Brevianamide F Exerts Antithrombotic Effects by Modulating the MAPK Signaling Pathway and Coagulation Cascade
Abstract
:1. Introduction
2. Results
2.1. Effect of Brevianamide F on Platelet Aggregation and Circulating Platelet Count in Thrombotic Zebrafish
2.2. Effect of Brevianamide F on the Staining Area and Intensity of Erythrocytes in the Heart of Thrombotic Zebrafish
2.3. Effect of Brevianamide F on Erythrocyte Fluorescence Area and Heart Rate of Thrombotic Zebrafish
2.4. Effect of Brevianamide F on Blood Flow Velocity in the Caudal Region of the Thrombotic Zebrafish
2.5. Effect of Brevianamide F on Thrombosis-Related Factors
2.6. Effect of Brevianamide F on Gene Expression Profile of Thrombotic Zebrafish
2.6.1. Differentially Expressed Genes (DEGs) Using Transcriptome Analysis
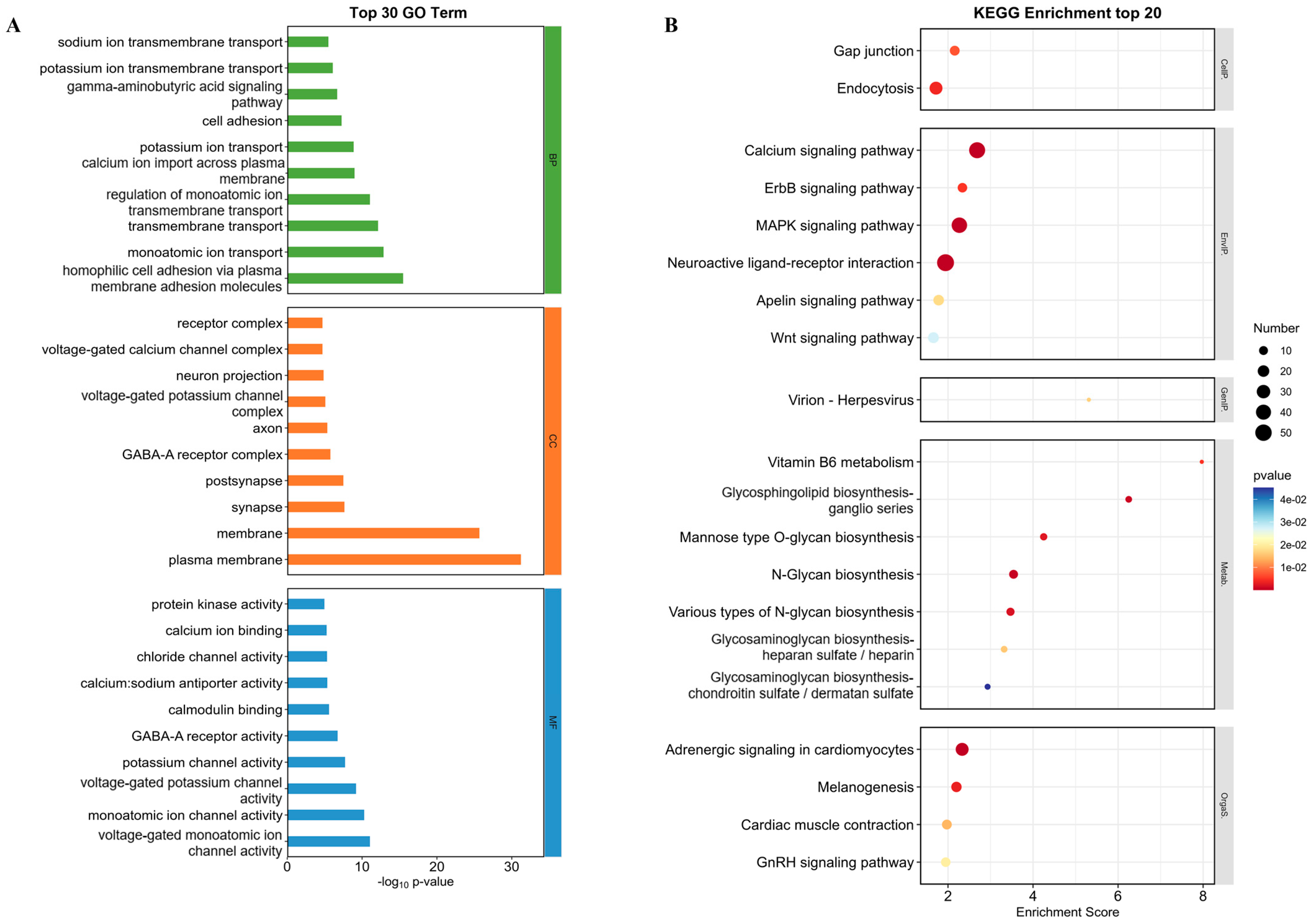
2.6.2. GO Function Enrichment Analysis and KEGG Enrichment Analysis
2.6.3. Effect of Brevianamide F on the Gene Expression Levels of Zebrafish
2.7. Interactions of Brevianamide F with Key Targets
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Zebrafish Maintenance
4.3. Chemical Treatment
4.4. Detection of Circulating Platelet Count
4.5. Erythrocyte Staining Area and Intensity Detection
4.6. Cardiac Erythrocyte Fluorescence Area and Heart Rate Detection
4.7. Caudal Blood Flow Velocity Detection
4.8. Detection of Thrombosis-Related Factors
4.9. Identification of Differentially Expressed Genes in Zebrafish Using RNA-Seq
4.10. qRT-PCR Assay for Expression Levels of Thrombus-Related Genes
4.11. Molecular Docking
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Freund, Y.; Cohen-Aubart, F.; Bloom, B. Acute pulmonary embolism: A review. JAMA 2022, 328, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Buxhofer-Ausch, V.; Wolf, D.; Sormann, S.; Forjan, E.; Schimetta, W.; Gisslinger, B.; Heibl, S.; Krauth, M.T.; Thiele, J.; Ruckser, R.; et al. Impact of Platelets on Major Thrombosis in Patients with a Normal White Blood Cell Count in Essential Thrombocythemia. Eur. J. Haematol. 2021, 106, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis. Arterioscl. Throm. Vas. 2014, 34, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Wendelboe, A.; Weitz, J.-I. Global Health Burden of Venous Thromboembolism. Arterioscl. Throm. Vas. 2024, 44, 1007–1011. [Google Scholar] [CrossRef]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J.L. Demographic and Epidemiologic Drivers of Global Cardiovascular Mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef]
- Huang, H.-K.; Liu, P.P.-S.; Lin, S.-M.; Yeh, J.-I.; Hsu, J.-Y.; Peng, C.C.-H.; Munir, K.M.; Loh, C.-H.; Tu, Y.-K. Risk of Serious Hypoglycemia in Patients with Atrial Fibrillation and Diabetes Concurrently Taking Antidiabetic Drugs and Oral Anticoagulants: A Nationwide Cohort Study. Eur. Heart J. Card. Pha. 2023, 9, 427–434. [Google Scholar] [CrossRef]
- Lee, J.Y.; Oh, I.-Y.; Lee, J.-H.; Kim, S.-Y.; Kwon, S.S.; Yang, H.-J.; Kim, Y.-K.; Bang, S.-M. The Increased Risk of Bleeding Due to Drug-Drug Interactions in Patients Administered Direct Oral Anticoagulants. Thromb. Res. 2020, 195, 243–249. [Google Scholar] [CrossRef]
- Swan, D.; Loughran, N.; Makris, M.; Thachil, J. Management of Bleeding and Procedures in Patients on Antiplatelet Therapy. Blood Rev. 2020, 39, 100619. [Google Scholar] [CrossRef]
- Jagadeeswaran, P.; Sheehan, J.P.; Craig, F.E.; Troyer, D. Identification and Characterization of Zebrafish Thrombocytes. Brit. J. Haematol. 1999, 107, 731–738. [Google Scholar] [CrossRef]
- Chandika, P.; Tennakoon, P.; Kim, T.-H.; Kim, S.-C.; Je, J.-Y.; Kim, J.-I.; Lee, B.; Ryu, B.; Kang, H.; Kim, H.-W.; et al. Marine Biological Macromolecules and Chemically Modified Macromolecules; Potential Anticoagulants. Mar. Drugs 2022, 20, 654. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Jia, X.; Li, R.; Chen, J.; Liu, X.; Song, B.; Zhong, S.; Qi, Y. Anticoagulant and Fibrinolytic Properties of Two Heparinoid Compounds Prepared from Shrimp Waste. Foods 2022, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liu, R.; Hu, X.; Liang, T.; Zhou, Z.; Huang, Z. MAPK Signaling Pathway-Targeted Marine Compounds in Cancer Therapy. J. Cancer Res. Clin. 2021, 147, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Linghu, K.-G.; Zhang, T.; Zhang, G.-T.; Lv, P.; Zhang, W.-J.; Zhao, G.-D.; Xiong, S.-H.; Ma, Q.-S.; Zhao, M.-M.; Chen, M.; et al. Small Molecule Deoxynyboquinone Triggers Alkylation and Ubiquitination of Keap1 at Cys489 on Kelch Domain for Nrf2 Activation and Inflammatory Therapy. J. Pharm. Anal. 2024, 14, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liang, J.; Zhang, B.; Huang, L.; Yu, J.; Xiao, X.; He, Z.; Tao, H.; Yuan, J. A Marine Natural Product, Harzianopyridone, as an Anti-ZIKV Agent by Targeting RNA-Dependent RNA Polymerase. Molecules 2024, 29, 978. [Google Scholar] [CrossRef]
- Sun, C.; Liu, Z.; Zhu, X.; Fan, Z.; Huang, X.; Wu, Q.; Zheng, X.; Qin, X.; Zhang, T.; Zhang, H.; et al. Antitubercular Ilamycins from Marine-Derived Streptomyces Atratus SCSIO ZH16 ΔilaR. J. Nat. Prod. 2020, 83, 1646–1657. [Google Scholar] [CrossRef]
- Shi, J.; Wang, C.; Xie, M.; Hao, Y.; Wang, N.; Ma, H.; Yang, X. Brefeldin A from the Deep-Sea-Derived Fungus Fusarium Sp. Targets on RIPK3 to Inhibit TNFα-Induced Necroptosis. Chem. Biodivers. 2022, 19, e202200696. [Google Scholar] [CrossRef]
- Huang, P.; Xie, F.; Ren, B.; Wang, Q.; Wang, J.; Wang, Q.; Abdel-Mageed, W.M.; Liu, M.; Han, J.; Oyeleye, A.; et al. Anti-MRSA and Anti-TB Metabolites from Marine-Derived Verrucosispora Sp. MS100047. Appl. Microbiol. Biot. 2016, 100, 7437–7447. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Afiyatullov, S.S.; Vishchuk, O.S.; Denisenko, V.A.; Slinkina, N.N.; Smetanina, O.F. Decumbenone C, a New Cytotoxic Decaline Derivative from the Marine Fungus Aspergillus Sulphureus KMM 4640. Arch. Pharm. Res. 2012, 35, 1757–1762. [Google Scholar] [CrossRef]
- Preciado, S.; Mendive-Tapia, L.; Torres-García, C.; Zamudio-Vázquez, R.; Soto-Cerrato, V.; Pérez-Tomás, R.; Albericio, F.; Nicolás, E.; Lavilla, R. Synthesis and Biological Evaluation of a Post-Synthetically Modified Trp-Based Diketopiperazine. MedChemComm 2013, 4, 1171. [Google Scholar] [CrossRef]
- Wauters, I.; Goossens, H.; Delbeke, E.; Muylaert, K.; Roman, B.I.; Van Hecke, K.; Van Speybroeck, V.; Stevens, C.V. Beyond the Diketopiperazine Family with Alternatively Bridged Brevianamide F Analogues. J. Org. Chem. 2015, 80, 8046–8054. [Google Scholar] [CrossRef]
- Li, L.; Chang, Q.-H.; Zhang, S.-S.; Yang, K.; Chen, F.-L.; Zhu, H.-J.; Cao, F.; Liu, Y.-F. (±)-Brevianamides Z and Z1, New Diketopiperazine Alkaloids from the Marine-Derived Fungus Aspergillus Versicolor. J. Mol. Struct. 2022, 1261, 132904. [Google Scholar] [CrossRef]
- Patel, P.; Naik, M.U.; Naik, U. Apoptosis Signal-Regulating Kinase (ASK1) Regulates Thrombosis in Part By Regulating cPLA2 Phosphorylation-Dependent TxA2 Generation. Blood 2016, 128, 3719. [Google Scholar] [CrossRef]
- Zhu, S.; Gilbert, J.C.; Hatala, P.; Harvey, W.; Liang, Z.; Gao, S.; Kang, D.; Jilma, B. The Development and Characterization of a Long Acting Anti-thrombotic von Willebrand Factor (VWF) Aptamer. J. Thromb. Haemost. 2020, 18, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Gotta, J.; Gruenewald, L.D.; Geyer, T.; Eichler, K.; Martin, S.-S.; Mahmoudi, S.; Booz, C.; Biciusca, T.; Reschke, P.; Juergens, L.-J.; et al. Indicators for Hospitalization in Acute Pulmonary Embolism: Uncover the Association Between D-dimer Levels, Thrombus Volume and Radiomics. Acad. Radiol. 2024, 31, 2610–2619. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-D.; Yang, Y.; Hengerjia, G.; Deng, Y. Exploring the mechanism of Liuwei Dihuang formula for promoting melanin synthesis in juvenile zebrafish based on network pharmacology and molecular docking. Heliyon 2023, 9, e21744. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Guo, Z.; Liu, Y. Analysis of the Potential Molecular Biology of Triptolide in the Treatment of Diabetic Nephropathy: A Narrative Review. Medicine 2022, 101, e31941. [Google Scholar] [CrossRef]
- Qiu, Y.; Ying, J.; Yan, F.; Yu, H.; Zhao, Y.; Li, H.; Xia, S.; Chen, J.; Zhu, J. Novel Antiosteoporotic Peptides Purified from Protein Hydrolysates of Taihe Black-Boned Silky Fowl: By Larval Zebrafish Model and Molecular Docking. Food Res. Int. 2023, 169, 112850. [Google Scholar] [CrossRef]
- Badimon, L.; Vilahur, G.; Rocca, B.; Patrono, C. The Key Contribution of Platelet and Vascular Arachidonic Acid Metabolism to the Pathophysiology of Atherothrombosis. Cardiovasc. Res. 2021, 117, 2001–2015. [Google Scholar] [CrossRef]
- Mi, Y.; Han, X.; Yu, X.; Li, L.; Tang, X.; Li, G. Sarcocinerenolides A, an open-loop decarbonizing cembranolide, and sarcocinerenolides B–I, eight polyoxygenated cembranolides with anti-thrombotic activity from the South China Sea soft coral Sarcophyton cinereum. Phytochemistry 2024, 223, 114109. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, P.; Hu, M.; Lu, S.; Lyu, Z.; Kou, Y.; Sun, Y.; Zhao, X.; Liu, F.; Tian, J. Naoxintong Restores Ischemia Injury and Inhibits Thrombosis via COX2-VEGF/NFκB Signaling. J. Ethnopharmacol. 2021, 270, 113809. [Google Scholar] [CrossRef]
- Yin, S.-J.; Luo, Y.-Q.; Zhao, C.-P.; Chen, H.; Zhong, Z.-F.; Wang, S.; Wang, Y.-T.; Yang, F.-Q. Antithrombotic Effect and Action Mechanism of Salvia Miltiorrhiza and Panax Notoginseng Herbal Pair on the Zebrafish. Chin. Med. 2020, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- García Suquia, A.; Alonso-Fernández, A.; de la Peña, M.; Romero, D.; Piérola, J.; Carrera, M.; Barceló, A.; Soriano, J.B.; Arque, M.; Fernández-Capitán, C.; et al. High D-Dimer Levels after Stopping Anticoagulants in Pulmonary Embolism with Sleep Apnoea. Eur. Resp. J. 2015, 46, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Misasi, R.; Capozzi, A.; Riitano, G.; Recalchi, S.; Manganelli, V.; Mattei, V.; Longo, A.; De Michele, M.; Garofalo, T.; Pulcinelli, F.M.; et al. Signal Transduction Pathway Involved in Platelet Activation in Immune Thrombotic Thrombocytopenia after COVID-19 Vaccination. Haematologica 2021, 107, 326–329. [Google Scholar] [CrossRef]
- Fan, X.; Wang, C.; Shi, P.; Gao, W.; Gu, J.; Geng, Y.; Yang, W.; Wu, N.; Wang, Y.; Xu, Y.; et al. Platelet MEKK3 Regulates Arterial Thrombosis and Myocardial Infarct Expansion in Mice. Blood Adv. 2018, 2, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Manne, B.K.; Münzer, P.; Badolia, R.; Walker-Allgaier, B.; Campbell, R.A.; Middleton, E.; Weyrich, A.S.; Kunapuli, S.P.; Borst, O.; Rondina, M.T. PDK1 Governs Thromboxane Generation and Thrombosis in Platelets by Regulating Activation of Raf1 in the MAPK Pathway. J. Thromb. Haemost. 2018, 16, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Larrivée, B.; Zhuang, Z.W.; Atri, D.; Moraes, F.; Prahst, C.; Eichmann, A.; Simons, M. Endothelial RAF1/ERK Activation Regulates Arterial Morphogenesis. Blood 2013, 121, 3988–3996. [Google Scholar] [CrossRef]
- Lima, A.M.; Wegner, S.V.; Martins Cavaco, A.C.; Estevão-Costa, M.I.; Sanz-Soler, R.; Niland, S.; Nosov, G.; Klingauf, J.; Spatz, J.P.; Eble, J.A. The Spatial Molecular Pattern of Integrin Recognition Sites and Their Immobilization to Colloidal Nanobeads Determine A2β1 Integrin-Dependent Platelet Activation. Biomaterials 2018, 167, 107–120. [Google Scholar] [CrossRef]
- Chung, C.-L.; Chen, J.-H.; Huang, W.-C.; Sheu, J.-R.; Hsia, C.-W.; Jayakumar, T.; Hsia, C.-H.; Chiou, K.-R.; Hou, S.-M. Glabridin, a Bioactive Flavonoid from Licorice, Effectively Inhibits Platelet Activation in Humans and Mice. Int. J. Mol. Sci. 2022, 23, 11372. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Chen, Q.-R.; Pan, W.-J.; Zhang, Y.; Li, J.-S.; Xue, X.-Y.; Lei, X.-H.; Wang, S.-M.; Meng, J. Moutan cortex exerts blood-activating and anti-inflammatory effects by regulating coagulation-inflammation cascades pathway in cells, rats and zebrafish. J. Ethnopharmacol. 2024, 320, 117398. [Google Scholar] [CrossRef]
- Woulfe, D.; Jiang, H.; Morgans, A.; Monks, R.; Birnbaum, M.; Brass, L.F. Defects in Secretion, Aggregation, and Thrombus Formation in Platelets from Mice Lacking Akt2. J. Clin. Investig. 2004, 113, 441–450. [Google Scholar] [CrossRef]
- Yin, H.; Stojanovic, A.; Hay, N.; Du, X. The Roles of Akt1 and Akt2 in GPIb-IX-Mediated Platelet Activation Signaling. Blood 2007, 110, 3635. [Google Scholar] [CrossRef]
- Ye, S.; Mao, B.; Yang, L.; Fu, W.; Hou, J. Thrombosis Recanalization by Paeoniflorin through the Upregulation of Urokinase-Type Plasminogen Activator via the MAPK Signaling Pathway. Mol. Med. Rep. 2016, 13, 4593–4598. [Google Scholar] [CrossRef] [PubMed]
- Giri, H.; Cai, X.; Panicker, S.R.; Biswas, I.; Rezaie, A.R. Thrombomodulin Regulation of Mitogen-Activated Protein Kinases. Int. J. Mol. Sci. 2019, 20, 1851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, T.; Zhou, Q.; Xin, G.; Niu, H.; Li, F.; Wang, Y.; Li, S.; Dong, Y.; Zhang, K.; et al. Endogenous SIRT6 in platelets negatively regulates platelet activation and thrombosis. Front. Pharmacol. 2023, 14, 1268708. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.-T.; Poole, A.-W. Isoform-Specific Functions of Protein Kinase C: The Platelet Paradigm. Biochem. Soc. Trans. 2007, 35, 1005–1008. [Google Scholar] [CrossRef]
- Kondreddy, V.; Keshava, S.; Das, K.; Magisetty, J.; Rao, L.V.M.; Pendurthi, U.R. The Gab2–MALT1 Axis Regulates Thromboinflammation and Deep Vein Thrombosis. Blood 2022, 140, 1549–1564. [Google Scholar] [CrossRef]
- Chion, A.; Byrne, C.; Atiq, F.; Doherty, D.; Aguila, S.; Fazavana, J.; Lopes, P.; Karampini, E.; Amin, A.; Roger, J.S.P.; et al. Aptamer BT200 blocks interaction of K1405-1408 in the VWF-A1 domain with macrophage LRP1. Blood, 2024; online ahead of print. [Google Scholar] [CrossRef]
- Yada, N.; Zhang, Q.; Bignotti, A.; Gralnek, S.-H.; Sosnovske, D.; Hogan, K.; Ye, Z.; Zheng, L.; Zheng, X.-L. Targeting neutrophil extracellular trap accumulation under flow in patients with immune-mediated thrombotic thrombocytopenic purpura. Blood Adv. 2024, 8, 2536–2551. [Google Scholar] [CrossRef]
- Duval, C.; Ariëns, R.A.S. Fibrinogen splice variation and cross-linking: Effects on fibrin structure/function and role of fibrinogen γ′ as thrombomobulin II. Matrix Biol. 2017, 60–61, 8–15. [Google Scholar] [CrossRef]
- Mangin, P.H.; Gardiner, E.E.; Ariëns, R.A.S.; Jandrot-Perrus, M. GPVI Interplay with Fibrin(Ogen) in Thrombosis. J. Thromb. Haemost. 2023, 21, 1703–1713. [Google Scholar] [CrossRef]
- Manz, X.D.; Bogaard, H.J.; Aman, J. Regulation of VWF (Von Willebrand Factor) in Inflammatory Thrombosis. Arterioscl. Throm. Vas. 2022, 42, 1307–1320. [Google Scholar] [CrossRef]
- Sheng, J.; Meng, Q.; Yang, Z.; Guan, J.; Zhao, Y.; Zhang, J.; Wang, Y.; Zhao, L.; Wang, Y. Identification of Cryptotanshinone from Tongmai to Inhibit Thrombosis in Zebrafish via Regulating Oxidative Stress and Coagulation Cascade. Phytomedicine 2020, 76, 153263. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Ho, C.-T.; Jun, M. Investigating the Potential Anti-Alzheimer’s Disease Mechanism of Marine Polyphenols: Insights from Network Pharmacology and Molecular Docking. Mar. Drugs 2023, 21, 580. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Yang, Z.; Yu, Q.; Zhao, L.; Wang, Y. Synergistic Effects of Cryptotanshinone and Senkyunolide I in Guanxinning Tablet Against Endogenous Thrombus Formation in Zebrafish. Front. Pharmacol. 2021, 11, 622787. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Zhang, M.; Li, P.; Wang, L.; Zhang, X.; Zhang, S.; Mu, Z.; Lin, H.; Li, X.; Liu, K. Marine-Fungus-Derived Natural Compound 4-Hydroxyphenylacetic Acid Induces Autophagy to Exert Antithrombotic Effects in Zebrafish. Mar. Drugs 2024, 22, 148. [Google Scholar] [CrossRef]

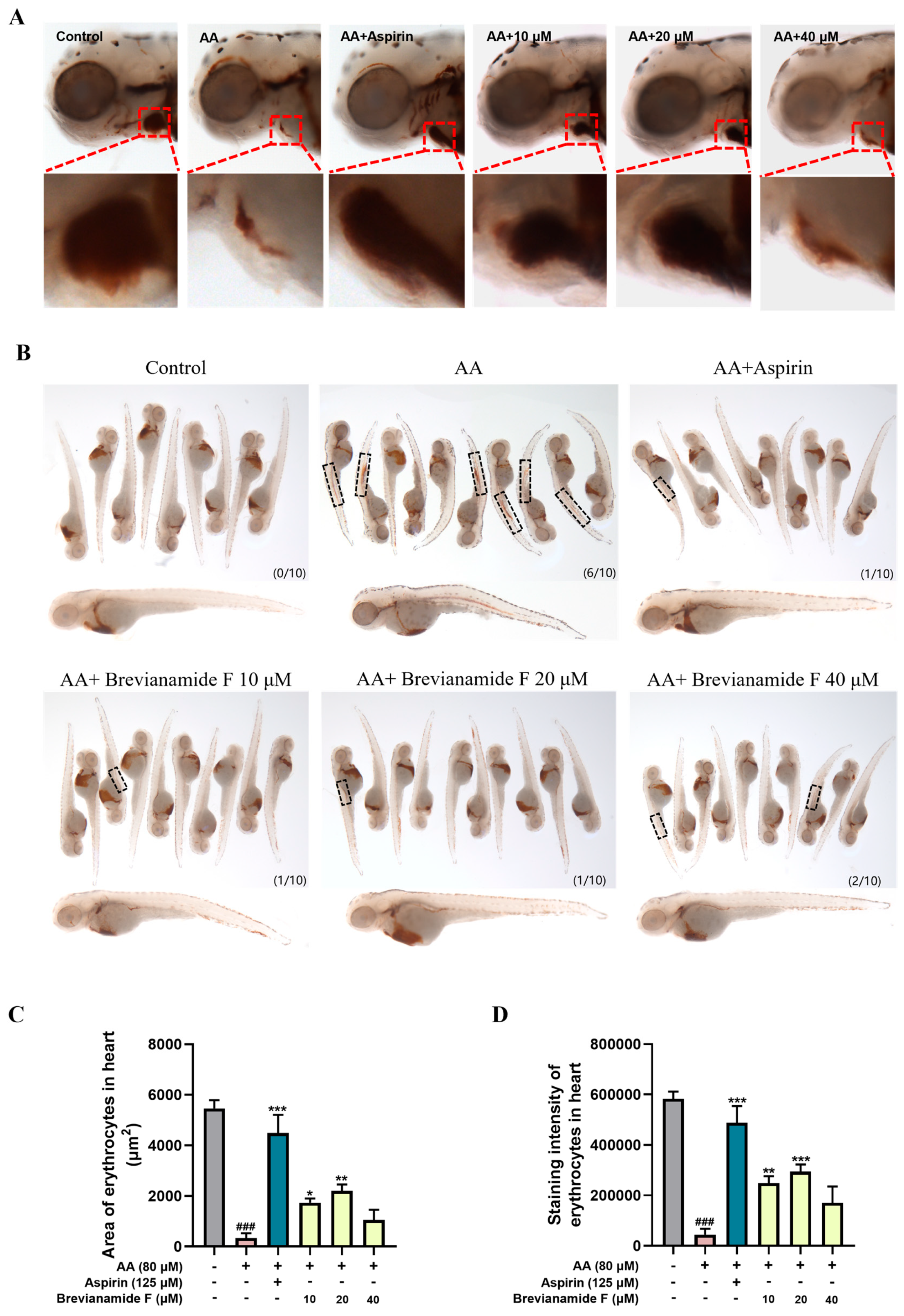






| Groups | Concentration (μM) | Cardiac Erythrocyte Staining Intensity (Pixel) | Thrombosis Prevention Rate (%) |
|---|---|---|---|
| Blank control group | – | 583494 ± 28378 | – |
| Thrombus model group (AA) | 80 | 44318 ± 23093 ### | – |
| Positive control group (AA + Aspirin) | 125 | 488484 ± 66153 *** | 76.69 |
| Brevianamide F treatment group (AA + Brevianamide F) | 10 | 249285 ± 27242 * | 35.39 |
| 20 | 294097 ± 29205 ** | 43.13 | |
| 40 | 171199 ± 64751 | 21.91 |
| Protein Target | PDB ID | Binding Energy (kJ/mol) |
|---|---|---|
| Brevianamide F | ||
| AKT2 | 8Q61 | −34.43 |
| MAPK14 | 6SFI | −36.94 |
| MAPK8 | 4QTD | −31.80 |
| MAP2K7 | 5Y90 | −34.81 |
| RAF1 | 6VJJ | −32.38 |
| MAPK1 | 6SLG | −28.79 |
| PKCα | 8U37 | −30.08 |
| PKCβ | 2I0E | −29.75 |
| PKCγ | 2UZP | −31.00 |
| VWF | 7EOW | −33.93 |
| F2 | 2AFQ | −32.13 |
| F7 | 5PAG | −40.75 |
| FGA | 3E1I | −25.19 |
| FGB | 2OYH | −28.79 |
| FGG | 3E1I | −29.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Sun, C.; Xia, Q.; Li, P.; Liu, K.; Zhang, Y. Brevianamide F Exerts Antithrombotic Effects by Modulating the MAPK Signaling Pathway and Coagulation Cascade. Mar. Drugs 2024, 22, 439. https://doi.org/10.3390/md22100439
Zhang H, Sun C, Xia Q, Li P, Liu K, Zhang Y. Brevianamide F Exerts Antithrombotic Effects by Modulating the MAPK Signaling Pathway and Coagulation Cascade. Marine Drugs. 2024; 22(10):439. https://doi.org/10.3390/md22100439
Chicago/Turabian StyleZhang, Huiwen, Chen Sun, Qing Xia, Peihai Li, Kechun Liu, and Yun Zhang. 2024. "Brevianamide F Exerts Antithrombotic Effects by Modulating the MAPK Signaling Pathway and Coagulation Cascade" Marine Drugs 22, no. 10: 439. https://doi.org/10.3390/md22100439






