Intense Sweeteners, Taste Receptors and the Gut Microbiome: A Metabolic Health Perspective
Abstract
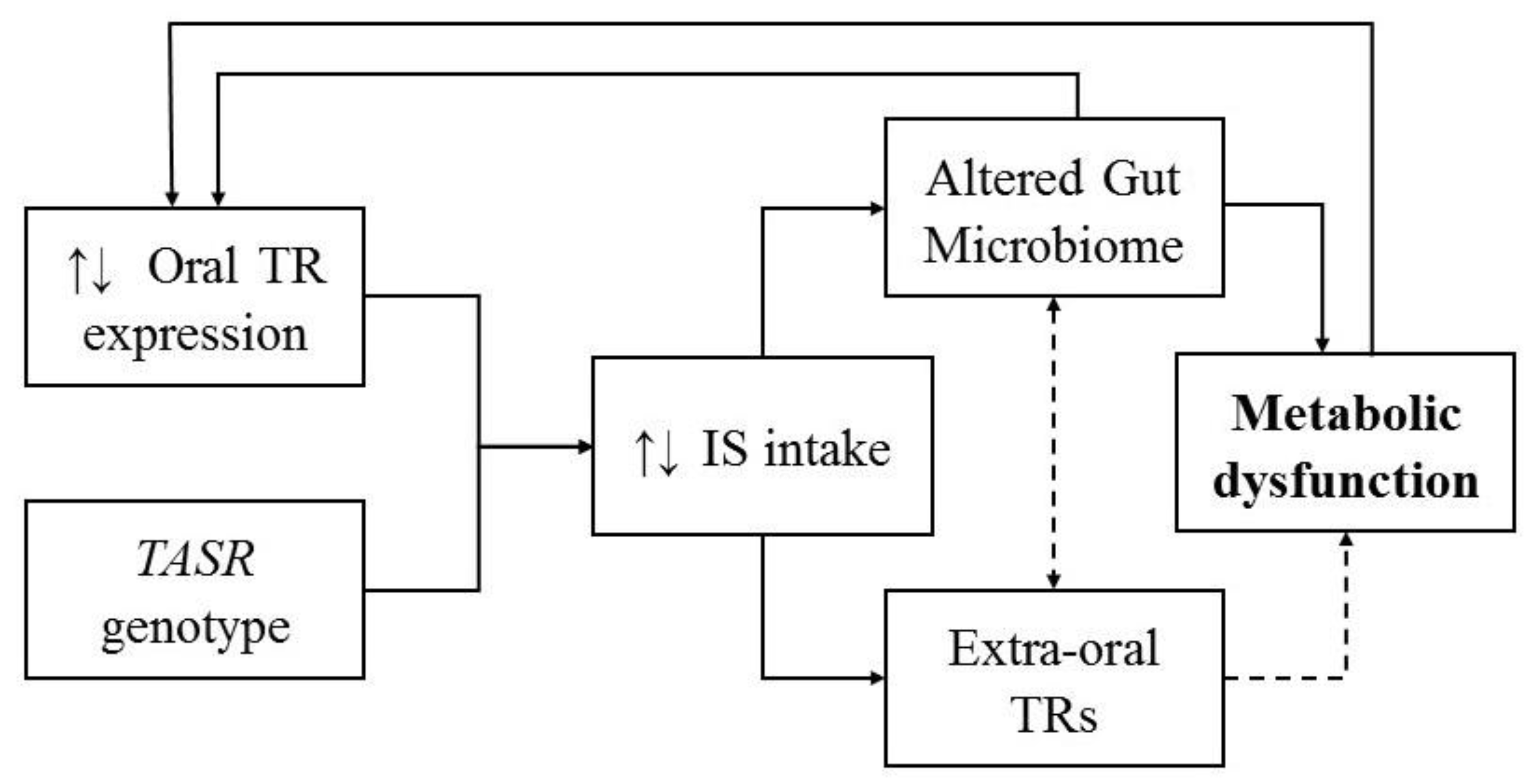
:1. Introduction
2. Weight and Intense Sweetener Consumption in Humans
3. The Metabolic Effects of Intense Sweeteners
4. Oral Detection of Intense Sweeteners
5. The Role of Extra-Oral Receptors in Detecting and Responding to Intense Sweeteners
6. Intense Sweeteners and The Gut Microbiome
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food Standards Australia and New Zealand. Intense Sweeteners. Available online: http://www.foodstandards.gov.au/consumer/additives/Pages/Sweeteners.aspx (accessed on 30 May 2020).
- Franz, M.J.; Powers, M.A.; Leontos, C.; Holzmeister, L.A.; Kulkarni, K.; Monk, A.; Wedel, N.; Gradwell, E. The Evidence for Medical Nutrition Therapy for Type 1 and Type 2 Diabetes in Adults. J. Am. Diet. Assoc. 2010, 110, 1852–1889. [Google Scholar] [CrossRef] [PubMed]
- Sylvetsky, A.C.; Jin, Y.; Clark, E.J.; Welsh, J.A.; Rother, K.I.; Talegawkar, S.A. Consumption of Low-Calorie Sweeteners among Children and Adults in the United States. J. Acad. Nutr. Diet. 2017, 117, 441–448.e2. [Google Scholar] [CrossRef] [PubMed]
- Swithers, S.E. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab. 2013, 24, 431–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepino, M.Y. Metabolic effects of non-nutritive sweeteners. Physiol. Behav. 2015, 152, 450–455. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.J.; Walter, M.; Rother, K.I. Effects of Diet Soda on Gut Hormones in Youths With Diabetes. Diabetes Care 2012, 35, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Hess, E.L.; Myers, E.A.; Swithers, S.E.; Hedrick, V.E. Associations Between Nonnutritive Sweetener Intake and Metabolic Syndrome in Adults. J. Am. Coll. Nutr. 2018, 37, 487–493. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Rother, K.I. Nonnutritive Sweeteners in Weight Management and Chronic Disease: A Review. Obesity 2018, 26, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Magnuson, B.A.; Carakostas, M.C.; Moore, N.H.; Poulos, S.; Renwick, A.G. Biological fate of low-calorie sweeteners. Nutr. Rev. 2016, 74, 670–689. [Google Scholar] [CrossRef] [Green Version]
- ҪiçekS, S. Structure-Dependent Activity of Plant-Derived Sweeteners. Molecules 2020, 25, 1946. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration. Additional Information about High-Intensity Sweeteners Permitted for Use in Food in the United States. Available online: https://www.fda.gov/food/food-additives-petitions/additional-information-about-high-intensity-sweeteners-permitted-use-food-united-states (accessed on 30 May 2020).
- European Commission Joint Research Centre. S&S Table 7: Acceptable Daily Intake (ADI) of Sweeteners in the EU. Available online: https://ec.europa.eu/jrc/en/page/ss-table-7-acceptable-daily-intake-adi-sweeteners-eu-182968 (accessed on 30 May 2020).
- Assadi-Porter, F.; Radek, J.; Rao, H.; Tonelli, M. Multimodal Ligand Binding Studies of Human and Mouse G-Coupled Taste Receptors to Correlate Their Species-Specific Sweetness Tasting Properties. Molecules 2018, 23, 2531. [Google Scholar] [CrossRef] [Green Version]
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.; Zuker, C.S. Mammalian Sweet Taste Receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Go, Y.; Satta, Y.; Takenaka, O.; Takahata, N. Lineage-Specific Loss of Function of Bitter Taste Receptor Genes in Humans and Nonhuman Primates. Genetics 2005, 170, 313–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochem, M. Type 1 Taste Receptors in Taste and Metabolism. Ann. Nutr. Metab. 2017, 70, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Avau, B.; Depoortere, I. The bitter truth about bitter taste receptors: Beyond sensing bitter in the oral cavity. Acta Physiol. 2015, 216, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Depoortere, I. Taste receptors of the gut: Emerging roles in health and disease. Gut 2013, 63, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Kok, B.; Galmozzi, A.; Littlejohn, N.; Albert, V.; Godio, C.; Kim, W.; Kim, S.; Bland, J.S.; Grayson, N.; Fang, M.; et al. Intestinal bitter taste receptor activation alters hormone secretion and imparts metabolic benefits. Mol. Metab. 2018, 16, 76–87. [Google Scholar] [CrossRef]
- Turner, A.; Veysey, M.; Keely, S.; Scarlett, C.J.; Lucock, M.; Beckett, E.L. Interactions between Bitter Taste, Diet and Dysbiosis: Consequences for Appetite and Obesity. Nutrients 2018, 10, 1336. [Google Scholar] [CrossRef] [Green Version]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef] [Green Version]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Zhu, C.; Li, H.; Yin, M.; Pan, C.; Huang, L.; Kong, C.; Wang, X.; Zhang, Y.; Qu, S.; et al. Dysbiosis Signatures of Gut Microbiota Along the Sequence from Healthy, Young Patients to Those with Overweight and Obesity. Obesity 2017, 26, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Abou-Setta, A.M.; Chauhan, B.F.; Rabbani, R.; Lys, J.; Copstein, L.; Mann, A.; Jeyaraman, M.M.; Reid, A.E.; Fiander, M.; et al. Nonnutritive sweeteners and cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. Can. Med. Assoc. J. 2017, 189, E929–E939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, S.P. Low-calorie sweetener use and energy balance: Results from experimental studies in animals, and large-scale prospective studies in humans. Physiol. Behav. 2016, 164, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Stellman, S.D.; Garfinkel, L. Artificial sweetener use and one-year weight change among women. Prev. Med. 1986, 15, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Grech, A.; Kam, C.O.; Gemming, L.; Rangan, A. Diet-Quality and Socio-Demographic Factors Associated with Non-Nutritive Sweetener Use in the Australian Population. Nutrients 2018, 10, 833. [Google Scholar] [CrossRef] [Green Version]
- Fowler, S.P.; Williams, K.; Resendez, R.G.; Hunt, K.J.; Hazuda, H.P.; Stern, M.P. Fueling the Obesity Epidemic? Artificially Sweetened Beverage Use and Long-term Weight Gain. Obesity 2008, 16, 1894–1900. [Google Scholar] [CrossRef]
- Gearon, E.; Peeters, A.; Ng, W.; Hodge, A.M.; Backholer, K. Diet and physical activity as possible mediators of the association between educational attainment and body mass index gain among Australian adults. Int. J. Public Health 2018, 63, 883–893. [Google Scholar] [CrossRef]
- Fowler, L.J.; Williams, K.; Hazuda, H.P. Diet soda intake is associated with long-term increases in waist circumference in a biethnic cohort of older adults: The San Antonio Longitudinal Study of Aging. J. Am. Geriatr. Soc. 2015, 63, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Madjd, A.; Taylor, M.A.; Delavari, A.; Malekzadeh, R.; Macdonald, I.A.; Farshchi, H.R. Effects on weight loss in adults of replacing diet beverages with water during a hypoenergetic diet: A randomized, 24-wk clinical trial. Am. J. Clin. Nutr. 2015, 102, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.; Wyatt, H.R.; Foster, G.D.; Pan, Z.; Wojtanowski, A.C.; Veur, S.S.V.; Herring, S.J.; Brill, C.; Hill, J.O. The effects of water and non-nutritive sweetened beverages on weight loss during a 12-week weight loss treatment program. Obesity 2014, 22, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, G.L.; Kanders, B.S.; Lavin, P.T.; Keller, S.D.; Whatley, J. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. Am. J. Clin. Nutr. 1997, 65, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Tate, D.; Turner-McGrievy, G.M.; Lyons, E.J.; Stevens, J.; Erickson, K.; Polzien, K.; Diamond, M.; Wang, X.; Popkin, B.M. Replacing caloric beverages with water or diet beverages for weight loss in adults: Main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am. J. Clin. Nutr. 2012, 95, 555–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maersk, M.; Belza, A.; Stødkilde-Jørgensen, H.; Ringgaard, S.; Chabanova, E.; Thomsen, H.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am. J. Clin. Nutr. 2011, 95, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-H.; Chan, P.; Sue, Y.-M.; Liu, J.-C.; Liang, T.H.; Huang, T.-Y.; Tomlinson, B.; Chow, M.S.S.; Kao, P.-F.; Chen, Y.-J. Efficacy and tolerability of oral stevioside in patients with mild essential hypertension: A two-year, randomized, placebo-controlled study. Clin. Ther. 2003, 25, 2797–2808. [Google Scholar] [CrossRef] [Green Version]
- Hunter, S.; Reister, E.; Cheon, E.; Mattes, R.D. Low Calorie Sweeteners Differ in Their Physiological Effects in Humans. Nutrients 2019, 11, 2717. [Google Scholar] [CrossRef] [Green Version]
- Pearlman, M.; Obert, J.; Casey, L. The Association between Artificial Sweeteners and Obesity. Curr. Gastroenterol. Rep. 2017, 19, 64. [Google Scholar] [CrossRef]
- Brown, R.J.; De Banate, M.A.; Rother, K.I. Artificial sweeteners: A systematic review of metabolic effects in youth. Pediatr. Obes. 2010, 5, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Burke, M.; Small, D.M. Physiological mechanisms by which non-nutritive sweeteners may impact body weight and metabolism. Physiol. Behav. 2015, 152, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Liauchonak, I.; Qorri, B.; Dawoud, F.; Riat, Y.; Szewczuk, M.R. Non-Nutritive Sweeteners and Their Implications on the Development of Metabolic Syndrome. Nutrients 2019, 11, 644. [Google Scholar] [CrossRef] [Green Version]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Swithers, S.E.; Laboy, A.F.; Clark, K.; Cooper, S.; Davidson, T.L. Experience with the high-intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behav. Brain Res. 2012, 233, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmnäs, M.S.A.; Cowan, T.E.; Bomhof, M.R.; Su, J.; Reimer, R.A.; Vogel, H.J.; Hittel, D.S.; Shearer, J. Low-Dose Aspartame Consumption Differentially Affects Gut Microbiota-Host Metabolic Interactions in the Diet-Induced Obese Rat. PLoS ONE 2014, 9, e109841. [Google Scholar] [CrossRef]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Fagherazzi, G.; Vilier, A.; Sartorelli, D.S.; Lajous, M.; Balkau, B.; Clavel-Chapelon, F. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l’Education Nationale–European Prospective Investigation into Cancer and Nutrition cohort. Am. J. Clin. Nutr. 2013, 97, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.J.; Walter, M.; Rother, K.I. Ingestion of Diet Soda Before a Glucose Load Augments Glucagon-Like Peptide-1 Secretion. Diabetes Care 2009, 32, 2184–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temizkan, S.; Deyneli, O.; Yasar, M.; Arpa, M.; Gunes, M.; Yazici, D.; Sirikçi, Ö.; Haklar, G.; Imeryuz, N.; Yavuz, D.G. Sucralose enhances GLP-1 release and lowers blood glucose in the presence of carbohydrate in healthy subjects but not in patients with type 2 diabetes. Eur. J. Clin. Nutr. 2014, 69, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Tiemann, C.D.; Patterson, B.W.; Wice, B.M.; Klein, S. Sucralose Affects Glycemic and Hormonal Responses to an Oral Glucose Load. Diabetes Care 2013, 36, 2530–2535. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Zhao, B.R.; Bound, M.J.; Checklin, H.L.; Bellon, M.; Little, T.; Young, R.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am. J. Clin. Nutr. 2011, 95, 78–83. [Google Scholar] [CrossRef]
- Rogers, P.J.; Carlyle, J.-A.; Hill, A.J.; Blundell, J.E. Uncoupling sweet taste and calories: Comparison of the effects of glucose and three intense sweeteners on hunger and food intake. Physiol. Behav. 1988, 43, 547–552. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Blau, J.E.; Rother, K.I. Understanding the metabolic and health effects of low-calorie sweeteners: Methodological considerations and implications for future research. Rev. Endocr. Metab. Disord. 2016, 17, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Kim, U.-K.; Jorgenson, E.; Coon, H.; Leppert, M.; Risch, N.; Drayna, D. Positional Cloning of the Human Quantitative Trait Locus Underlying Taste Sensitivity to Phenylthiocarbamide. Science 2003, 299, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E.; Bartoshuk, L.M.; Kidd, J.R.; Duffy, V.B. Supertasting and PROP Bitterness Depends on More than the TAS2R38 Gene. Chem. Senses 2008, 33, 255–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepper, B.J.; White, E.A.; Koelliker, Y.; Lanzara, C.; D’Adamo, P.; Gasparini, P.; D’Adamo, P. Genetic Variation in Taste Sensitivity to 6-n-Propylthiouracil and Its Relationship to Taste Perception and Food Selection. Ann. N. Y. Acad. Sci. 2009, 1170, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Diószegi, J.; Llanaj, E.; Ádány, R. Genetic Background of Taste Perception, Taste Preferences, and Its Nutritional Implications: A Systematic Review. Front. Genet. 2019, 10, 1272. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-H. Variation in the TAS2R38 Bitterness Receptor Gene Was Associated with Food Consumption and Obesity Risk in Koreans. Nutrients 2019, 11, 1973. [Google Scholar] [CrossRef] [Green Version]
- Tepper, B.J.; Koelliker, Y.; Zhao, L.; Ullrich, N.V.; Lanzara, C.; D’Adamo, P.; Ferrara, A.; Ulivi, S.; Esposito, L.; Gasparini, P.; et al. Variation in the Bitter-taste Receptor GeneTAS2R38, and Adiposity in a Genetically Isolated Population in Southern Italy. Obesity 2008, 16, 2289–2295. [Google Scholar] [CrossRef]
- Pawellek, I.; Grote, V.; Rzehak, P.; Xhonneux, A.; Verduci, E.; Stolarczyk, A.; Closa-Monasterolo, R.; Reischl, E.; Koletzko, B. Association of TAS2R38 variants with sweet food intake in children aged 1–6 years. Appetite 2016, 107, 126–134. [Google Scholar] [CrossRef]
- Mikołajczyk-Stecyna, J.; Malinowska, A.; Chmurzyńska, A. TAS2R38 and CA6 genetic polymorphisms, frequency of bitter food intake, and blood biomarkers among elderly woman. Appetite 2017, 116, 57–64. [Google Scholar] [CrossRef]
- Lumeng, J.C.; Cardinal, T.M.; Sitto, J.R.; Kannan, S. Ability to taste 6-n-propylthiouracil and BMI in low-income preschool-aged children. Obesity 2008, 16, 1522–1528. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.J.; Agüera, Z.; Sabater, M.; Moreno-Navarrete, J.M.; Alonso-Ledesma, I.; Xifra, G.; Botas, P.; Delgado, E.; Jiménez-Murcia, S.; Fernández-García, J.C.; et al. Genetic variations of the bitter taste receptor TAS2R38 are associated with obesity and impact on single immune traits. Mol. Nutr. Food Res. 2016, 60, 1673–1683. [Google Scholar] [CrossRef]
- Kuhn, C.; Bufe, B.; Winnig, M.; Hofmann, T.; Frank, O.; Behrens, M.; Lewtschenko, T.; Slack, J.P.; Ward, C.D.; Meyerhof, W. Bitter Taste Receptors for Saccharin and Acesulfame K. J. Neurosci. 2004, 24, 10260–10265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, A.L.; McGeary, J.E.; Knopik, V.S.; Hayes, J.E. Bitterness of the Non-nutritive Sweetener Acesulfame Potassium Varies With Polymorphisms in TAS2R9 and TAS2R31. Chem. Senses 2013, 38, 379–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The Molecular Receptive Ranges of Human TAS2R Bitter Taste Receptors. Chem. Senses 2009, 35, 157–170. [Google Scholar] [CrossRef]
- Behrens, M.; Blank, K.; Meyerhof, W. Blends of Non-caloric Sweeteners Saccharin and Cyclamate Show Reduced Off-Taste due to TAS2R Bitter Receptor Inhibition. Cell Chem. Boil. 2017, 24, 1199–1204.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo, W.; González-Nilo, F.; Agosin, E. Docking and Molecular Dynamics of Steviol Glycoside–Human Bitter Receptor Interactions. J. Agric. Food Chem. 2016, 64, 7585–7596. [Google Scholar] [CrossRef] [PubMed]
- Hellfritsch, C.; Brockhoff, A.; Stähler, F.; Meyerhof, W.; Hofmann, T.F. Human Psychometric and Taste Receptor Responses to Steviol Glycosides. J. Agric. Food Chem. 2012, 60, 6782–6793. [Google Scholar] [CrossRef]
- Chao, D.H.M.; Argmann, C.; Van Eijk, M.; Boot, R.; Ottenhoff, R.; Van Roomen, C.; Foppen, E.; Siljee, J.E.; Unmehopa, U.A.; Kalsbeek, A.; et al. Impact of obesity on taste receptor expression in extra-oral tissues: Emphasis on hypothalamus and brainstem. Sci. Rep. 2016, 6, 29094. [Google Scholar] [CrossRef] [Green Version]
- Sylvetsky, A.C.; Figueroa, J.; Zimmerman, T.; Swithers, S.E.; Welsh, J.A. Consumption of low-calorie sweetened beverages is associated with higher total energy and sugar intake among children, NHANES 2011-2016. Pediatr. Obes. 2019, 14, e12535. [Google Scholar] [CrossRef]
- Mahar, A.; Duizer, L. The Effect of Frequency of Consumption of Artificial Sweeteners on Sweetness Liking by Women. J. Food Sci. 2007, 72, S714–S718. [Google Scholar] [CrossRef]
- Rother, K.I.; Conway, E.M.; Sylvetsky, A.C. How Non-nutritive Sweeteners Influence Hormones and Health. Trends Endocrinol. Metab. 2018, 29, 455–467. [Google Scholar] [CrossRef]
- Bachmanov, A.; Bosak, N.P.; Lin, C.; Matsumoto, I.; Ohmoto, M.; Reed, D.; Nelson, T.M. Genetics of Taste Receptors. Curr. Pharm. Des. 2014, 20, 2669–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernstrom, J.D.; Munger, S.D.; Sclafani, A.; De Araujo, I.E.; Roberts, A.; Molinary, S. Mechanisms for sweetness. J. Nutr. 2012, 142, 1134S–1141S. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bachmanov, A.; Maehashi, K.; Li, W.; Lim, R.; Brand, J.G.; Beauchamp, G.K.; Reed, D.; Thai, C.; Floriano, W. Sweet taste receptor gene variation and aspartame taste in primates and other species. Chem. Senses 2011, 36, 453–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Bailo, B.; Toguri, C.; Eny, K.M.; El-Sohemy, A. Genetic Variation in Taste and Its Influence on Food Selection. OMICS J. Integr. Boil. 2009, 13, 69–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamoun, E.; Mutch, D.; Allen-Vercoe, E.; Buchholz, A.C.; Duncan, A.M.; Spriet, L.L.; Haines, J.; Ma, W.L.D. On behalf of the Guelph Family Health Study A review of the associations between single nucleotide polymorphisms in taste receptors, eating behaviors, and health. Crit. Rev. Food Sci. Nutr. 2017, 58, 194–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pronin, A.N.; Xu, H.; Tang, H.; Zhang, L.; Li, Q.; Li, X. Specific Alleles of Bitter Receptor Genes Influence Human Sensitivity to the Bitterness of Aloin and Saccharin. Curr. Boil. 2007, 17, 1403–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, R.M.; Lee, R.J.; Cohen, N.A.; Woodworth, B.; Poetker, D.; Reh, D. Taste Receptors in Upper Airway Immunity. Adv. Oto Rhino Laryngol. 2016, 79, 91–102. [Google Scholar] [CrossRef]
- Freund, J.; Lee, R.J. Taste receptors in the upper airway. World J. Otorhinolaryngol. Head Neck Surg. 2018, 4, 67–76. [Google Scholar] [CrossRef]
- Shah, A.S.; Ben-Shahar, Y.; Moninger, T.O.; Kline, J.N.; Welsh, M.J. Motile Cilia of Human Airway Epithelia Are Chemosensory. Science 2009, 325, 1131–1134. [Google Scholar] [CrossRef] [Green Version]
- Tizzano, M.; Gulbransen, B.; Vandenbeuch, A.; Clapp, T.R.; Herman, J.P.; Sibhatu, H.M.; Churchill, M.; Silver, W.L.; Kinnamon, S.C.; Finger, T. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc. Natl. Acad. Sci. USA 2010, 107, 3210–3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adappa, N.D.; Zhang, Z.; Palmer, J.N.; Kennedy, D.W.; Doghramji, L.; Lysenko, A.; Reed, D.; Scott, T.; Zhao, N.W.; Owens, D.; et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int. Forum Allergy Rhinol. 2013, 4, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.J.; Xiong, G.; Kofonow, J.M.; Chen, B.; Lysenko, A.; Jiang, P.; Abraham, V.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Investig. 2012, 122, 4145–4159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Wang, X.; Young, R.; Horowitz, M.; Rayner, C.K.; Wu, T. Role of Intestinal Bitter Sensing in Enteroendocrine Hormone Secretion and Metabolic Control. Front. Endocrinol. 2018, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Dotson, C.D.; Zhang, L.; Xu, H.; Shin, Y.-K.; Vigues, S.; Ott, S.H.; Elson, A.; Choi, H.J.; Shaw, H.; Egan, J.M.; et al. Bitter Taste Receptors Influence Glucose Homeostasis. PLoS ONE 2008, 3, e3974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, S.; Laermans, J.; Verhulst, P.-J.; Thijs, T.; Tack, J.; Depoortere, I. Bitter taste receptors and α-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc. Natl. Acad. Sci. USA 2011, 108, 2094–2099. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.-J.; Kokrashvili, Z.; Theodorakis, M.J.; Carlson, O.D.; Kim, B.-J.; Zhou, J.; Kim, H.H.; Xu, X.; Chan, S.L.; Juhaszova, M.; et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA 2007, 104, 15069–15074. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Bagenna, B.; Fu, M. The sweet taste signalling pathways in the oral cavity and the gastrointestinal tract affect human appetite and food intake: A review. Int. J. Food Sci. Nutr. 2018, 70, 125–135. [Google Scholar] [CrossRef]
- Andreozzi, P.; Sarnelli, G.; Pesce, M.; Zito, F.P.; Alessandro, A.D.; Verlezza, V.; Palumbo, I.; Turco, F.; Esposito, K.; Cuomo, R. The Bitter Taste Receptor Agonist Quinine Reduces Calorie Intake and Increases the Postprandial Release of Cholecystokinin in Healthy Subjects. J. Neurogastroenterol. Motil. 2015, 21, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3–36): Secretory Controls and Physiological Roles in Eating and Glycemia in Health, Obesity, and After RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Gerspach, A.C.; Biesiekierski, J.R.; Deloose, E.; Clevers, E.; Rotondo, A.; Rehfeld, J.F.; Depoortere, I.; Van Oudenhove, L.; Tack, J. Effects of caloric and noncaloric sweeteners on antroduodenal motility, gastrointestinal hormone secretion and appetite-related sensations in healthy subjects. Am. J. Clin. Nutr. 2018, 107, 707–716. [Google Scholar] [CrossRef] [Green Version]
- Rosales, C.; Martinez-Carrillo, B.E.; Reséndiz-Albor, A.A.; Ramírez-Duran, N.; Valdés-Ramos, R.; Mondragón-Velásquez, T.; Escoto-Herrera, J.A. Chronic Consumption of Sweeteners and Its Effect on Glycaemia, Cytokines, Hormones, and Lymphocytes of GALT in CD1 Mice. BioMed. Res. Int. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gerspach, A.C.; Steinert, R.E.; Schönenberger, L.; Graber-Maier, A.; Beglinger, C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am. J. Physiol. Metab. 2011, 301, E317–E325. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Nagasawa, M.; Yamada, S.; Hara, A.; Mogami, H.; Nikolaev, V.O.; Lohse, M.J.; Shigemura, N.; Ninomiya, Y.; Kojima, I. Sweet Taste Receptor Expressed in Pancreatic β-Cells Activates the Calcium and Cyclic AMP Signaling Systems and Stimulates Insulin Secretion. PLoS ONE 2009, 4, e5106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sternini, C.; Anselmi, L.; Rozengurt, E. Enteroendocrine cells: A site of ‘taste’ in gastrointestinal chemosensing. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsutomi, K.; Masaki, T.; Shimasaki, T.; Gotoh, K.; Chiba, S.; Kakuma, T.; Shibata, H. Effects of a nonnutritive sweetener on body adiposity and energy metabolism in mice with diet-induced obesity. Metabolism 2014, 63, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Avau, B.; Bauters, D.; Steensels, S.; Vancleef, L.; Laermans, J.; Lesuisse, J.; Buyse, J.; Lijnen, H.R.; Tack, J.; Depoortere, I. The Gustatory Signaling Pathway and Bitter Taste Receptors Affect the Development of Obesity and Adipocyte Metabolism in Mice. PLoS ONE 2015, 10, e0145538. [Google Scholar] [CrossRef] [Green Version]
- Simon, B.R.; Parlee, S.D.; Learman, B.S.; Mori, H.; Scheller, E.L.; Cawthorn, W.P.; Ning, X.; Gallagher, K.; Tyrberg, B.; Assadi-Porter, F.M.; et al. Artificial Sweeteners Stimulate Adipogenesis and Suppress Lipolysis Independently of Sweet Taste Receptors. J. Boil. Chem. 2013, 288, 32475–32489. [Google Scholar] [CrossRef] [Green Version]
- Cancello, R.; Micheletto, G.; Meta, D.; Lavagno, R.; Bevilacqua, E.; Panizzo, V.; Invitti, C. Expanding the role of bitter taste receptor in extra oral tissues: TAS2R38 is expressed in human adipocytes. Adipocyte 2020, 9, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.J.; Rother, K.I. Non-nutritive sweeteners and their role in the gastrointestinal tract. J. Clin. Endocrinol. Metab. 2012, 97, 2597–2605. [Google Scholar] [CrossRef] [Green Version]
- Bryant, C.; McLaughlin, J. Low calorie sweeteners: Evidence remains lacking for effects on human gut function. Physiol. Behav. 2016, 164, 482–485. [Google Scholar] [CrossRef]
- Lertrit, A.; Srimachai, S.; Saetung, S.; Chanprasertyothin, S.; Chailurkit, L.-O.; Areevut, C.; Katekao, P.; Ongphiphadhanakul, B.; Sriphrapradang, C. Effects of sucralose on insulin and glucagon-like peptide-1 secretion in healthy subjects: A randomized, double-blind, placebo-controlled trial. Nutrition 2018, 55–56, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Sylvetsky, A.C.; Brown, R.J.; Blau, J.E.; Walter, M.; Rother, K.I. Hormonal responses to non-nutritive sweeteners in water and diet soda. Nutr. Metab. 2016, 13, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Bellon, M.; Wishart, J.M.; Young, R.; Blackshaw, A.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am. J. Physiol. Liver Physiol. 2009, 296, G735–G739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.W.; Brown, M.M.B.; Onken, K.L.; Beitz, D.C. Short-term consumption of sucralose, a nonnutritive sweetener, is similar to water with regard to select markers of hunger signaling and short-term glucose homeostasis in women. Nutr. Res. 2011, 31, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chang, J.; Checklin, H.L.; Young, R.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effect of the artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. Br. J. Nutr. 2010, 104, 803–806. [Google Scholar] [CrossRef] [Green Version]
- Steinert, R.E.; Frey, F.; Töpfer, A.; Drewe, J.; Beglinger, C. Effects of carbohydrate sugars and artificial sweeteners on appetite and the secretion of gastrointestinal satiety peptides. Br. J. Nutr. 2011, 105, 1320–1328. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, F.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Castañer, O.; Goday, A.; Park, Y.-M.M.; Lee, S.H.; Magkos, F.; Shiow, S.-A.T.E.; Schröder, H. The Gut Microbiome Profile in Obesity: A Systematic Review. Int. J. Endocrinol. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Gültekin, F.; Oner, M.E.; Savaş, H.B.; Dogan, B. Food additives and microbiota. North. Clin. Istanb. 2019, 7, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-P.; Browman, D.; Herzog, H.; Neely, G.G. Non-nutritive sweeteners possess a bacteriostatic effect and alter gut microbiota in mice. PLoS ONE 2018, 13, e0199080. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, J.E.; Cho, N.A.; Klancic, T.; Nicolucci, A.C.; Shearer, J.; Borgland, S.L.; Johnston, L.A.; Ramay, H.R.; Tuplin, E.N.; Chleilat, F.; et al. Maternal low-dose aspartame and stevia consumption with an obesogenic diet alters metabolism, gut microbiota and mesolimbic reward system in rat dams and their offspring. Gut 2020, 10, 1136. [Google Scholar] [CrossRef] [Green Version]
- Nettleton, J.E.; Klancic, T.; Schick, A.; Choo, A.C.; Shearer, J.; Borgland, S.L.; Chleilat, F.; Mayengbam, S.; Reimer, R.A. Low-Dose Stevia (Rebaudioside A) Consumption Perturbs Gut Microbiota and the Mesolimbic Dopamine Reward System. Nutrients 2019, 11, 1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Ojeda, F.J.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Adv. Nutr. 2019, 10, S31–S48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, S.M.S.; Francisco, M.G.; Higashi, B.; Almeida, R.R.; Krausová, G.; Pilau, E.; Gonçalves, J.E.; Gonçalves, R.A.C.; De Oliveira, A.J.B. Chemical characterization and prebiotic activity of fructo-oligosaccharides from Stevia rebaudiana (Bertoni) roots and in vitro adventitious root cultures. Carbohydr. Polym. 2016, 152, 718–725. [Google Scholar] [CrossRef]
- Polyák, É.; Gombos, K.; Hajnal, B.; Bonyár-Müller, K.; Szabó, S.; Gubicskó-Kisbenedek, A.; Marton, K.; Ember, I. Effects of artificial sweeteners on body weight, food and drink intake. Acta Physiol. Hung. 2010, 97, 401–407. [Google Scholar] [CrossRef]
- Alcock, J.; Maley, C.C.; Aktipis, C.A. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. BioEssays 2014, 36, 940–949. [Google Scholar] [CrossRef]
- Miras, A.D.; Le Roux, C.W. Mechanisms underlying weight loss after bariatric surgery. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 575–584. [Google Scholar] [CrossRef]
- Swartz, T.D.; Duca, F.A.; De Wouters, T.; Sakar, Y.; Covasa, M. Up-regulation of intestinal type 1 taste receptor 3 and sodium glucose luminal transporter-1 expression and increased sucrose intake in mice lacking gut microbiota. Br. J. Nutr. 2011, 107, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Beckett, E.L.; Martin, C.; Yates, Z.; Veysey, M.; Duesing, K.; Lucock, M. Bitter taste genetics—The relationship to tasting, liking, consumption and health. Food Funct. 2014, 5, 3040–3054. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Workman, A.D.; Chen, B.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Lee, R.J.; Cohen, N.A. Staphylococcus aureus triggers nitric oxide production in human upper airway epithelium. Int. Forum Allergy Rhinol. 2015, 5, 808–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.H.; Hahn, S.; McMahon, D.; Bonislawski, D.; Kennedy, D.W.; Adappa, N.D.; Palmer, J.N.; Jiang, P.; Lee, R.J.; Cohen, N.A. Nitric oxide production is stimulated by bitter taste receptors ubiquitously expressed in the sinonasal cavity. Am. J. Rhinol. Allergy 2017, 31, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Name | Sweetener Type | Chemical Components [1] | Sweetness Relative to Sucrose [1] | AU ADI [1] (mg/ kg bw/d) | US ADI [11] (mg/ kg bw/ d) | EU ADI [12] (mg/ kg bw/ d) |
|---|---|---|---|---|---|---|
| Acesulphame K | Artificial | Acetoacetic acid and potassium | 200× | 15 | 15 | 9 |
| Advantame | Artificial | Aspartame (below) and vanillin | 20,000× | 5 | 32.8 | 5 |
| Alitame | Artificial | Aspartic acid and alanine | 2000× | 1 | Not approved | Not approved |
| Aspartame | Artificial | Aspartic acid and phenylalanine | 200× | 40 | 50 | 40 |
| Aspartame-acesulphame K salt | Artificial | Aspartame and acesulphame-K | 350× | As respective elements | Not approved | As respective elements |
| Cyclamate | Artificial | Salt of cyclohexylsulfamic acid | 30–50× | 11 | Not approved | 7 |
| Monk fruit extract | Natural | Siraitia grosvenorii fruit extract | 250–400× | No ADI | No ADI | Not approved |
| Neotame | Artificial | Modified version of aspartame | 7000–13,000× | 2 | 0.3 | 2 |
| Neohesperidine DC | Artificial | Modified Neohesperidin from citrus | 1000× | Not approved | Not approved | 5 |
| Saccharin | Artificial | Forms: acid saccharin, sodium saccharin, potassium saccharin and calcium saccharin | 300× | 5 | 15 | 5 |
| Stevia | Natural | Steviol glycosides from Stevia rebaudiana | 200–300× | 4 | 4 | 4 |
| Sucralose | Artificial | Sucralose | 600× | 15 | 5 | 15 |
| Thaumatin | Natural | Thaumatococcus daniellii fruit extract | 2000–3000× | No ADI | Not approved | No ADI |
| Name | Known to Activate T2Rs | Sources |
|---|---|---|
| Acesulphame potassium | T2R9, T2R43, T2R31 | Allen et al. 2013 [65]; Kuhn et al., 2004 [64]; Meyerhof et al., 2010 [66] |
| Advantame | No | |
| Alitame | No | |
| Aspartame | No | |
| Aspartame-acesulphame salt | No | |
| Cyclamate | T2R1, T2R31, T2R38 T2R43 | Behrens et al., 2017 [67]; Meyerhof et al., 2010 [66] |
| Monk fruit extract | No | |
| Neotame | No | |
| Neohesperidine DC | No | |
| Saccharin | T2R8, T2R43, T2R31 | Kuhn et al., 2004 [64]; Meyerhof et al., 2010 [66] |
| Stevia | T2R4, T2R14 | Acevedo et al., 2016 [68]; Hellfritsch et al., 2012 [69] |
| Sucralose | No | |
| Thaumatin | No | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, A.; Veysey, M.; Keely, S.; Scarlett, C.J.; Lucock, M.; Beckett, E.L. Intense Sweeteners, Taste Receptors and the Gut Microbiome: A Metabolic Health Perspective. Int. J. Environ. Res. Public Health 2020, 17, 4094. https://doi.org/10.3390/ijerph17114094
Turner A, Veysey M, Keely S, Scarlett CJ, Lucock M, Beckett EL. Intense Sweeteners, Taste Receptors and the Gut Microbiome: A Metabolic Health Perspective. International Journal of Environmental Research and Public Health. 2020; 17(11):4094. https://doi.org/10.3390/ijerph17114094
Chicago/Turabian StyleTurner, Alexandria, Martin Veysey, Simon Keely, Christopher J. Scarlett, Mark Lucock, and Emma L. Beckett. 2020. "Intense Sweeteners, Taste Receptors and the Gut Microbiome: A Metabolic Health Perspective" International Journal of Environmental Research and Public Health 17, no. 11: 4094. https://doi.org/10.3390/ijerph17114094
APA StyleTurner, A., Veysey, M., Keely, S., Scarlett, C. J., Lucock, M., & Beckett, E. L. (2020). Intense Sweeteners, Taste Receptors and the Gut Microbiome: A Metabolic Health Perspective. International Journal of Environmental Research and Public Health, 17(11), 4094. https://doi.org/10.3390/ijerph17114094






