Fermented Fish Collagen Attenuates Melanogenesis via Decreasing UV-Induced Oxidative Stress
Abstract
:1. Introduction
2. Results
2.1. FC Increased the Expressions of GlyR and GlyT in the UV-Irradiated Keratinocyte
2.2. FC Decreased the Expression of NOXs and Oxidative Stress in UV-Irradiated Keratinocytes
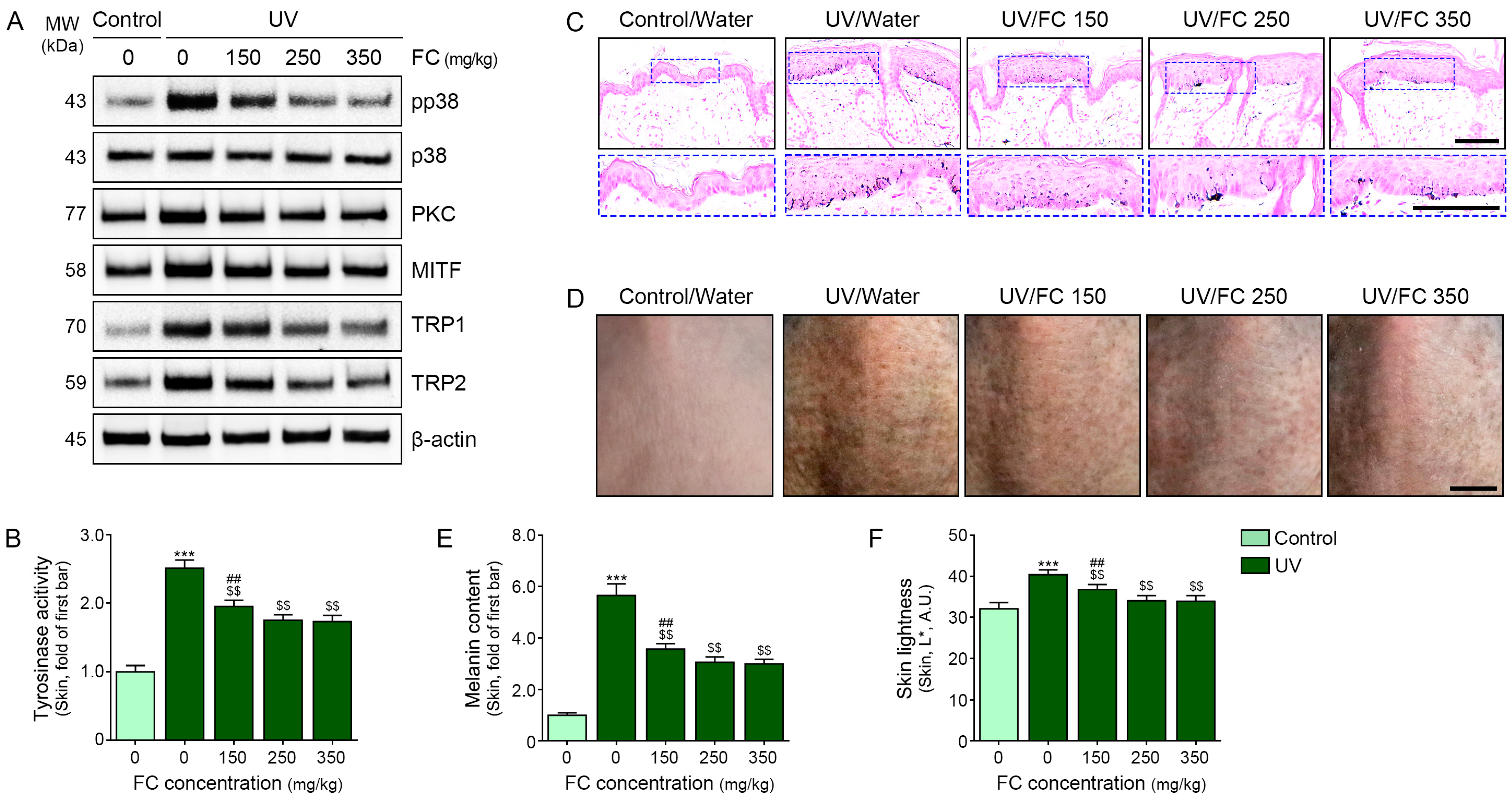
2.3. FC Treatment Decreased the Expression of p38 along with PKC, MITF, TRP1, TRP2, and TYR Activity in Melanocytes
2.4. FC Increased GlyR and GlyT and Decreased Oxidative Stress in the UV-Irradiated Animal Skin
2.5. FC Decreased Melanogenesis in UV-Irradiated Animal Skin
3. Discussion
4. Materials and Methods
4.1. FC Preparation and Analysis
4.1.1. FC Preparation
4.1.2. Amino Acid Analysis
4.1.3. High-Performance Liquid Chromatography (HPLC) Analysis
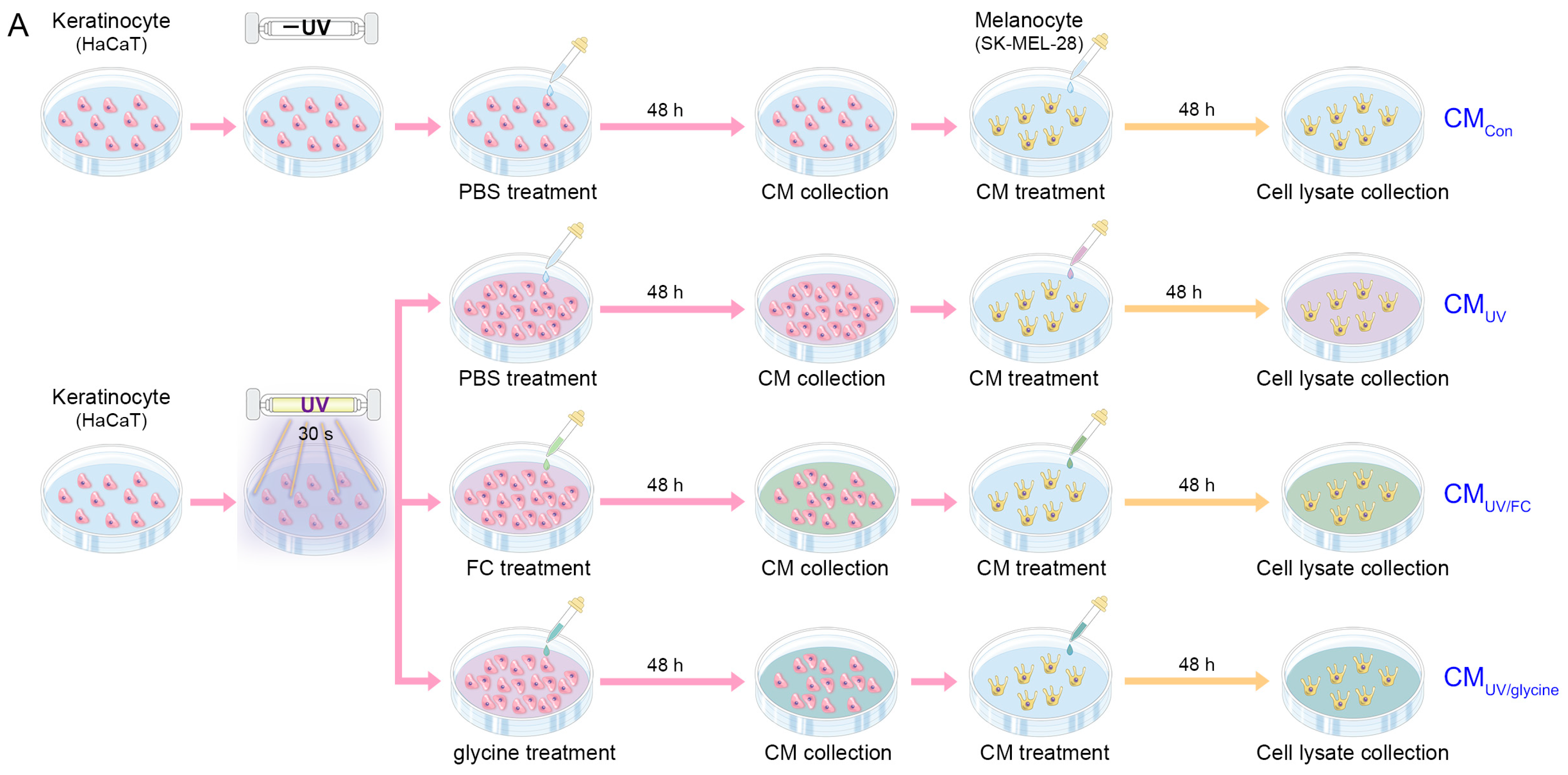
4.2. In Vitro Model
4.3. In Vivo Model
4.3.1. Mouse Model and Maintenance
4.3.2. Experimental Design
4.3.3. Skin Lightness
4.4. Sample Preparation
4.4.1. Protein Isolation
4.4.2. Paraffin-Embedded Skin Tissue Block
4.5. Cell Viability
4.6. GSH/GSSG Ratio, SOD Activity, and Tyrosinase Activity
4.7. Western Blot
4.8. Enzyme-Linked Immunosorbent Assay (ELISA)
4.9. Fontana–Masson Staining
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jurkiewicz, B.A.; Buettner, G.R. EPR detection of free radicals in UV-irradiated skin: Mouse versus human. Photochem. Photobiol. 1996, 64, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L. Pollution and Sun Exposure: A Deleterious Synergy. Mechanisms and Opportunities for Skin Protection. Curr. Med. Chem. 2018, 25, 5469–5486. [Google Scholar] [CrossRef]
- Masaki, H.; Atsumi, T.; Sakurai, H. Detection of hydrogen peroxide and hydroxyl radicals in murine skin fibroblasts under UVB irradiation. Biochem. Biophys. Res. Commun. 1995, 206, 474–479. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Scharffetter-Kochanek, K.; Brenneisen, P.; Wenk, J.; Herrmann, G.; Ma, W.; Kuhr, L.; Meewes, C.; Wlaschek, M. Photoaging of the skin from phenotype to mechanisms. Exp. Gerontol. 2000, 35, 307–316. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Riley, P.A.; Borovansky, J. Melanins and Melanosomes: Biosynthesis, Biogenesis, Physiological, and Pathological Functions; Wiley-VCH: Weinheim, Germany, 2011; pp. 343–381. [Google Scholar]
- Fitzpatrick, T.B.; Breathnach, A.S. The epidermal melanin unit system. Dermatol. Wochenschr. 1963, 147, 481–489. [Google Scholar]
- Fitzpatrick, T.B.; Lerner, A.B. Biochemical basis of human melanin pigmentation. AMA Arch. Derm. Syphilol. 1954, 69, 133–149. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Kamiya, T.; Kawakami, A.; Ogino, J.; Sohma, H.; Uhara, H.; Jimbow, K. Elucidation of Melanogenesis Cascade for Identifying Pathophysiology and Therapeutic Approach of Pigmentary Disorders and Melanoma. Int. J. Mol. Sci. 2020, 21, 6129. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today 2017, 22, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Kundu, J.K.; Shin, J.W.; Na, H.K.; Surh, Y.J. Docosahexaenoic acid inhibits UVB-induced activation of NF-κB and expression of COX-2 and NOX-4 in HR-1 hairless mouse skin by blocking MSK1 signaling. PLoS ONE 2011, 6, 28065. [Google Scholar] [CrossRef]
- Rezvani, H.R.; Dedieu, S.; North, S.; Belloc, F.; Rossignol, R.; Letellier, T.; de Verneuil, H.; Taïeb, A.; Mazurier, F. Hypoxia-inducible factor-1alpha, a key factor in the keratinocyte response to UVB exposure. J. Biol. Chem. 2007, 282, 16413–16422. [Google Scholar] [CrossRef]
- Ryu, H.C.; Kim, C.; Kim, J.Y.; Chung, J.H.; Kim, J.H. UVB radiation induces apoptosis in keratinocytes by activating a pathway linked to “BLT2-reactive oxygen species”. J. Investig. Dermatol. 2010, 130, 1095–1106. [Google Scholar] [CrossRef]
- Pelle, E.; Mammone, T.; Maes, D.; Frenkel, K. Keratinocytes act as a source of reactive oxygen species by transferring hydrogen peroxide to melanocytes. J. Investig. Dermatol. 2005, 124, 793–797. [Google Scholar] [CrossRef]
- Kim, H.E.; Lee, S.G. Induction of ATP synthase β by H2O2 induces melanogenesis by activating PAH and cAMP/CREB/MITF signaling in melanoma cells. Int. J. Biochem. Cell Biol. 2013, 45, 1217–1222. [Google Scholar] [CrossRef]
- Kemmerling, U.; Muñoz, P.; Müller, M.; Sánchez, G.; Aylwin, M.L.; Klann, E.; Carrasco, M.A.; Hidalgo, C. Calcium release by ryanodine receptors mediates hydrogen peroxide-induced activation of ERK and CREB phosphorylation in N2a cells and hippocampal neurons. Cell Calcium 2007, 41, 491–502. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Wazir, U.; Kothari, S.; Gibbons, N.C.; Moore, J.; Wood, J.M. Human phenylalanine hydroxylase is activated by H2O2: A novel mechanism for increasing the L-tyrosine supply for melanogenesis in melanocytes. Biochem. Biophys. Res. Commun. 2004, 322, 88–92. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.Y.; Huang, Y.L.; Pu, C.M.; Kang, Y.N.; Hoang, K.D.; Chen, K.H.; Chen, C. Effects of Oral Collagen for Skin Anti-Aging: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2080. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005, 53, 6531–6536. [Google Scholar] [CrossRef]
- Ohara, H.; Ichikawa, S.; Matsumoto, H.; Akiyama, M.; Fujimoto, N.; Kobayashi, T.; Tajima, S. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J. Dermatol. 2010, 37, 330–338. [Google Scholar] [CrossRef]
- Daniel, H. Molecular and integrative physiology of intestinal peptide transport. Annu. Rev. Physiol. 2004, 66, 361–384. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Zuniga-Munoz, A.M.; Guarner-Lans, V. Beneficial Effects of the Amino Acid Glycine. Mini Rev. Med. Chem. 2017, 17, 15–32. [Google Scholar] [CrossRef]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxid. Med. Cell Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef]
- Zhou, X.; Han, D.; Xu, R.; Wu, H.; Qu, C.; Wang, F.O.; Wang, X.; Zhao, Y. Glycine protects against high sucrose and high fat-induced non-alcoholic steatohepatitis in rats. Oncotarget 2016, 7, 80223–80237. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, A.; Ortiz-Balderas, E.; Cardozo-Saldaña, G.; Diaz-Diaz, E.; El-Hafidi, M. Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin. Sci. 2014, 126, 19–29. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Lin, G.; Hu, S.; Wang, B.; Dai, Z.; Wu, G. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J. Nutr. 2014, 144, 1540–1548. [Google Scholar] [CrossRef]
- Howard, A.; Tahir, I.; Javed, S.; Waring, S.M.; Ford, D.; Hirst, B.H. Glycine transporter GLYT1 is essential for glycine-mediated protection of human intestinal epithelial cells against oxidative damage. J. Physiol. 2010, 588, 995–1009. [Google Scholar] [CrossRef]
- Van den Eynden, J.; SahebAli, S.; Horwood, N.; Carmans, S.; Brône, B.; Hellings, N.; Steels, P.; Harvey, R.J.; Rigo, J.-M. Glycine and glycine receptor signalling in non-neuronal cells. Front. Mol. Neurosci. 2009, 2, 9. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007, 113, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, J.; Li, C.; Wang, Z.; Li, J.; Zhao, D.; Wang, S.; Zhang, H.; Huang, Y.; Guo, X. Glycine Transporter-1 and glycine receptor mediate the antioxidant effect of glycine in diabetic rat islets and INS-1 cells. Free Radic. Biol. Med. 2018, 123, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, H.F. Catalysis of thiol/disulfide exchange: Single-turnover reduction of protein disulfide-isomerase by glutathione and catalysis of peptide disulfide reduction. Biochemistry 1989, 28, 7298–7305. [Google Scholar] [CrossRef]
- Shigenaga, M.K.; Gimeno, C.J.; Ames, B.N. Urinary 8-hydroxy-2’-deoxyguanosine as a biological marker of in vivo oxidative DNA damage. Proc. Natl. Acad. Sci. USA 1989, 86, 9697–9701. [Google Scholar] [CrossRef]
- Kasai, H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2’-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat. Res. 1997, 387, 147–163. [Google Scholar] [CrossRef]
- Nahhas, A.F.; Abdel-Malek, Z.A.; Kohli, I.; Braunberger, T.L.; Lim, H.W.; Hamzavi, I.H. The potential role of antioxidants in mitigating skin hyperpigmentation resulting from ultraviolet and visible light-induced oxidative stress. Photodermatol. Photoimmunol. Photomed. 2019, 35, 420–428. [Google Scholar] [CrossRef]
- Eastoe, J.E. The amino acid composition of mammalian collagen and gelatin. Biochem. J. 1955, 61, 589–600. [Google Scholar] [CrossRef]
- Eastoe, J.E. The amino acid composition of fish collagen and gelatin. Biochem. J. 1957, 65, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Holwerda, A.M.; van Loon, L.J.C. The impact of collagen protein ingestion on musculoskeletal connective tissue remodeling: A narrative review. Nutr. Rev. 2022, 80, 1497–1514. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Mikhal’chik, E.V.; Suprun, M.V.; Papacharalambous, M.; Truhanov, A.I.; Korkina, L.G. Skin Antiageing and Systemic Redox Effects of Supplementation with Marine Collagen Peptides and Plant-Derived Antioxidants: A Single-Blind Case-Control Clinical Study. Oxid. Med. Cell Longev. 2016, 2016, 4389410. [Google Scholar] [CrossRef]
- Song, H.; Zhang, S.; Zhang, L.; Li, B. Effect of Orally Administered Collagen Peptides from Bovine Bone on Skin Aging in Chronologically Aged Mice. Nutrients 2017, 9, 1209. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.A. Blood glutathione in severe malnutrition in childhood. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.A.; Gibson, N.R.; Lu, Y.; Jahoor, F. Synthesis of erythrocyte glutathione in healthy adults consuming the safe amount of dietary protein. Am. J. Clin. Nutr. 2004, 80, 101–107. [Google Scholar] [CrossRef]
- Jiang, L.; Qin, X.; Zhong, X.; Liu, L.; Jiang, L.; Lu, Y.; Fan, L.; He, Z.; Chen, Q. Glycine-induced cytoprotection is mediated by ERK1/2 and AKT in renal cells with ATP depletion. Eur. J. Cell Biol. 2011, 90, 333–341. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Ma, B.; Li, K.; Li, X.; Bai, H.; Yang, Q.; Zhu, X.; Ben, J.; Chen, Q. Glycine attenuates cerebral ischemia/reperfusion injury by inhibiting neuronal apoptosis in mice. Neurochem. Int. 2012, 61, 649–658. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, X.; Li, J.; Fang, R.; Wang, Z.; Zhang, J.; Li, K.; Li, X.; Bai, H.; Yang, Q.; et al. Glycine prevents pressure overload induced cardiac hypertrophy mediated by glycine receptor. Biochem. Pharmacol. 2017, 123, 40–51. [Google Scholar] [CrossRef]
- Wheeler, M.D.; Thurman, R.G. Production of superoxide and TNF-alpha from alveolar macrophages is blunted by glycine. Am. J. Physiol. 1999, 277, 952–959. [Google Scholar]
- Sarma, N.; Chakraborty, S.; Poojary, S.A.; Rathi, S.; Kumaran, S.; Nirmal, B.; Felicita, J.; Sarkar, R.; Jaiswal, P.; D’Souza, P.; et al. Evidence-based Review, Grade of Recommendation, and Suggested Treatment Recommendations for Melasma. Indian. Dermatol. Online J. 2017, 8, 406–442. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, B.P.; Aman, T.; Alexis, A.F. Postinflammatory Hyperpigmentation: Epidemiology, Clinical Presentation, Pathogenesis and Treatment. Am. J. Clin. Dermatol. 2018, 19, 489–503. [Google Scholar] [CrossRef]
- Taraz, M.; Niknam, S.; Ehsani, A.H. Tranexamic acid in treatment of melasma: A comprehensive review of clinical studies. Dermatol. Ther. 2017, 30, 10. [Google Scholar] [CrossRef]
- Roberts, W.E. Chemical peeling in ethnic/dark skin. Dermatol. Ther. 2004, 17, 196–205. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Yang, F.C.; Cho, B.K. A review of laser and light therapy in melasma. Int. J. Womens Dermatol. 2017, 3, 11–20. [Google Scholar] [CrossRef]
- Chan, R.; Park, K.C.; Lee, M.H.; Lee, E.S.; Chang, S.E.; Leow, Y.H.; Tay, Y.K.; Legarda-Montinola, F.; Tsai, R.Y.; Tsai, T.H.; et al. A randomized controlled trial of the efficacy and safety of a fixed triple combination (fluocinolone acetonide 0.01%, hydroquinone 4%, tretinoin 0.05%) compared with hydroquinone 4% cream in Asian patients with moderate to severe melasma. Br. J. Dermatol. 2008, 159, 697–703. [Google Scholar]
- Taylor, S.C.; Torok, H.; Jones, T.; Lowe, N.; Rich, P.; Tschen, E.; Menter, A.; Baumann, L.; Wieder, J.J.; Jarratt, M.M.; et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis 2003, 72, 67–72. [Google Scholar] [PubMed]
- Grimes, P.; Kelly, A.P.; Torok, H.; Willis, I. Community-based trial of a triple-combination agent for the treatment of facial melasma. Cutis 2006, 77, 177–184. [Google Scholar]
- Rajaratnam, R.; Halpern, J.; Salim, A.; Emmett, C. Interventions for melasma. Cochrane Database Syst. Rev. 2010, 7, 003583. [Google Scholar] [CrossRef]
- Kimbrough-Green, C.K.; Griffiths, C.E.; Finkel, L.J.; Hamilton, T.A.; Bulengo-Ransby, S.M.; Ellis, C.N.; Voorhees, J.J. Topical retinoic acid (tretinoin) for melasma in black patients. A vehicle-controlled clinical trial. Arch. Dermatol. 1994, 130, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Callender, V.D. Acne in ethnic skin: Special considerations for therapy. Dermatol. Ther. 2004, 17, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Grimes, P.E.; Ijaz, S.; Nashawati, R.; Kwak, D. New oral and topical approaches for the treatment of melasma. Int. J. Womens Dermatol. 2018, 5, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Bala, H.R.; Lee, S.; Wong, C.; Pandya, A.G.; Rodrigues, M. Oral Tranexamic Acid for the Treatment of Melasma: A Review. Dermatol. Surg. 2018, 44, 814–825. [Google Scholar] [CrossRef]
- Mathe, N.; Balogun, M.; Yoo, J. A case report on the use of topical cysteamine 5% cream in the management of refractory postinflammatory hyperpigmentation (PIH) resistant to triple combination cream (hydroquinone, topical corticosteroids, and retinoids). J. Cosmet. Dermatol. 2021, 20, 204–206. [Google Scholar] [CrossRef]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef]
- Chung, K.W.; Jeong, H.O.; Jang, E.J.; Choi, Y.J.; Kim, D.H.; Kim, S.R.; Lee, K.J.; Lee, H.J.; Chun, P.; Byun, Y.; et al. Characterization of a small molecule inhibitor of melanogenesis that inhibits tyrosinase activity and scavenges nitric oxide (NO). Biochim. Biophys. Acta 2013, 1830, 4752–4761. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, K.-A.; Lee, S.Y.; Oh, S.; Batsukh, S.; Jang, J.-W.; Lee, B.-J.; Rheu, K.-m.; Li, S.; Jeong, M.-S.; Son, K.H.; et al. Fermented Fish Collagen Attenuates Melanogenesis via Decreasing UV-Induced Oxidative Stress. Mar. Drugs 2024, 22, 421. https://doi.org/10.3390/md22090421
Byun K-A, Lee SY, Oh S, Batsukh S, Jang J-W, Lee B-J, Rheu K-m, Li S, Jeong M-S, Son KH, et al. Fermented Fish Collagen Attenuates Melanogenesis via Decreasing UV-Induced Oxidative Stress. Marine Drugs. 2024; 22(9):421. https://doi.org/10.3390/md22090421
Chicago/Turabian StyleByun, Kyung-A, So Young Lee, Seyeon Oh, Sosorburam Batsukh, Jong-Won Jang, Bae-Jin Lee, Kyoung-min Rheu, Sichao Li, Min-Seok Jeong, Kuk Hui Son, and et al. 2024. "Fermented Fish Collagen Attenuates Melanogenesis via Decreasing UV-Induced Oxidative Stress" Marine Drugs 22, no. 9: 421. https://doi.org/10.3390/md22090421





