudah, S. Extraction of carotenoids and lipids from algae by supercritical CO2 and subcritical dimethyl ether. J. Supercrit. Fluids 2015, 96, 245–251. [Google Scholar] [CrossRef]
- Tanaka, Y.; Uemori, C.; Kon, T.; Honda, M.; Wahyudiono; Machmudah, S.; Kanda, H.; Goto, M. Preparation of liposomes encapsulating β–carotene using supercritical carbon dioxide with ultrasonication. J. Supercrit. Fluids 2020, 161, 104848. [Google Scholar] [CrossRef]
- Ono, M.; Honda, M.; Wahyudiono; Yasuda, K.; Kanda, H.; Goto, M. Production of β-carotene nanosuspensions using supercritical CO2 and improvement of its efficiency by Z-isomerization pre-treatment. J. Supercrit. Fluids 2018, 138, 124–131. [Google Scholar] [CrossRef]
- Abu-Rezq, T.; Al-Hooti, S.; Jacob, D.A.; Al-Shamali, M.; Ahmed, N. Induction and extraction of β-carotene from locally isolated Dunaliella salina. J. Algal Biomass Utln. 2010, 1, 58–83. [Google Scholar]
- Lamers, P.P.; van de Laak, C.C.W.; Kaasenbrood, P.S.; Lorier, J.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol. Bioeng. 2010, 106, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.; Vitali, C.; Sánchez-Martínez, J.D.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E.; Herrero, M. Compressed CO2 technologies for the recovery of carotenoid-enriched extracts from Dunaliella salina with potential neuroprotective activity. ACS Sustain. Chem. Eng. 2020, 8, 11413–11423. [Google Scholar] [CrossRef]
- Hosseinim, S.R.P.; Tavakoli, O.; Sarrafzadeh, M.H. Experimental optimization of SC-CO2 extraction of carotenoids from Dunaliella salina. J. Supercrit. Fluids 2017, 121, 89–95. [Google Scholar] [CrossRef]
- Tirado, D.F.; Calvo, L. The Hansen theory to choose the best cosolvent for supercritical CO2 extraction of β-carotene from Dunaliella salina. J. Supercrit. Fluids 2019, 145, 211–218. [Google Scholar] [CrossRef]
- Ludwig, K.; Rihko-Struckmann, L.; Brinitzer, G.; Unkelbach, G.; Sundmacher, K. β-Carotene extraction from Dunaliella salina by supercritical CO2. J. Appl. Phycol. 2021, 33, 1435–1445. [Google Scholar] [CrossRef]
- Harvey, P.J.; Ben-Amotz, A. Towards a sustainable Dunaliella salina microalgal biorefinery for 9-cis β-carotene production. Algal Res. 2020, 50, 102002. [Google Scholar] [CrossRef]
- Honda, M.; Sato, H.; Takehara, M.; Inoue, Y.; Kitamura, C.; Takemura, R.; Fukaya, T.; Wahyudiono Kanda, H.; Goto, M. Microwave-accelerated Z-isomerization of (all-E)-lycopene in tomato oleoresin and enhancement of the conversion by vegetable oils containing disulfide compounds. Eur. J. Lipid Sci. Technol. 2018, 120, 1800060. [Google Scholar] [CrossRef]
- Kodama, T.; Honda, M.; Takemura, R.; Fukaya, T.; Uemori, C.; Kanda, H.; Goto, M. Effect of the Z-isomer content on nanoparticle production of lycopene using solution-enhanced dispersion by supercritical fluids (SEDS). J. Supercrit. Fluids 2018, 133, 291–296. [Google Scholar] [CrossRef]
- Honda, M.; Watanabe, Y.; Murakami, K.; Hoang, N.N.; diono, W.; Kanda, H.; Goto, M. Enhanced lycopene extraction from gac (Momordica cochinchinensis Spreng.) by the Z-isomerization induced with microwave irradiation pre-treatment. Eur. J. Lipid Sci. Technol. 2018, 120, 1700293. [Google Scholar]
- Dey, S.; Rathod, V.K. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason. Sonochem. 2013, 20, 271–276. [Google Scholar] [CrossRef]
- Pirwitz, K.; Rihko-Struckmann, L.; Sundmacher, K. Valorization of the aqueous phase obtained from hydrothermally treated Dunaliella salina remnant biomass. Bioresour. Technol. 2016, 219, 64–71. [Google Scholar] [CrossRef]
- Wetterwald, L.; Leybros, A.; Fleury, G.; Delrue, F.; Dimitriades-Lemaire, A.; Chambonniere, P.; Hertz, A. Supercritical CO2 extraction of neutral lipids from dry and wet Chlorella vulgaris NIES 227 microalgae for biodiesel production. J. Environ. Chem. Eng. 2023, 11, 110628. [Google Scholar] [CrossRef]
- Aljabri, H.; Cherif, M.; Siddiqui, S.A.; Bounnit, T.; Saadaoui, I. Evidence of the drying technique’s impact on the biomass quality of Tetraselmis subcordiformis (Chlorophyceae). Biotechnol Biofuels 2023, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, Y.; Lemmon, E.W. An equation of state for the thermodynamic properties of dimethyl ether. J. Phys. Chem. Ref. Data 2011, 40, 023104. [Google Scholar] [CrossRef]
- Kanda, H.; Zhu, L.; Xu, B.; Kusumi, K.; Wang, T. Extraction of fucoxanthin, antioxidants and lipid from wet diatom Chaetoceros simplex var. calcitrans by liquefied dimethyl ether. Arab. J. Chem. 2024, 17, 105538. [Google Scholar] [CrossRef]
- Tatamitani, Y.; Liu, B.; Shimada, J.; Ogata, T.; Ottaviani, P.; Maris, A.; Caminati, W.; Alonso, J.L. Weak, improper, C−O...H−C hydrogen bonds in the dimethyl ether dimer. J. Am. Chem. Soc. 2002, 124, 2739–2743. [Google Scholar] [CrossRef]
- Chai, M.; Yang, M.; Qi, R.; Chen, Z.; Li, J. Vapor-liquid equilibrium (VLE) prediction for dimethyl ether (DME) and water system in DME injection process with Peng-Robinson equation of state and composition dependent binary interaction coefficient. J. Pet. Sci. Eng. 2022, 211, 110172. [Google Scholar] [CrossRef]
- Holldorff, H.; Knapp, H. Binary vapor-liquid-liquid equilibrium of dimethyl ether-water and mutual solubilities of methyl chloride and water: Experimental results and data reduction. Fluid Phase Equilib. 1988, 44, 195–209. [Google Scholar] [CrossRef]
- Tallon, S.; Fenton, K. The solubility of water in mixtures of dimethyl ether and carbon dioxide. Fluid Phase Equilib. 2010, 298, 60–66. [Google Scholar] [CrossRef]
- Watanabe, Y.; Honda, M.; Higashiura, T.; Fukaya, T.; Machmudah, S.; Kanda, H.; Goto, M. Rapid and selective concentration of lycopene Z-isomers from tomato pulp by supercritical CO2 with co-solvents. Solvent Extr. Res. Dev. Jpn. 2018, 25, 47–57. [Google Scholar]
- Lecksiwilai, N.; Gheewala, S.H.; Sagisaka, M.; Yamaguchi, K. Net Energy Ratio and life cycle greenhouse gases (GHG) assessment of bio-dimethyl ether (DME) produced from various agricultural residues in Thailand. J. Clean. Prod. 2016, 134, 523–531. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Chemical recycling of carbon dioxide to methanol and dimethyl ether: From greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. J. Org. Chem. 2009, 74, 487–498. [Google Scholar] [CrossRef]
- European Commission. Commission Directive (EU) 2016/1855 of 19 October 2016 amending Directive 2009/32/EC of the European Parliament and of the Council on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients. Off. J. Eur. Union. 2016, 284, 19–20. [Google Scholar]
- Food and Drug Administration. GRAS Notice for the Use of Dimethyl Ether as an Extraction Solvent. GRAS Notice No. GRN 000741. 2017. Available online: https://www.fda.gov/media/115219/download (accessed on 24 September 2024).
- Naito, M.; Radcliffe, C.; Wada, Y.; Hoshino, T.; Liu, X.; Arai, M.; Tamura, M. A comparative study on the autoxidation of dimethyl ether (DME) comparison with diethyl ether (DEE) and diisopropyl ether (DIPE). J. Loss Prev. Process Ind. 2005, 18, 469–473. [Google Scholar] [CrossRef]
- Kanda, H.; Wahyudiono; Machmudah, S.; Goto, M. Direct extraction of lutein from wet macroalgae by liquefied dimethyl ether without any pretreatment. ACS Omega 2020, 5, 24005–24010. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Kamo, Y.; Machmudah, S.; Wahyudiono; Goto, M. Extraction of Fucoxanthin from raw macroalgae excluding drying and cell wall disruption by liquefied dimethyl ether. Mar. Drugs 2014, 12, 2383–2396. [Google Scholar] [CrossRef]
- Kanda, H.; Oshita, K.; Takeda, K.; Takaoka, M.; Makino, H.; Morisawa, S.; Takeda, N. Dewatering a superabsorbent polymer using liquefied dimethyl ether. Dry. Technol. 2010, 28, 30–35. [Google Scholar]
- Kanda, H.; Mei, L.; Yamamoto, T.; Wang, T.; Zhu, L. Preparation of β-carotene nanoparticles using supercritical CO2 anti-solvent precipitation by injection of liquefied gas feed solution. J. CO2 Util. 2024, 83, 102831. [Google Scholar] [CrossRef]
- Dimić, I.; Pezo, L.; Rakić, D.; Teslić, N.; Zeković, Z.; Pavlić, B. Supercritical fluid extraction kinetics of cherry seed oil: Kinetics modeling and ANN optimization. Foods 2021, 10, 1513. [Google Scholar] [CrossRef]
- Saha, N.; Samanta, A.K.; Chaudhuri, S.; Dutta, D. Characterization and antioxidant potential of a carotenoid from a newly isolated yeast. Food Sci. Biotechnol. 2015, 8, 117–124. [Google Scholar] [CrossRef]
- Rubio-Diaz, D.E.; De Nardo, T.; Santos, A.; De Jesus, S.; Francis, D.; Rodriguez-Saona, L.E. Profiling of nutritionally important carotenoids from genetically-diverse tomatoes by infrared spectroscopy. Food Chem. 2010, 120, 282–289. [Google Scholar] [CrossRef]
- Liu, J.; Mukherjee, J.; Hawkes, J.J.; Wilkinson, S.J. Optimization of lipid production for algal biodiesel in nitrogen stressed cells of Dunaliella salina using FTIR analysis. J. Chem. Technol. Biotechnol. 2013, 88, 1807–1814. [Google Scholar] [CrossRef]
- Murdock, J.N.; Wetzel, D.L. FT-IR microspectroscopy enhances biological and ecological analysis of algae. Appl. Spectrosc. Rev. 2009, 44, 335–361. [Google Scholar] [CrossRef]
- Scarsini, M.; Thurotte, A.; Veidl, B.; Amiard, F.; Niepceron, F.; Badawi, M.; Lagarde, F.; Schoefs, B.; Marchand, J. Metabolite quantification by fourier transform infrared spectroscopy in diatoms: Proof of concept on Phaeodactylum tricornutum. Front. Plant Sci. 2021, 12, 756421. [Google Scholar] [CrossRef] [PubMed]
- Dahlquist, E. Technologies for Converting Biomass to Useful Energy: Combustion, Gasification, Pyrolysis, Torrefaction and Fermentation; CRC Press Inc.: London, UK, 2013. [Google Scholar]
- Lamers, P.P.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in nitrogen-starved Dunaliella salina, a unicellular green microalga. J. Biotechnol. 2012, 162, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Hernández, J.A.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; Ortiz-Basurto, R.I.; Prieto, C.; Lagaron, J.M. Use of electrosprayed agave fructans as nanoencapsulating hydrocolloids for bioactives. Nanomaterials 2018, 8, 868. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. The [DPPH●/DPPH-H]-HPLC-DAD method on tracking the antioxidant activity of pure antioxidants and goutweed (Aegopodium podagraria L.) hydroalcoholic extracts. Molecules. 2020, 25, 6005. [Google Scholar] [CrossRef]
- Fernández-Agulló, A.; Pereira, E.; Freire, M.S.; Valentão, P.; Andrade, P.B.; González-Álvarez, J.; Pereira, J.A. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind. Crops Prod. 2013, 42, 126–132. [Google Scholar] [CrossRef]
- Waterbury, J.B.; Sranier, R.Y. Isolation and growth of cyanobacteria from marine and hypersaline environments. In The Prokaryotes, A Handbook on Habitats, Isolation and Identification of Bacteria; Springer-Verlag: Berlin/Heidelberg, Germany, 1981; pp. 221–223. ISBN 978-3-662-13187-9. [Google Scholar] [CrossRef]

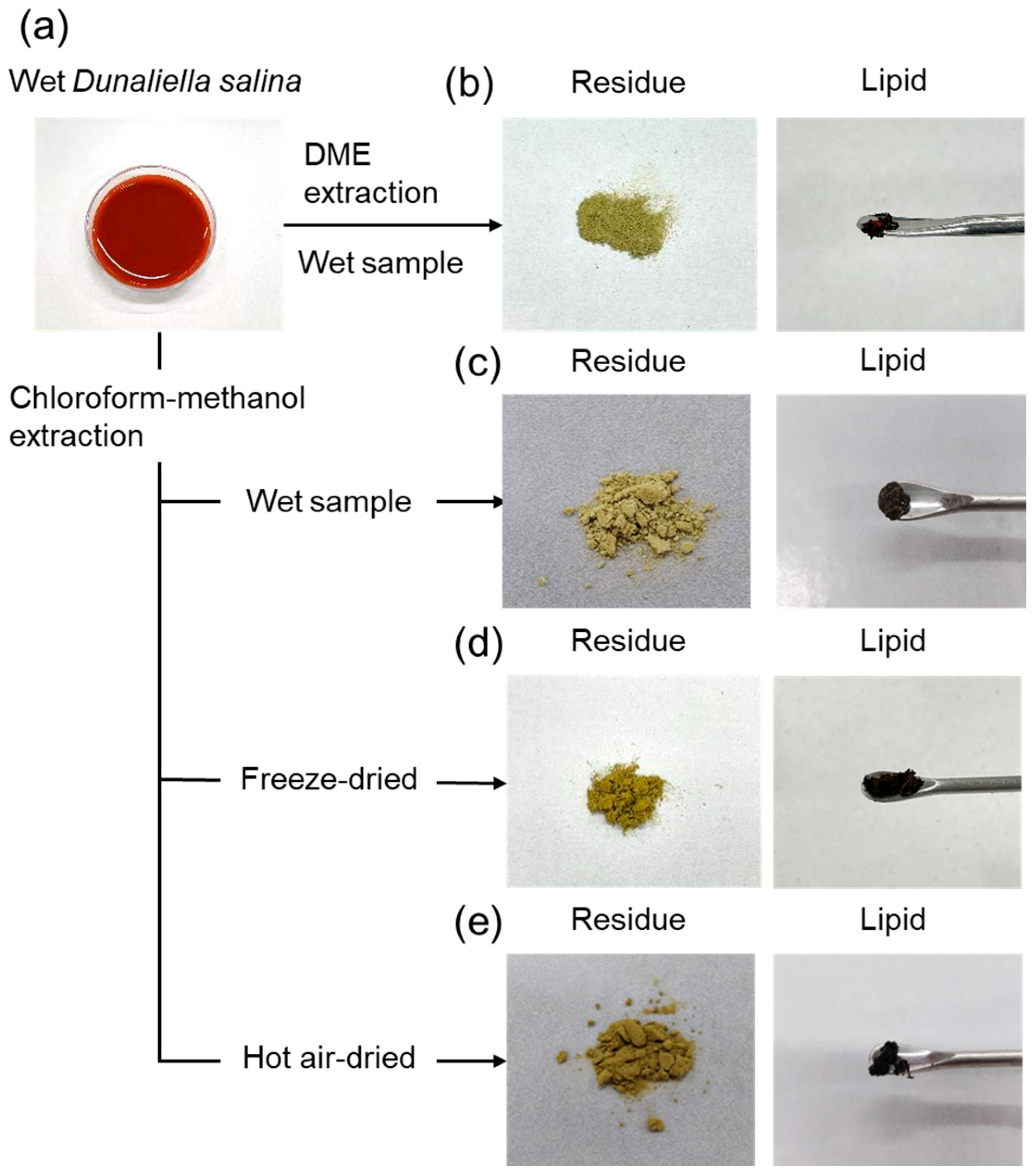



| Solvent | Sample | Lipid (wt%) * | β-Carotene (mg/g) * |
|---|---|---|---|
| Liquefied DME | Wet | 29.2 ± 4.8 | 7.0 ± 0.7 |
| Chloroform–methanol | Wet | 20.1 ± 1.0 | 1.8 ± 0.2 |
| Freeze-dried | 28.7 ± 1.6 | 4.4 ± 0.8 | |
| Hot-air-dried | 27.3 ± 1.8 | 1.8 ± 0.3 |
| Peak | Wave Number (cm−1) | Functional Groups | Compounds | Samples with Peaks Detected |
|---|---|---|---|---|
| 1 | 3392 | CH=CH stretching | Lipid | Chloroform–methanol extract DME extract |
| 2 | 3281 | O-H, N-H symmetric stretching | Protein Water | Chloroform–methanol residue DME residue Original sample |
| 3 | 2923 | C–H stretching | Lipid β-Carotene | All samples |
| 4 | 2851 | CH3, CH2 symmetry stretching | Lipid β-Carotene | Chloroform–methanol residue DME residue Original sample |
| 5 | 1722 | C=O stretching | Lipid | Chloroform–methanol extract DME extract |
| 6 | 1633 | C=O stretching | Protein | Chloroform–methanol residue DME residue Original sample |
| 7 | 1530 | Amide II C-N stretching N-H angular vibration | Protein | Chloroform–methanol residue DME residue Original sample |
| 8 | 1458 | CH2 symmetry in-plane variation, CH3 inverse symmetry variable angle | Lipid β-Carotene | All samples |
| 9 | 1377 | C–H stretching | β-Carotene | All samples |
| 10 | 1164 | C-O stretching | Lipid | Chloroform–methanol extract DME extract |
| 11 | 1046 | C-O symmetric stretching | Polysaccharides | All samples |
| 12 | 965 | C=C out of plane bending vibration | β-Carotene | Chloroform–methanol extract DME extract |
| Solvent | Sample | C (wt%) * | H (wt%) * | N (wt%) * | O (wt%) *,** | S (wt%) * | C/N (−) |
|---|---|---|---|---|---|---|---|
| Original | 51.4 ± 0.2 | 6.1 ± 0.1 | 7.8 ± 0.0 | 34.0 ± 0.0 | 0.8 ± 0.1 | 6.6 | |
| Liquefied DME | Residue | 46.5 ± 0.1 | 6.1 ± 0.1 | 10.7 ± 0.1 | 35.9 ± 0.2 | 0.9 ± 0.0 | 4.5 |
| Lipid | 72.8 ± 0.1 | 10.6 ± 0.2 | 0.7 ± 0.0 | 16.0 ± 0.1 | 0.5 ± 0.1 | 112.0 | |
| Chloroform–methanol | Residue | 42.4 ± 0.1 | 6.8 ± 0.0 | 9.3 ± 0.1 | 40.9 ± 0.1 | 0.6 ± 0.0 | 4.5 |
| Lipid | 59.7 ± 0.1 | 8.4 ± 0.2 | 0.9 ± 0.0 | 30.7 ± 0.2 | 0.3 ± 0.1 | 70.0 | |
| p-Value | Residue | 0.0000 | 0.0033 | 0.0001 | 0.0000 | 0.0001 | 0.0043 |
| Lipid | 0.0000 | 0.0002 | 0.0092 | 0.0000 | 0.2375 | 0.0001 |
| Solvent | Sample | TPC (mg GAE/Dry-g) * | IC50 (mg/mL-Lipid) * |
|---|---|---|---|
| Liquefied DME | Wet | 6.11 ± 1.01 | 0.66 ± 0.04 |
| Chloroform–methanol | Freeze-dried | 5.59 ± 0.34 | 0.73 ± 0.21 |
| Solvent | Sample | Na (wt%) * | Mg (wt%) * |
|---|---|---|---|
| Original | 0.43 ± 0.04 | 0.23 ± 0.05 | |
| Liquefied DME | Residue | 0.40 ± 0.14 | 0.34 ± 0.14 |
| Lipid | 0.03 ± 0.01 | 0.02 ± 0.01 | |
| Chloroform–methanol | Residue | 0.23 ± 0.02 | 0.35 ± 0.00 |
| Lipid | 0.44 ± 0.01 | 0.05 ± 0.01 | |
| p-Value | Residue | 0.810 | 0.484 |
| Lipid | 0.004 | 0.051 |
| Fatty Acid | Liquefied DME (wt%) * | Chloroform–Methanol (wt%) * | p-Value |
|---|---|---|---|
| C12:0 | 1.56 ± 0.97 | 0.60 ± 0.40 | 0.3339 |
| C14:0 | 1.73 ± 0.31 | 1.47 ± 0.31 | 0.3472 |
| C16:0 | 27.03 ± 6.15 | 46.63 ± 5.00 | 0.0289 |
| C16:1 (n − 7) | 4.50 ± 0.34 | 2.36 ± 0.58 | 0.0169 |
| C16:3 (n − 4) | 5.80 ± 2.54 | 0.00 ± 0.00 | 0.0854 |
| C18:0 | 2.38 ± 0.23 | 2.19 ± 0.53 | 0.4589 |
| C18:1 (n − 9) | 4.42 ± 0.41 | 0.00 ± 0.00 | 0.0157 |
| C18:2 (n − 6) | 14.31 ± 3.96 | 12.98 ± 1.79 | 0.6838 |
| C18:3 (n − 6) | 34.72 ± 3.57 | 32.63 ± 2.10 | 0.4836 |
| Saturated fatty acid | 34.76 ± 7.97 | 50.89 ± 4.70 | 0.0387 |
| Unsaturated fatty acid | 65.25 ± 11.44 | 49.11 ± 4.70 | 0.0387 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, H.; Kusumi, K.; Zhu, L.; Wang, T. Direct Extraction of Lipids, β-Carotene, and Polyphenolic Compounds from Wet Microalga Dunaliella salina by Liquefied Dimethyl Ether. Mar. Drugs 2024, 22, 438. https://doi.org/10.3390/md22100438
Kanda H, Kusumi K, Zhu L, Wang T. Direct Extraction of Lipids, β-Carotene, and Polyphenolic Compounds from Wet Microalga Dunaliella salina by Liquefied Dimethyl Ether. Marine Drugs. 2024; 22(10):438. https://doi.org/10.3390/md22100438
Chicago/Turabian StyleKanda, Hideki, Kaito Kusumi, Li Zhu, and Tao Wang. 2024. "Direct Extraction of Lipids, β-Carotene, and Polyphenolic Compounds from Wet Microalga Dunaliella salina by Liquefied Dimethyl Ether" Marine Drugs 22, no. 10: 438. https://doi.org/10.3390/md22100438







