Temporal Evolution of PAHs Bioaccessibility in an Aged-Contaminated Soil during the Growth of Two Fabaceae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Material
2.2. PAHs Bioaccessibility Measurement
2.2.1. PAHs Desorption Kinetics
2.2.2. PAHs Desorption Modelling
2.2.3. PAHs Desorption Parameters
2.3. Rhizoremediation Experiment
2.4. Chemical Analyses
2.4.1. Dry Weight Determination
2.4.2. Bioaccessible PAHs Determination in Soil Samples
2.4.3. Total PAHs Determination in Soil Samples
2.4.4. PAHs Analysis
2.5. Statistics
3. Results
3.1. PAHs Bioaccessibility Measurement
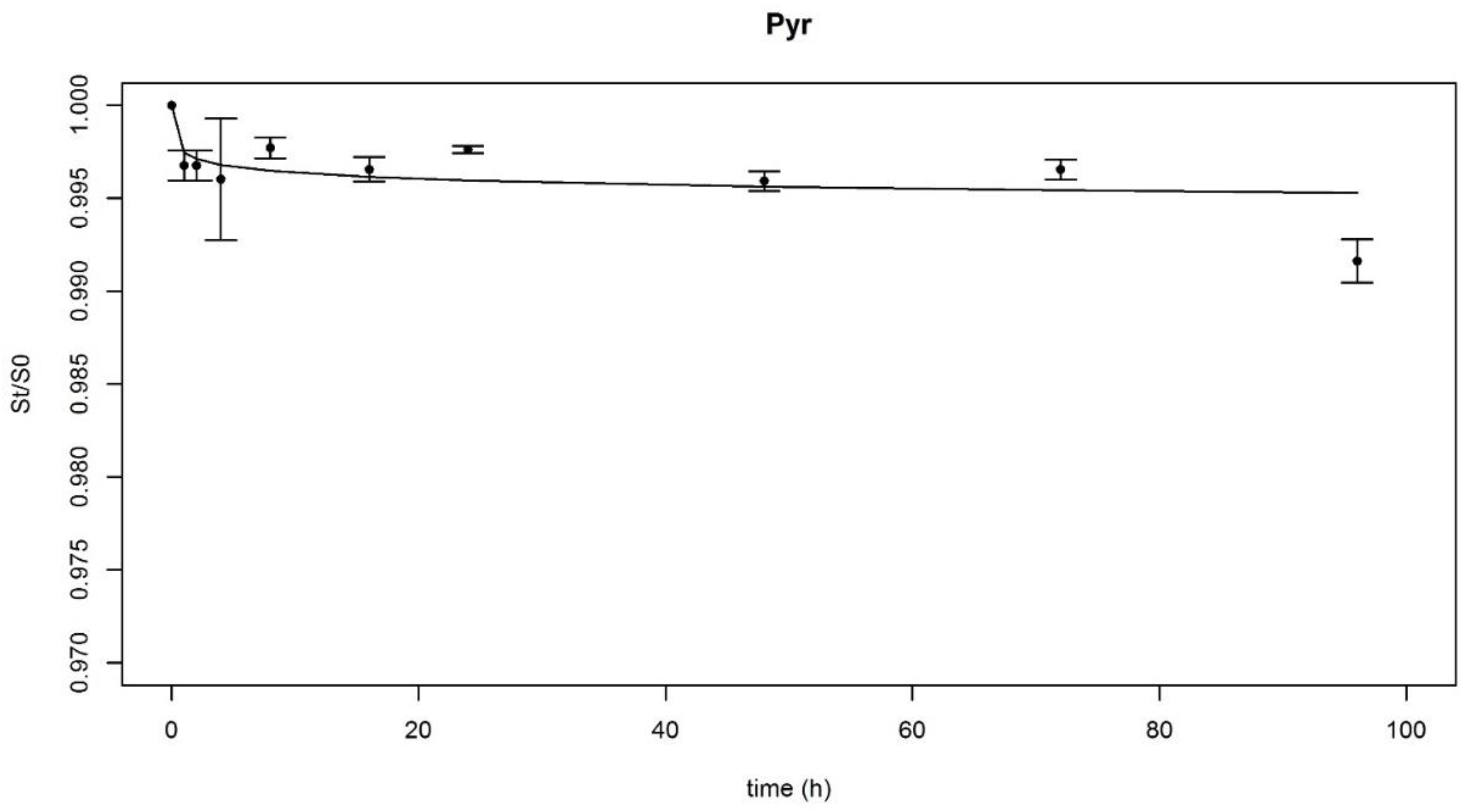
3.1.1. Modelling PAHs Desorption Kinetics
3.1.2. PAHs Desorption Parameters
3.2. PAHs Rhizoremediation
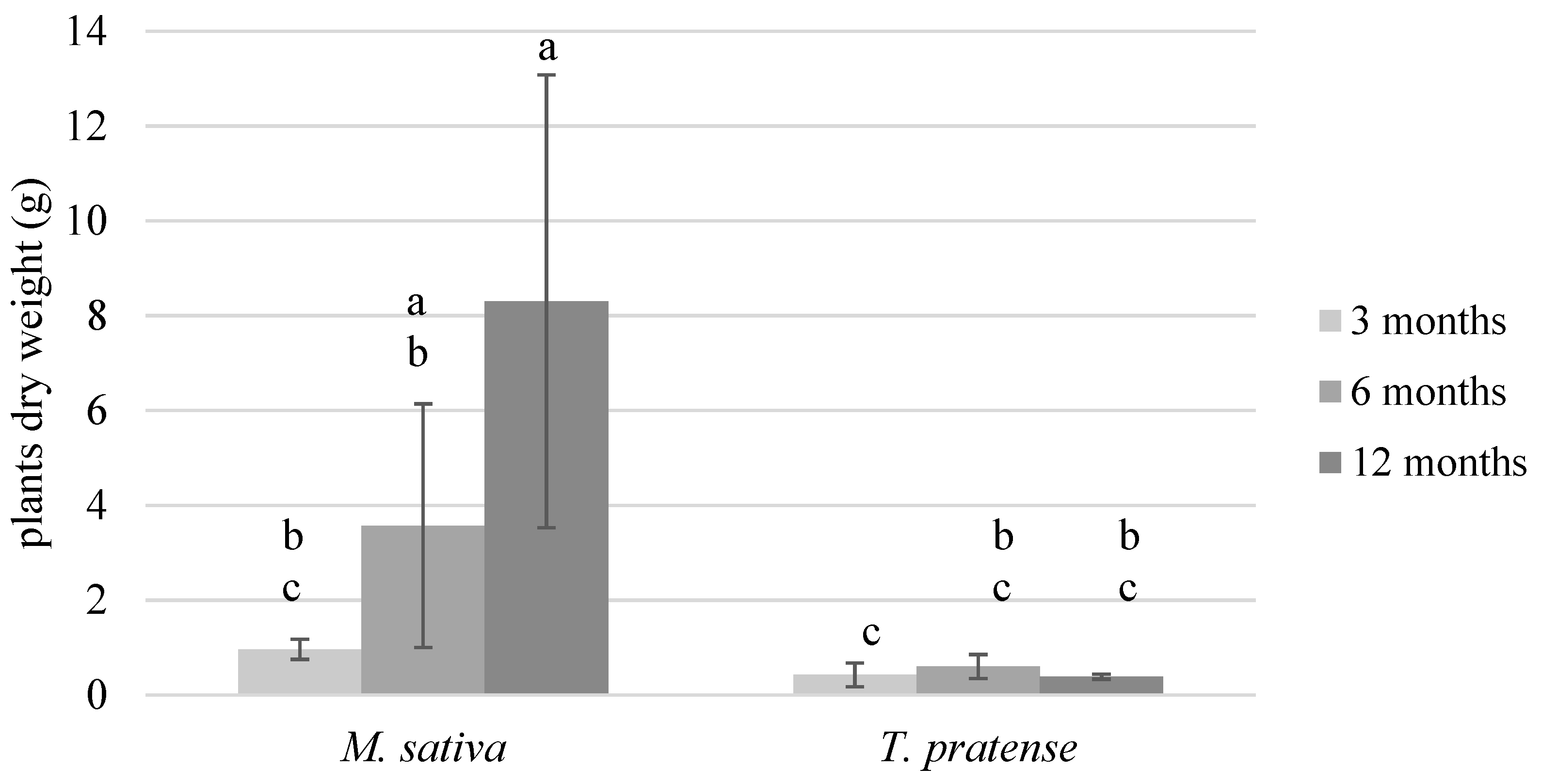
3.2.1. Plant Biomass
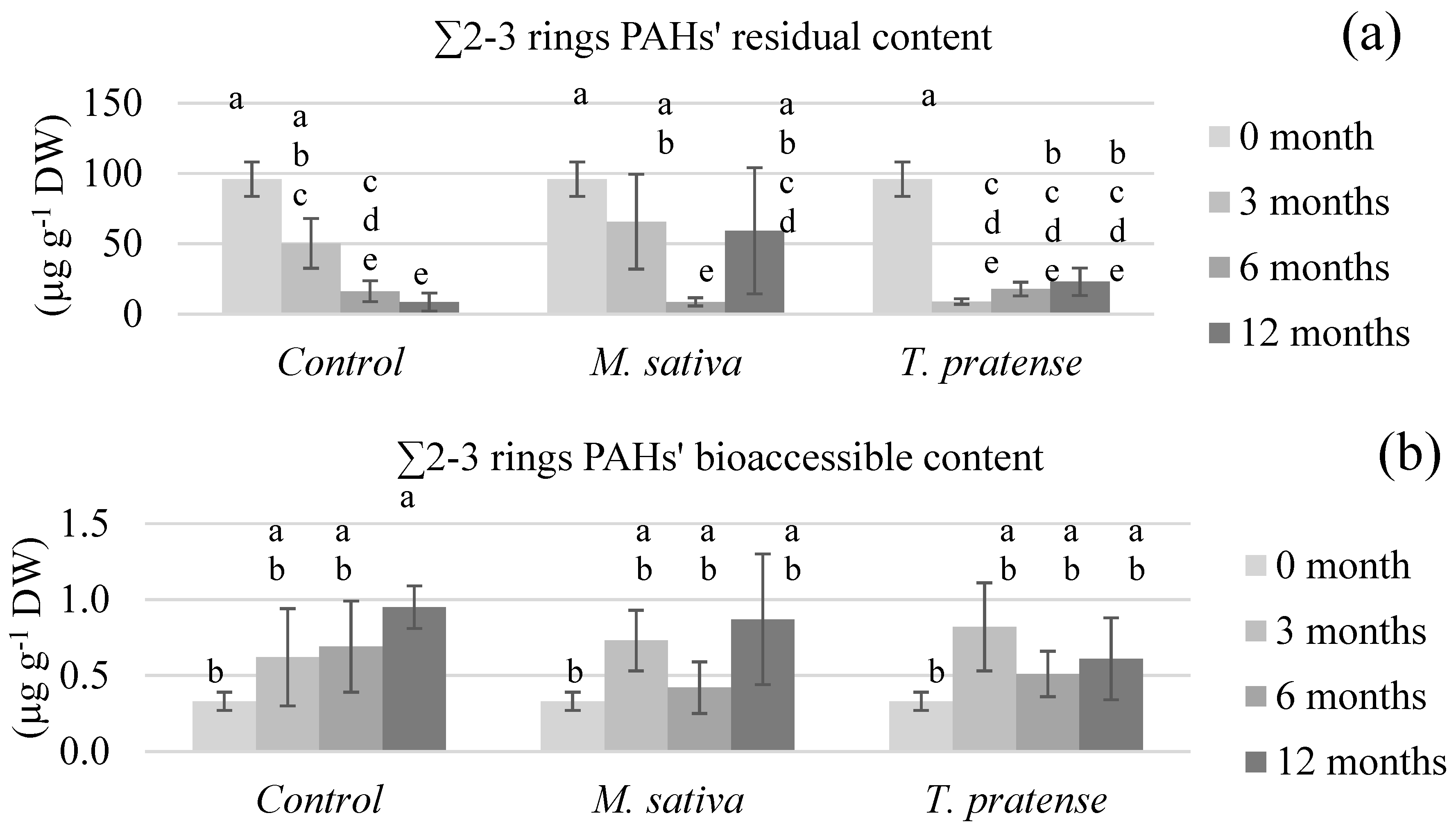
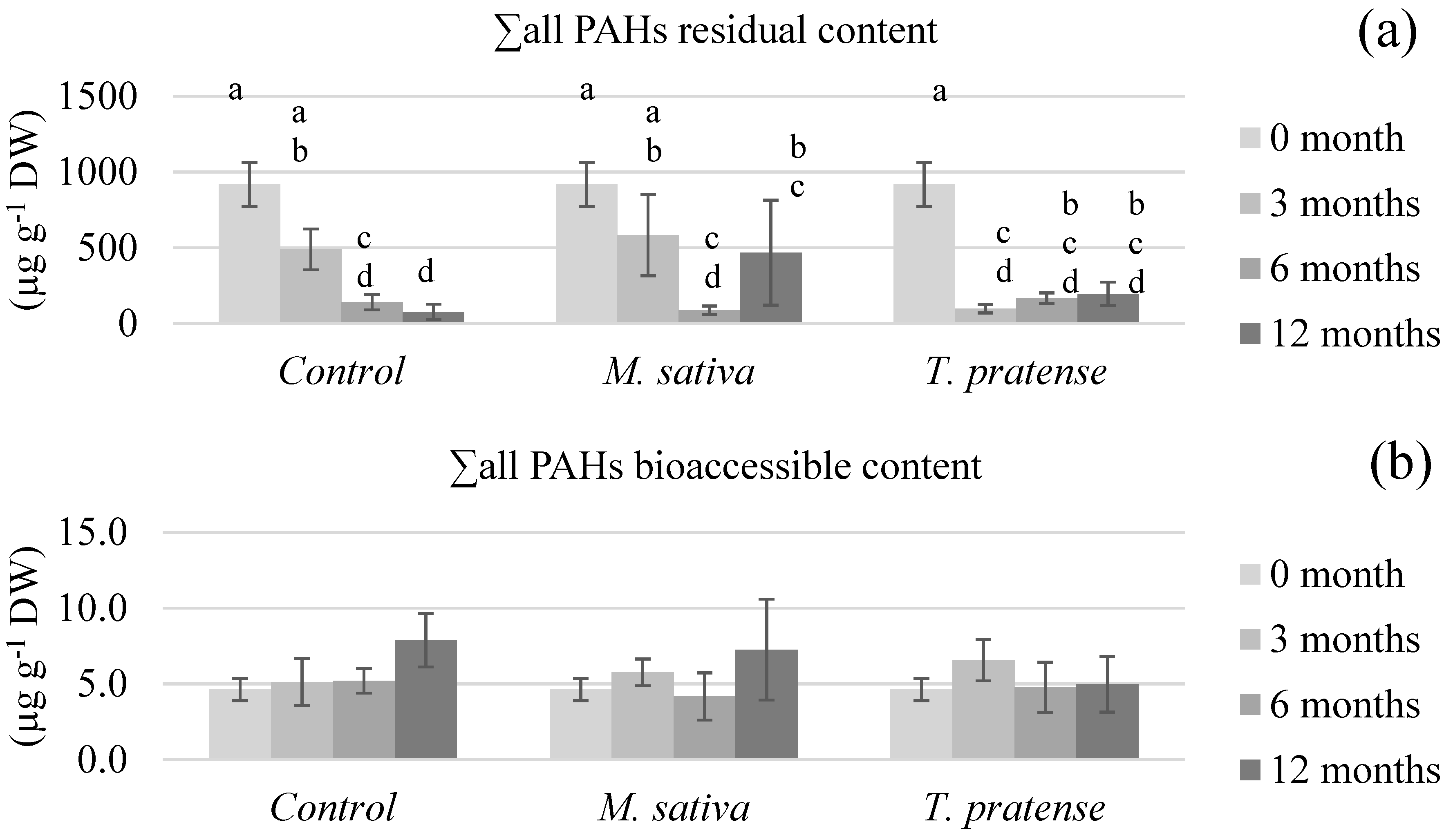
3.2.2. PAHs’ Bioaccessible and Residual Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Model | ||||
|---|---|---|---|---|
| PAHs | First Order | First-Order | First-Order | Site Distribution |
| Two-Compartment | Three-Compartment | |||
| N | −268 | −273 | −266 | −278 * |
| Ace | −233 | −255 | −248 | −261 * |
| Fle | −342 | −363 | −368 * | −365 |
| Phen | −355 | −370 | −371 | −372 * |
| Anthr | −362 | −390 | −400 * | −397 |
| F | −373 | −388 | −390 | −390 * |
| Pyr | −419 | −439 | −443 | −445 * |
| BaA | −354 | −377 | −382 | −388 * |
| Chrys | −339 | −380 * | −357 | −377 |
| BbF | −340 | −360 | −352 | −376 * |
| BkF | −341 | −369 | −378 | −386 * |
| BaP | −354 | −378 | −392 | −397 * |
| DBahA | −329 | −351 | −342 | −370 * |
| BghiP | −318 | −360 | −335 | −365 * |
| IcdP | −338 | −353 | −356 | −369 * |
| ∑2–3 rings | −365 | −380 | −382 * | −378 |
| ∑4 rings | −392 | −410 | −412 | −412 * |
| ∑4–6 rings | −342 | −365 | −371 | −383 * |
| ∑all | −357 | −381 | −386 | −394 * |
References
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A review. Front. Microbiol. 2016, 7, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhar, K.; Subashchandrabose, S.R.; Venkateswarlu, K.; Krishnan, K.; Megharaj, M. Anaerobic microbial degradation of polycyclic aromatic hydrocarbons: A comprehensive review. Rev. Environ. Contam. Toxicol. 2020, 251, 25–108. [Google Scholar] [CrossRef]
- Keith, L.H. The source of U.S. EPA’s sixteen PAH priority pollutants. Polycycl. Aromat. Compd. 2014, 35, 147–160. [Google Scholar] [CrossRef]
- Nzila, A. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons under anaerobic conditions: Overview of studies, proposed pathways and future perspectives. Environ. Pollut. 2018, 239, 788–802. [Google Scholar] [CrossRef]
- Johnsen, A.; Wick, L.Y.; Harms, H. Principles of microbial PAH-degradation in soil. Environ. Pollut. 2005, 133, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Semple, K.T.; Doick, K.J.; Jones, K.C.; Burauel, P.; Craven, A.; Harms, H. Peer reviewed: Defining bioavailability and bioaccessibility of contaminated soil and sediment is complicated. Environ. Sci. Technol. 2004, 38, 228A–231A. [Google Scholar] [CrossRef] [Green Version]
- Semple, K.T.; Morriss, A.W.J.; Paton, G. Bioavailability of hydrophobic organic contaminants in soils: Fundamental concepts and techniques for analysis. Eur. J. Soil Sci. 2003, 54, 809–818. [Google Scholar] [CrossRef]
- Cui, X.; Mayer, P.; Gan, J.J. Methods to assess bioavailability of hydrophobic organic contaminants: Principles, operations, and limitations. Environ. Pollut. 2012, 172, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Reichenauer, T.G.; Germida, J.J. Phytoremediation of organic contaminants in soil and groundwater. ChemSusChem 2008, 1, 708–717. [Google Scholar] [CrossRef]
- Ouvrard, S.; Leglize, P.; Morel, J.L. PAH phytoremediation: Rhizodegradation or rhizoattenuation? Int. J. Phytoremediation 2013, 16, 46–61. [Google Scholar] [CrossRef]
- Alagić, S.; Maluckov, B.S.; Radojičić, V.B. How can plants manage polycyclic aromatic hydrocarbons? May these effects represent a useful tool for an effective soil remediation? A review. Clean Technol. Environ. Policy 2014, 17, 597–614. [Google Scholar] [CrossRef]
- Martin, B.; George, S.J.; Price, C.A.; Ryan, M.H.; Tibbett, M. The role of root exuded low molecular weight organic anions in facilitating petroleum hydrocarbon degradation: Current knowledge and future directions. Sci. Total Environ. 2014, 472, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Plant Physiology; Sinauer: Sunderland, MA, USA, 2006. [Google Scholar]
- Oleszek, W.; Bialy, Z. Chromatographic determination of plant saponins—An update (2002–2005). J. Chromatogr. A 2006, 1112, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Davin, M.; Starren, A.; Marit, E.; Lefébure, K.; Fauconnier, M.-L.; Colinet, G. Investigating the effect of medicago sativa L. and trifolium pratense L. root exudates on PAHs bioremediation in an aged-contaminated soil. Water Air Soil Pollut. 2019, 230, 296. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.; Soole, K.; Bentham, R. Hydrocarbon phytoremediation in the family fabacea—A review. Int. J. Phytoremediation 2011, 13, 317–332. [Google Scholar] [CrossRef]
- Sparg, S.; Light, M.E.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- INERIS. Hydrocarbures Aromatiques Polycyliques-Guide Méthodologique-acquisition Des Données D’entrée Des Modèles Analytiques Ou Numériques De Transferts Dans Les Sols Et Les Eaux Souterraines. 2005. Available online: https://www.ineris.fr (accessed on 10 March 2019).
- Cornelissen, G.; van Noort, P.C.M.; Govers, H.A.J. Desorption kinetics of chlorobenzenes, polycyclic aromatic hydrocarbons, and polychlorinated biphenyls: Sediment extraction with Tenax(R) and effects of contact time and solute hydrophobicity. Environ. Toxicol. Chem. 1997, 16, 1351–1357. [Google Scholar] [CrossRef]
- Barnier, C.; Ouvrard, S.; Robin, C.; Morel, J.L. Desorption kinetics of PAHs from aged industrial soils for availability assessment. Sci. Total Environ. 2014, 470, 639–645. [Google Scholar] [CrossRef]
- Prague, M.; Diakite, A.; Commenges, D. Package’ marqLevAlg ’-Algorithme de Levenberg-Marquardt en R: Une Alternative à’ Optimx’ Pour des Problèmes de Minimisation. HAL. 2012. Available online: https://hal.archives-ouvertes.fr (accessed on 11 March 2019).
- ISO. Soil Quality-Determination of Dry Matter and Water Content on a Mass Basis-Gravimetric Method; The International Organization for Standardization: Geneva, Switzerland, 1993. [Google Scholar]
- ISO. Soil Quality-Determination of Polynuclear Aromatic Hydrocarbons-Method Using High-Performance Liquid Chromatography; The International Organization for Standardization: Geneva, Switzerland, 1998. [Google Scholar]
- Kass, R.E.; Raftery, A.E. Bayes factors. J. Am. Stat. Assoc. 1995, 90, 773–795. [Google Scholar] [CrossRef]
- Müntz, K.; Belozersky, M.; Dunaevsky, Y.; Schlereth, A.; Tiedemann, J. Stored proteinases and the initiation of storage protein mobilization in seeds during germination and seedling growth. J. Exp. Bot. 2001, 52, 1741–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO/TS. Soil Quality-Environmental Availability of Non-Polar Organic Compounds-Determination of the Potential Bioavailable Fraction and the Non-Bioavailable Fraction Using a Strong Adsorbent or Complexing Agent; The International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- Smith, M.J.; Flowers, T.; Duncan, H.; Alder, J. Effects of polycyclic aromatic hydrocarbons on germination and subsequent growth of grasses and legumes in freshly contaminated soil and soil with aged PAHs residues. Environ. Pollut. 2006, 141, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Sverdrup, L.E.; Krogh, P.H.; Nielsen, T.; Kjær, C.; Stenersen, J. Toxicity of eight polycyclic aromatic compounds to red clover (trifolium pratense), ryegrass (lolium perenne), and mustard (sinapsis alba). Chemosphere 2003, 53, 993–1003. [Google Scholar] [CrossRef]
- Henner, P.; Schiavon, M.; Druelle, V.; Lichtfouse, E. Phytotoxicity of ancient gaswork soils. Effect of polycyclic aromatic hydrocarbons (PAHs) on plant germination. Org. Geochem. 1999, 30, 963–969. [Google Scholar] [CrossRef] [Green Version]
- Afegbua, S.L.; Batty, L. Effect of single and mixed polycyclic aromatic hydrocarbon contamination on plant biomass yield and PAH dissipation during phytoremediation. Environ. Sci. Pollut. Res. 2018, 25, 18596–18603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genot, V.; Colinet, G.; Brahy, V.; Bock, L. L’état de fertilité des terres agricoles et forestières en région wallonne (adapté du chapitre 4-sol 1 de «L’État de l’Environnement wallon 2006–2007»). Biotechnol. Agron. Soc. Environ. 2009, 13, 121–138. [Google Scholar]
- Olson, P.E.; Castro, A.; Joern, M.; DuTeau, N.M.; Pilon-Smits, E.A.; Reardon, K.F. Comparison of plant families in a greenhouse phytoremediation study on an aged polycyclic aromatic hydrocarbon-contaminated soil. J. Environ. Qual. 2007, 36, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Fan, S.; Li, P.-J.; Gong, Z.; Ren, W.; He, N. Promotion of pyrene degradation in rhizosphere of alfalfa (Medicago sativa L.). Chemosphere 2008, 71, 1593–1598. [Google Scholar] [CrossRef]
- Hamdi, H.; Benzarti, S.; Aoyama, I.; Jedidi, N. Rehabilitation of degraded soils containing aged PAHs based on phytoremediation with alfalfa (Medicago sativa L.). Int. Biodeterior. Biodegrad. 2012, 67, 40–47. [Google Scholar] [CrossRef]
- Teng, Y.; Shen, Y.; Luo, Y.; Sun, X.; Sun, M.; Fu, D.; Li, Z.; Christie, P. Influence of rhizobium meliloti on phytoremediation of polycyclic aromatic hydrocarbons by alfalfa in an aged contaminated soil. J. Hazard. Mater. 2011, 186, 1271–1276. [Google Scholar] [CrossRef]
- Schwab, P.; Al-Assi, A.A.; Banks, M.K. Adsorption of naphthalene onto plant roots. J. Environ. Qual. 1998, 27, 220–224. [Google Scholar] [CrossRef]
- Kang, F.; Chen, D.; Gao, Y.; Zhang, Y. Distribution of polycyclic aromatic hydrocarbons in subcellular root tissues of ryegrass (Lolium multiflorum Lam.). BMC Plant Boil. 2010, 10, 210. [Google Scholar] [CrossRef] [Green Version]
- Leigh, M.B.; Fletcher, J.S.; Fu, X.; Schmitz, F.J. Root turnover: An important source of microbial substrates in rhizosphere remediation of recalcitrant contaminants. Environ. Sci. Technol. 2002, 36, 1579–1583. [Google Scholar] [CrossRef]
- Baquero, R.P.; Martín, M.L.; Ortega-Calvo, J.-J. Implementing standardized desorption extraction into bioavailability-oriented bioremediation of PAH-polluted soils. Sci. Total Environ. 2019, 696, 134011. [Google Scholar] [CrossRef]
- Posada-Baquero, R.; Jiménez-Volkerink, S.N.; García, J.L.; Vila, J.; Cantos, M.; Grifoll, M.; Ortega-Calvo, J.J. Rhizosphere-enhanced biosurfactant action on slowly desorbing PAHs in contaminated soil. Sci. Total Environ. 2020, 720, 137608. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.; Fernández-López, M.; García-Rodríguez, F.M.; Villadas, P.J.; Rosso, J.A.; Fernández-López, M.; Del Panno, M.T. Exploring the effect of composting technologies on the recovery of hydrocarbon contaminated soil post chemical oxidative treatment. Appl. Soil Ecol. 2020, 150, 103459. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, J.; Lou, L.; Zhu, L. Solubilization properties of polycyclic aromatic hydrocarbons by saponin, a plant-derived biosurfactant. Environ. Pollut. 2011, 159, 1198–1204. [Google Scholar] [CrossRef]
- Laha, S.; Tansel, B.; Ussawarujikulchai, A. Surfactant–soil interactions during surfactant-amended remediation of contaminated soils by hydrophobic organic compounds: A review. J. Environ. Manag. 2009, 90, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Davin, M.; Starren, A.; Deleu, M.; Lognay, G.; Colinet, G.; Fauconnier, M.-L. Could saponins be used to enhance bioremediation of polycyclic aromatic hydrocarbons in aged-contaminated soils? Chemosphere 2018, 194, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| PAHs | Abbreviation | µg g−1 DW |
|---|---|---|
| Naphthalene | N | 20.2 ± 2.4 |
| Acenaphthene | Ace | 1.0 ± 0.4 |
| Fluorene | Fle | 5.1 ± 0.9 |
| Phenanthrene | Phen | 45.5 ± 7.2 |
| Anthracene | Anthr | 24.1 ± 3.6 |
| Light PAHs | ∑2–3 rings | 95.9 ± 12.2 |
| Fluoranthene | F | 139 ± 36.6 |
| Pyrene | Pyr | 117 ± 20.5 |
| Intermediate PAHs | ∑4 rings | 256 ± 47.9 |
| Benzo(a)anthracene | BaA | 79.2 ± 10.5 |
| Chrysene | Chrys | 73.6 ± 8.5 |
| Benzo(b)fluoranthene | BbF | 96.0 ± 19.4 |
| Benzo(k)fluoranthene | BkF | 48.1 ± 5.0 |
| Benzo(a)pyrene | BaP | 95.2 ± 15.6 |
| Dibenzo(ah)anthracene | DBahA | 12.1 ± 1.3 |
| Benzo(ghi)perylene | BghiP | 66.3 ± 25.3 |
| Indeno(123-c,d)pyrene | IcdP | 94.3 ± 21.7 |
| Heavy PAHs | ∑4–6 rings | 565 ± 90.0 |
| Total PAHs | ∑all | 917 ± 146 |
| First-Order Model | (1 Parameter) |
| First-Order Two-Compartment Model | (4 Parameters) |
| First-Order Three-Compartment Model | (6 Parameters) |
| Site Distribution Model | (2 Parameters) |
| β (h) | α (-) | tex (h) | |
|---|---|---|---|
| N | 1.86 × 100 | 4.41 × 10−3 | 24 |
| Ace | 7.30 × 10−2 | 3.72 × 10−3 | 24 |
| Fle | 1.06 × 10−1 | 2.05 × 10−3 | 24 |
| Phen | 4.39 × 10−2 | 1.33 × 10−3 | 24 |
| Anthr | 6.94 × 10−2 | 1.53 × 10−3 | 24 |
| F | 1.09 × 10−1 | 1.37 × 10−3 | 24 |
| Pyr | 4.92 × 10−3 | 4.77 × 10−4 | 24 |
| BaA | 8.84 × 10−2 | 1.62 × 10−3 | 24 |
| Chrys | 1.32 × 10−1 | 2.20 × 10−3 | 24 |
| BbF | 6.57 × 10−3 | 1.04 × 10−3 | 24 |
| BkF | 2.13 × 10−2 | 1.41 × 10−3 | 24 |
| BaP | 6.09 × 10−4 | 6.84 × 10−4 | 24 |
| DBahA | 2.17 × 10−7 | 4.40 × 10−4 | 24 |
| BghiP | 5.93 × 10−7 | 5.38 × 10−4 | 24 |
| IcdP | 6.73 × 10−5 | 5.61 × 10−4 | 24 |
| ∑2–3 rings | 2.47 × 10−1 | 1.95 × 10−3 | 24 |
| ∑4 rings | 4.97 × 10−2 | 9.47 × 10−4 | 24 |
| ∑4–6 rings | 6.84 × 10−3 | 1.07 × 10−3 | 24 |
| ∑all | 1.84 × 10−2 | 1.11 × 10−3 | 24 |
| Reference | Tested Plant (s) | Germination/Growing Conditions | Germination/Growing Outcomes | Germination/Growing Conditions in Presented Experimental Soil (Table 1) |
|---|---|---|---|---|
| Sverdrup et al. [29] | T. pratense L. | Soil freshly spiked with Fle, Phen, F, and Pyr at individual concentrations up to 1000 mg kg−1 DW | No seed emergence inhibition; 20% plant growth inhibition starting at concentrations: 55 mg kg−1 DW (Fle), 37 mg kg−1 DW (Phen), 140 mg kg−1 DW (F), and 49 mg kg−1 DW (Pyr). | Individual concentrations of: 20.2 mg kg−1 DW (N); 5.1 mg kg−1 DW (Fle); 45.5 mg kg−1 DW (Phen); 139 mg kg−1 DW (F); 117 mg kg−1 DW (Pyr); 79.2 mg kg−1 DW (BaA); 73.6 mg kg−1 DW (Chrys). The total concentration of 15 PAHs was 917 mg kg−1 DW. |
| Smith et al. [28] | T. pratense L. | Soil spiked with seven PAHs and aged for 4 weeks (total concentration was 450 mg kg−1 DW after the ageing process) | Germination was not affected; growth was significantly reduced (70%). | |
| Aged-contaminated soil (total concentration of 16 PAHs was 5300 mg kg−1 DW) | Germination was not affected; growth was significantly reduced (65%). | |||
| Henner et al. [30] | M. sativa L.; T. pratense L. | Pure saturated solutions of N, Phen, F, Chrys and BaA | Similar germination levels as in the absence of PAHs. | |
| Aged-contaminated soil (total concentration of 16 PAHs was 1500 mg kg−1 DW) | Germination slowed (3–4 days) but reached similar levels as in uncontaminated soil; plant growth was inhibited (80%) for M. sativa; No information for T. pratense. | |||
| Afegbua and Batty [31] | M. sativa L. | Soil spiked with Phen (300 mg kg−1 DW), F (200 mg kg−1 DW), and BaA (5 mg kg−1 DW) then aged for 4 weeks. | Shoots and roots dry biomass respectively increased by 110% and 40% when PAHs were mixed. |
| Reference | Tested Plant (s) | Phytoremediation Conditions | Phytoremediation Outcomes |
|---|---|---|---|
| Fan et al. [34] | M. sativa L. | Soil freshly spiked with Pyr (500 mg kg−1 DW). | 6% better removal in the rhizosphere compared to the non-rhizosphere soil. |
| Hamdi et al. [35] | M. sativa L. | Soil spiked with BaA (100 mg kg−1 DW) + 15-month landfarming (bioremediation process) had brought content down to 9 mg kg−1 DW. Then soil was planted 5 months in controlled conditions. | BaA content lowered to 4.3 mg kg−1 DW. No unplanted control to compare results. |
| Teng et al. [36] | M. sativa L. | Agricultural weathered soil (total concentration of 16 PAHs was 10 mg kg−1 DW) was planted for 3 months. | 45% lowering of the 16 PAHs mixture. |
| Olson et al. [33] | M. sativa L.; T. pratense L. | Weathered soil (total concentration of 17 PAHs was 753 mg kg−1 DW) was planted 14 months in controlled conditions. | Total PAHs dissipation was not different from unplanted control samples, after 7 and 14 months. |
| Reference | Initial Soil Concentrations | Remediation Conditions | PAHs Residual Concentrations Evolution | PAHs Bioaccessible Concentrations Evolution |
|---|---|---|---|---|
| Posada-Baquero et al. [40] | Phen and BaA concentrations were 843.10 and 56.5 mg kg−1, respectively; Phen and BaA bioaccessible concentrations were 0.75 and 0.10 mg kg−1, respectively. | 5 months biostimulation in a biopile amended with urea and KH2PO4; No reported control. | Phen diminished by over 94%; BaA diminished by about 35%. | Phen diminished by almost 90%; BaA diminished by 30%. |
| Phen and BaA concentrations were 197.10 and 4.12 mg kg−1, respectively; Phen and BaA bioaccessible concentrations were 0.42 and 0.20 mg kg−1, respectively. | 60 days sunflowers phytoremediation in a greenhouse; No reported control. | Phen diminished by over 97%; BaA diminished by about 46%. | Phen diminished by over 86%; BaA diminished by 70%. | |
| Phen and BaA concentrations were 36.7 and 0.64 mg kg−1, respectively; Phen and BaA bioaccessible concentrations were 0.23 and 0.03 mg kg−1, respectively. | 60 days bioaugmentation with specialized strains; No reported control. | Phen diminished by over 30%; BaA diminished by over 10%. | Phen raised by over 140%; BaA raised by 300%. | |
| Phen and BaA concentrations were 46.3 and 1.40 mg kg−1, respectively; Phen and BaA bioaccessible concentrations were 0.27 and 0.024 mg kg−1, respectively. | 60 days bioaugmentation with specialized strains; No reported control. | Phen diminished by 60%; BaA did not diminish. | Phen raised by over 35%; BaA raised by over 200%. | |
| Medina et al. [42] | Aged-contaminated soil (PAHs concentration was 214 mg kg−1 and bioaccessible PAHs fraction was 1%). | Chemical oxidation with ammonium persulfate; No reported control. | PAHs diminished by almost 30%. | PAHs raised to a 19% fraction of remaining total PAHs. |
| Aged-contaminated soil (PAHs concentration was 151 mg kg−1 and bioaccessible PAHs fraction was 19%). | 12 months incubation (served as control). | PAHs diminished by 25%. | PAHs raised to a 30% fraction of remaining total PAHs. | |
| 12 months biostimulation through composting with amended goat manure. | PAHs diminished by 33%. | PAHs raised to a 56% fraction of remaining total PAHs. | ||
| Posada-Baquero et al. [41] | Aged-contaminated soil (PAHs concentration was 513 mg kg−1 and bioaccessible PAHs fraction were 60 and 40% for light and heavy PAHs, respectively). | 210 days of sunflower phytoremediation in a greenhouse combined to a biosurfactant amendment after 75 days. | Light and heavy PAHs diminished by over 90% and 70% in (un)planted soil samples, respectively; Biosurfactant addition had no effect. | Light and heavy PAHs diminished under 10 and around 10% in (un)planted soil samples, respectively; Biosurfactant addition enhanced all PAHs bioaccessible fractions in planted samples for a few days; At the end, bioaccessible fractions were similar in all samples. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davin, M.; Renard, E.; Lefébure, K.; Fauconnier, M.-L.; Colinet, G. Temporal Evolution of PAHs Bioaccessibility in an Aged-Contaminated Soil during the Growth of Two Fabaceae. Int. J. Environ. Res. Public Health 2020, 17, 4016. https://doi.org/10.3390/ijerph17114016
Davin M, Renard E, Lefébure K, Fauconnier M-L, Colinet G. Temporal Evolution of PAHs Bioaccessibility in an Aged-Contaminated Soil during the Growth of Two Fabaceae. International Journal of Environmental Research and Public Health. 2020; 17(11):4016. https://doi.org/10.3390/ijerph17114016
Chicago/Turabian StyleDavin, Marie, Elisa Renard, Kévin Lefébure, Marie-Laure Fauconnier, and Gilles Colinet. 2020. "Temporal Evolution of PAHs Bioaccessibility in an Aged-Contaminated Soil during the Growth of Two Fabaceae" International Journal of Environmental Research and Public Health 17, no. 11: 4016. https://doi.org/10.3390/ijerph17114016
APA StyleDavin, M., Renard, E., Lefébure, K., Fauconnier, M. -L., & Colinet, G. (2020). Temporal Evolution of PAHs Bioaccessibility in an Aged-Contaminated Soil during the Growth of Two Fabaceae. International Journal of Environmental Research and Public Health, 17(11), 4016. https://doi.org/10.3390/ijerph17114016





