A Review of the Sustainable Utilization of Rice Residues for Bioenergy Conversion Using Different Valorization Techniques, Their Challenges, and Techno-Economic Assessment
Abstract
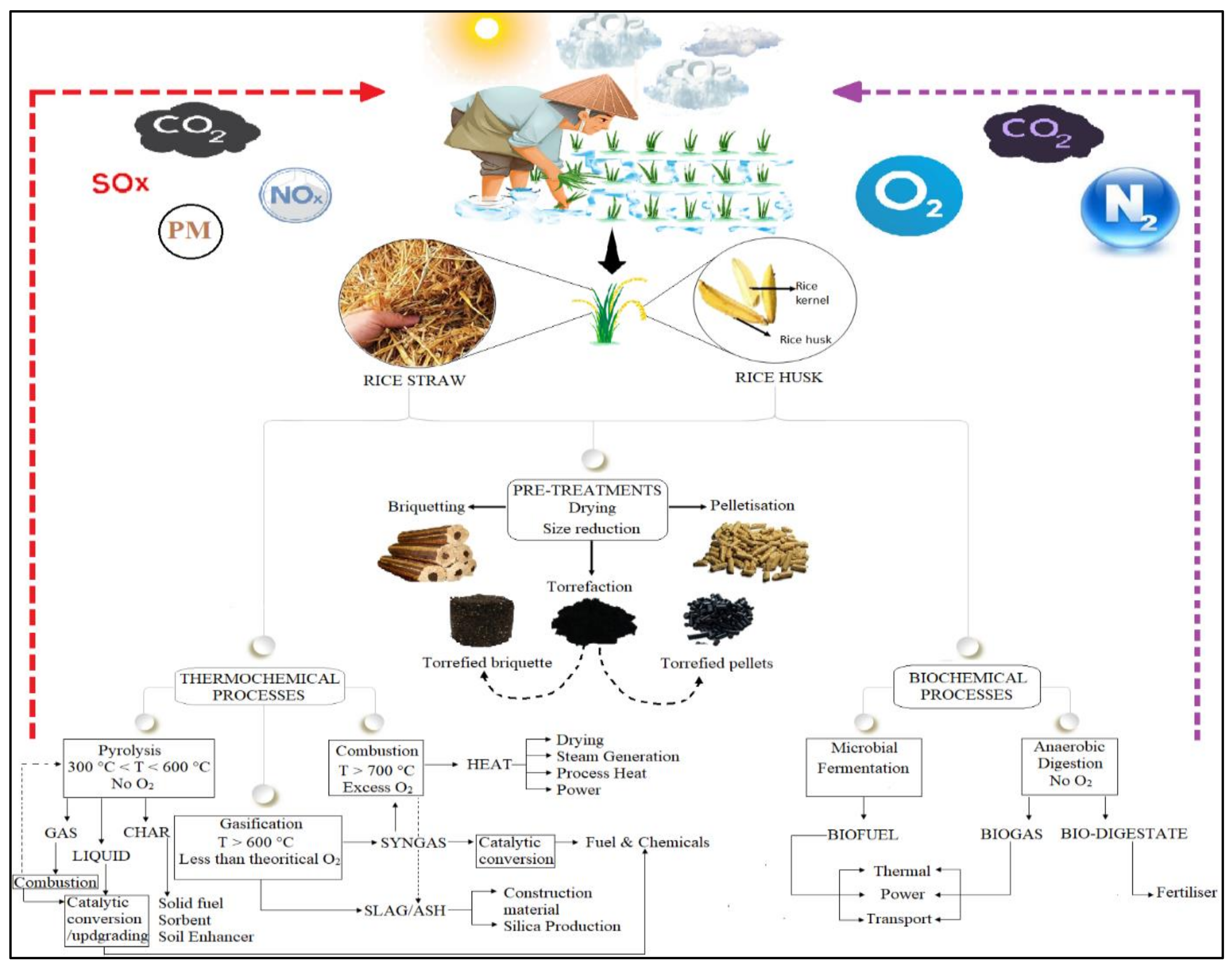
:1. Introduction
2. Physico-Chemical Characteristic of Rice Residues
2.1. Structural Characteristics
2.2. Proximate Analysis of Rice Residues
2.3. Ultimate Analysis and HHV of Rice Residues
3. Pre-Treatment of Rice Residues
3.1. Briquetting
3.2. Pelletization
3.3. Torrefaction
3.4. Hydrothermal Carbonization
4. Valorization of Rice Residues
4.1. Direct Combustion
4.2. Gasification
4.3. Pyrolysis
4.4. Anaerobic Digestion
4.5. Microbial Fermentation
5. Techno-Economic Consideration of Rice Residues for Energy Augmentation
Carbon Footprint Recovery via Rice Residue Conversions to Energy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABE | Acetone, Butanol and Ethanol |
| AC | Ash Content |
| AD | Anaerobic Digestion |
| APRS | NaOH Pre-treated Rice Straw |
| ATP | Adenosine Triphosphates |
| AU | Australia |
| BEP | Bioethanol Plant |
| BT | Temperature Distributions of Fixed Bed |
| C3H4O3 | Pyruvate Molecules |
| CC | Corn Cobs |
| Char | Gaseous, Aqueous Chemicals and Solid Fuel |
| CH4 | Methane |
| CS | Coconut Shells |
| CSP | Concentrating Solar Power |
| CXHY, | Hydrocarbons |
| DADT | Dry AD Technology |
| DORB | De-oiled Rice Bran |
| EO | Excess Oxygen Ratio |
| ER | Equivalence Ratio |
| FBT | Freeboard |
| FC | Fixed Carbon |
| GDP | Gross Domestic Product |
| GHG | Greenhouse Gas |
| GWh | Gigawatt Hours |
| HHV | Higher Heating Value |
| HRT | Hydraulic Retention Time |
| HTC | Hydrothermal Carbonization |
| HTG | Hydrothermal Gasification |
| HTL | Hydrothermal Liquefaction |
| IBSO | In-bed Stoichiometric Oxygen |
| IEA | International Energy Agency |
| IFC | International Finance Corporation |
| IRR | Internal Rate of Return |
| LCA | Life Cycle Assessment |
| LCOE | Levelized Cost of Energy |
| LHV | Lower Heating Value |
| MC | Moisture Content |
| MF | Microbial Fermentation |
| MIHTC | Microwave-Induced Hydrothermal Carbonization |
| NA | Not Available |
| NAD+ | Nicotinamide Adenine Dinucleotide |
| NADH | Nicotinamide Adenine Dinucleotide (NAD) + Hydrogen (H) |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NPE | Nonylphenol Ethoxylate |
| NPV | Net Present Value |
| OLR | Organic Loading Rate |
| PAPRS | Phosphoric Acid Pre-treated Rice Straw |
| PFBD | Paddy Flatbed Dryer |
| PKS | Palm Kernel Shell |
| PM | Particulate Matter |
| PV | Photovoltaic |
| RB | Rice Bran |
| RH | Rice Husk |
| RHA | Rice Husk Ash |
| RR | Rice Residues |
| RRS | Non-Pre-Treated Rice Straw |
| RS | Rice Straw |
| SAARS | South Asian Association for Regional Cooperation |
| SB | Sugarcane Bagasse |
| SBET | Specific Surface Area |
| SD | Sawdust |
| SS | Sewage Sludge |
| Tar | Bio-oil/liquid |
| TRS | Torrefied Rice Straw |
| TS | Total Solid |
| VFA | Volatile Fatty Acid |
| VFBC | Vortexing Fluidized-bed Combustor |
| VM | Volatile Matter |
| VS | Volatile Solids |
| WH | Wheat Husk |
| WS | Wheat Straw |
References
- Banks, C.J.; Heaven, S.; Zhang, Y.; Baier, U. Food Waste Digestion: Anaerobic Digestion of Food Waste for a Circular Economy, 12th ed.; Murphy, J.D., Ed.; IEA Bioenergy: Richland, WA, USA, 2018; Volume 37, pp. 1–40. [Google Scholar]
- World Bank Gross Domestic Product for Low Income Countries. Available online: https://fred.stlouisfed.org/series/NYGDPMKTPCDLIC (accessed on 3 December 2021).
- Gerbens-Leenes, P.W.; Nonhebel, S.; Krol, M.S. Food consumption patterns and economic growth. Increasing affluence and the use of natural resources. Appetite 2010, 55, 597–608. [Google Scholar] [CrossRef] [PubMed]
- OECD-FAO. Agricultural Outlook 2017–2026. Available online: https://stats.oecd.org/Index.aspx?DataSetCode=HIGH_AGLINK_2017 (accessed on 29 September 2021).
- Southeast Asia: Satellite Data for Rice Cultivation. Available online: https://www.giz.de/en/workingwithgiz/43933.html (accessed on 19 February 2022).
- Esa, N.M.; Ling, T.B.; Peng, L.S. By-products of rice processing: An overview of health benefits and applications. Rice Res. Open Access 2013, 1, 1–11. [Google Scholar] [CrossRef]
- Evi, A. Cementitious Materials; Ningrum, H.F., Ed.; Media Sains Indonesia: Kota Bandung, Indonesia, 2021; pp. 1–81. [Google Scholar]
- Cheewaphongphan, P.; Junpen, A.; Kamnoet, O.; Garivait, S. Study on the potential of rice straws as a supplementary fuel in very small power plants in Thailand. Energies 2018, 11, 270. [Google Scholar] [CrossRef] [Green Version]
- Goswami, S.B.; Mondal, R.; Mandi, S.K. Crop residue management options in rice–rice system: A review. Arch. Agron. Soil Sci. 2020, 66, 1218–1234. [Google Scholar] [CrossRef]
- Azza, E.; Hideto, U.A.G. Impact of rice residues application on rice growth, yield and some paddy soil properties. Int. J. Agric. Res. 2007, 2, 1030–1036. [Google Scholar] [CrossRef] [Green Version]
- Silalertruksa, T.; Gheewala, S.H. A comparative LCA of rice straw utilization for fuels and fertilizer in Thailand. Bioresour. Technol. 2013, 150, 412–419. [Google Scholar] [CrossRef]
- Lasko, K.; Vadrevu, K.P.; Tran, V.T.; Ellicott, E.; Nguyen, T.T.N.; Bui, H.Q.; Justice, C. Satellites may underestimate rice residue and associated burning emissions in Vietnam. Environ. Res. Lett. 2017, 12, 085006. [Google Scholar] [CrossRef]
- Junpen, A.; Pansuk, J.; Kamnoet, O.; Cheewaphongphan, P.; Garivait, S. Emission of air pollutants from rice residue open burning in Thailand, 2018. Atmosphere 2018, 9, 449. [Google Scholar] [CrossRef] [Green Version]
- Quispe, I.; Navia, R.; Kahhat, R. Energy potential from rice husk through direct combustion and fast pyrolysis: A review. Waste Manag. 2017, 59, 200–210. [Google Scholar] [CrossRef]
- Singh, J. Paddy and wheat stubble blazing in Haryana and Punjab states of India: A menace for environmental health. Environ. Qual. Manag. 2018, 28, 47–53. [Google Scholar] [CrossRef]
- Golshan, M.; Faghihi, M.; Roushan-Zamir, T.; Masood, M.M.; Farahmand-Far, H.; Rahmati, S.; Islami, F. Early effects of burning rice farm residues on respiratory symptoms of villagers in suburbs of Isfahan, Iran. Int. J. Environ. Health Res. 2002, 12, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Dobermann, A.T.H.F. Rice straw management. Better Crop. Int. 2002, 16, 7–11. [Google Scholar]
- Charles, R.K.J.; Majid, M.A. Renewable energy for sustainable development in India: Current status, future prospects, challenges, employment, and investment opportunities. Energy. Sustain. Soc. 2020, 10, 1–36. [Google Scholar] [CrossRef]
- Syahirah, M.Z.N.; Azam, A.S.; Hidayu, J.N. Extraction of Silica from Rice Husk via Acid Leaching Treatment. In Proceedings of the 2nd AIMC 2018 Asia International Multidisciplinary Conference, Universiti Teknologi Malaysia Johor Bahru, Malaysia, 12–13 May 2018. [Google Scholar]
- Wang, S.; Wang, N.; Yao, K.; Fan, Y.; Li, W.; Han, W.; Yin, X.; Chen, D. Characterization and interpretation of Cd (II) adsorption by different modified rice straws under contrasting conditions. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sobky, E.S.E.A. Effect of burned rice straw, phosphorus and nitrogen fertilization on wheat (Triticum aestivum L.). Ann. Agric. Sci. 2017, 62, 113–120. [Google Scholar] [CrossRef]
- Biswas, M.K. Cultivation of paddy straw mushrooms (Volvariella volvacea) in the lateritic zone of West Bengal-A healthy food for rural people. Int. J. Econ. Plants 2014, 23, 23–27. [Google Scholar]
- Olusesi, O.S.; Udoye, N.E. Development and characterization of AA6061 aluminium alloy /clay and rice husk ash composite. Manuf. Lett. 2021, 29, 34–41. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Wu, D.; Bai, X.; Lin, Y.; Wu, T.; Zhang, C.; Chen, D.; Li, H. Improving the supercapacitor performance of activated carbon materials derived from pretreated rice husk. J. Energy Storage 2021, 44, 103432. [Google Scholar] [CrossRef]
- Guo, W.; Li, G.; Zheng, Y.; Li, K. Nano-silica extracted from rice husk and its application in acetic acid steam reforming. RSC Adv. 2021, 11, 34915–34922. [Google Scholar] [CrossRef]
- Jiang, D.; Jiang, D.; Lv, S.; Cui, S.; Sun, S.; Song, X.; He, S.; Zhang, J. Effect of flame-retardant rice straw fibers on properties of fiber cement-based composites at high temperatures. J. Build. Eng. 2021, 44, 102923. [Google Scholar] [CrossRef]
- Natarajan, N.; Vasudevan, M.; Vivekk, V.; Velusamy, M. Selvaraj Eco-friendly and edible waste cutlery for sustainable environment. Int. J. Eng. Adv. Technol. 2019, 9, 615–624. [Google Scholar] [CrossRef]
- Muthuraj, R.; Lacoste, C.; Lacroix, P.; Bergeret, A. Sustainable thermal insulation biocomposites from rice husk, wheat husk, wood fibers and textile waste fibers: Elaboration and performances evaluation. Ind. Crop. Prod. 2019, 135, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Le Guen, M.-J.; Hill, S.; Smith, D.; Theobald, B.; Gaugler, E.; Barakat, A.; Mayer-Laigle, C. Influence of rice husk and wood biomass properties on the manufacture of filaments for fused deposition modeling. Front. Chem. 2019, 7, 735. [Google Scholar] [CrossRef] [PubMed]
- Hafez, A.M.; Khedr, M.A.; Amin, S.K.; Sabry, R.M.; Osman, R.M. Utilitization of agricultural residues of rice cultivation in manufacturing of light fired clay bricks. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 2588–2600. [Google Scholar]
- Hossain, N.; Nizamuddin, S.; Selvakannan, P.; Griffin, G.; Madapusi, S.; Shah, K. The effect of KOH activation and Ag nanoparticle incorporation on rice husk-based porous materials for wastewater treatment. Chemosphere 2021, 291, 132760. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Elliston, A.; Le Gall, G.; Colquhoun, I.J.; Collins, S.R.A.; Wood, I.P.; Dicks, J.; Roberts, I.N.; Waldron, K.W. Optimising conditions for bioethanol production from rice husk and rice straw: Effects of pre-treatment on liquor composition and fermentation inhibitors. Biotechnol. Biofuels 2018, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Matin, H.H.A.; Hadiyanto. Biogas production from rice husk waste by using solid state anaerobic digestion (SSAD) method. In Proceedings of the The 2nd International Conference on Energy, Environmental and Information System (ICENIS 2017), Semarang, Indonesia, 15–16 August 2017. [Google Scholar]
- Afzal, A.; Mohibullah, M.; Sharma, V.K. Performance analysis of a rice husk power generating system: A case study. Int. J. Sustain. Energy 2011, 30, 1–10. [Google Scholar] [CrossRef]
- Silvy, N.; Shamim Reza, M.; Nazim Uddin, M.; Akther, M. Comparison between different components of some available hardwood and softwood in Bangladesh. IOSR J. Biotechnol. Biochem. 2018, 4, 1–5. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.I.; Mohapatra, S.K.; Gangacharyulu, D. Fluidised bed combustion and gasification of rice husk and rice straw—A state of art review. Int. J. Renew. Energy Technol. 2011, 2, 345. [Google Scholar] [CrossRef]
- Jenkins, B.; Baxter, L.; Miles, T.R., Jr.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Arjmandi, R.; Hassan, A.; Majeed, K.; Zakaria, Z. Rice husk filled polymer composites. Int. J. Polym. Sci. 2015, 2015, 1–32. [Google Scholar] [CrossRef]
- Luo, K.; Wang, Y.; Xiao, H.; Song, G.; Cheng, Q.; Fan, G. Preparation of convertible cellulose from rice straw using combined organosolv fractionation and alkaline bleaching. IOP Conf. Ser. Earth Environ. Sci. 2019, 237, 052053. [Google Scholar] [CrossRef]
- Shariff, A.; Syairah, M.A.N.; Ismiza Ismail, N.; Abdullah, N. Corn cob as a potential feedstock for slow pyrolysis of biomass. J. Phys. Sci. 2016, 27, 123–137. [Google Scholar] [CrossRef]
- Danish, M.; Naqvi, M.; Farooq, U.; Naqvi, S. Characterization of South Asian agricultural residues for potential utilization in future “energy mix” Selection and/or peer-review under responsibility of ICAE. Energy Procedia 2015, 75, 2974–2980. [Google Scholar] [CrossRef] [Green Version]
- Mohomane, S.M.; Linganiso, L.Z.; Buthelezi, T.; Motaung, T.E. Effect of extraction period on properties of sugarcane bagasse and softwood chips cellulose. Wood Res. 2017, 62, 931–938. [Google Scholar]
- Vaskalis, I.; Skoulou, V.; Stavropoulos, G.; Zabaniotou, A. Towards circular economy solutions for the management of rice processing residues to bioenergy via gasification. Sustainability 2019, 11, 6433. [Google Scholar] [CrossRef] [Green Version]
- Fang, M.; Yang, L.; Chen, G.; Shi, Z.; Luo, Z.; Cen, K. Experimental study on rice husk combustion in a circulating fluidized bed. Fuel Process. Technol. 2004, 85, 1273–1282. [Google Scholar] [CrossRef]
- Qi, R.; Chen, Z.; Wang, M.; Wu, R.; Jiang, E. Prediction method for torrefied rice husk based on gray-scale analysis. ACS Omega 2019, 4, 17837–17842. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Hamidin, N.; Md Ali, U.F. Effect of dolomite on pyrolysis of rice straw. Adv. Mater. Res. 2013, 795, 170–173. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Qureshi, S.S.; Baloch, H.A.; Siddiqui, M.T.H.; Takkalkar, P.; Mubarak, N.M.; Dumbre, D.K.; Griffin, G.J.; Madapusi, S.; Tanksale, A. Microwave hydrothermal carbonization of rice straw: Optimization of process parameters and upgrading of chemical, fuel, structural and thermal properties. Materials 2019, 12, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, K.Y.; Liao, C.K.; Lu, C.H. The effects of prepared iron-based catalyst on the energy yield in gasification of rice straw. Int. J. Hydrogen Energy 2016, 41, 21747–21754. [Google Scholar] [CrossRef]
- Supramono, D.; Julianto; Haqqyana, H.; Setiadi, H.; Nasikin, M. Phase separation of bio-oil produced by co-pyrolysis of corn cobs and polypropylene. IOP Conf. Ser. Earth Environ. Sci. 2017, 93, 012072. [Google Scholar] [CrossRef]
- Kpalo, S.Y.; Zainuddin, M.F.; Manaf, L.A.; Roslan, A.M. Production and characterization of hybrid briquettes from corncobs and oil palm trunk bark under a low pressure densification technique. Sustainability 2020, 12, 2466. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Kumar, N.B. Combustion characteristics of high ash Indian coal, wheat straw, wheat husk and their blends. Mater. Sci. Energy Technol. 2021, 4, 274–281. [Google Scholar] [CrossRef]
- Singh, H.; Sapra, K.; Sidhu, B.S. Evaluation and characterization of different biomass residues through proximate & ultimate analysis and heating value. Asian J. Eng. Appl. Technol. 2012, 2, 6–10. [Google Scholar]
- Pedroso, D.T.; Machin, E.B.; Cabrera-Barjas, G.; Flores, M.; Urra, H.G.; De Carvalho, F.S.; Silva Dos Santos, M.I.; Machín, A.B.; Canettieri, E.V.; Pérez, N.P.; et al. Sugarcane bagasse torrefaction for fluidized bed gasification. Appl. Sci. 2021, 11, 6105. [Google Scholar] [CrossRef]
- Kumar, A.S.; Sudhir, Y.A. Comparative study of sugarcane bagasse gasification and direct combustion. J. Ind. Pollut. Control 2018, 34, 2063–2074. [Google Scholar]
- Garca-Pèrez, M.; Chaala, A.; Yang, J.; Roy, C. Co-pyrolysis of sugarcane bagasse with petroleum residue. Part I: Thermogravimetric analysis. Fuel 2001, 80, 1245–1258. [Google Scholar] [CrossRef]
- Kaniapan, S.; Suhaimi, H.; Hamdan, Y.; Pasupuleti, J. Experiment analysis on the characteristic of empty fruit bunch, palm kernel shell, coconut shell, and rice husk for biomass boiler fuel. J. Mech. Eng. Sci. 2021, 15, 8300–8309. [Google Scholar] [CrossRef]
- Patel, B. Biomass Characterization and its use as solid fuel for combustion. Iran. J. Energy Environ. 2012, 3, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, B.M.; Bakker, R.R.; Wei, J.B. On the properties of washed straw. Biomass Bioenergy 1996, 10, 177–200. [Google Scholar] [CrossRef]
- Hu, Q.; Shao, J.; Yang, H.; Yao, D.; Wang, X.; Chen, H. Effects of binders on the properties of bio-char pellets. Appl. Energy 2015, 157, 508–516. [Google Scholar] [CrossRef]
- Yerrayya, A.; Shree, V.A.K.; Shreyas, S.; Chakravarthy, S.R.; Vinu, R. Hydrothermal liquefaction of rice straw using methanol as co-solvent. Energies 2020, 13, 2618. [Google Scholar] [CrossRef]
- White, R.H. Effect of lignin content and extractives on the higher heating of wood. Wood Fiber Sci. 1987, 19, 446–452. [Google Scholar]
- Demirbas, A. Relationships between heating value and lignin, moisture, ash and extractive contents of biomass fuels. Energy Explor. Exploit. 2002, 20, 105–111. [Google Scholar] [CrossRef]
- Maksum, A.; Husein, M.K.E.; Permana, S.; Rustandi, A.; Soedarsono, J.W. A preliminary study on the reduction of limonite ore by using rice husk as a reducing agent. IOP Conf. Ser. Mater. Sci. Eng. 2018, 316. [Google Scholar] [CrossRef]
- Maksum, A.; Rustandi, A.; Permana, S.; Soedarsono, J.W. Roasting-quenching pretreatment in the calcination process to improve the purity of rice husk bio-silica. JP J. Heat Mass Transf. 2019, 16, 313–326. [Google Scholar] [CrossRef]
- Natarajan, E.; Öhman, M.; Gabra, M.; Nordin, A.; Liliedahl, T.; Rao, A.N. Experimental determination of bed agglomeration tendencies of some common agricultural residues in fluidized bed combustion and gasification. Biomass Bioenergy 1998, 15, 163–169. [Google Scholar] [CrossRef]
- Chandak, S.P. Characterization of Waste Agricultural Biomass for Energy Applications; United Nations Environment Programme (UNEP DTIE IETC): San José, Costa Rica, 2013. [Google Scholar]
- Rice/Straw. Available online: http://cdmaster2.vt.tuwien.ac.at/biobib/fuel210.html (accessed on 26 February 2022).
- Matali, S.; Rahman, N.A.; Idris, S.S.; Yaacob, N.; Alias, A.B. Lignocellulosic biomass solid fuel properties enhancement via torrefaction. Procedia Eng. 2016, 148, 671–678. [Google Scholar] [CrossRef] [Green Version]
- Rangabhashiyam, S.; Balasubramaniam, P. The potential of lignocellulosic biomass precursors for biochar production: Performance, mechanism and wastewater application—A review. Ind. Crop. Prod. 2019, 128, 405–423. [Google Scholar] [CrossRef]
- Nhuchhen, D.R. Prediction of carbon, hydrogen, and oxygen compositions of raw and torrefied biomass using proximate analysis. Fuel 2016, 180, 348–356. [Google Scholar] [CrossRef]
- Van Hung, N.; Maguyon-Detras, M.C.; Migo, M.V.; Quilloy, R.; Balingbing, C.; Chivenge, P.; Gummert, M. Rice straw overview: Availability, properties, and management practices. In Sustainable Rice Straw Management; Gummert, M., Van Hung, N., Chivenge, P., Douthwaite, B., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; p. 13. [Google Scholar]
- Kargbo, F.; Xing, J.; Zhang, Y. Property analysis and pretreatment of rice straw for energy use in grain drying: A review. Agric. Biol. J. N. Am. 2010, 1, 195–200. [Google Scholar] [CrossRef]
- Yuhong, F.; Yafei, S.; Zhendong, Z.; Xinlei, G.; Mindong, C. Activated bio-chars derived from rice husk via one- and two-step KOH-catalyzed pyrolysis for phenol adsorption. Sci. Total Environ. 2018, 646, 1567–1577. [Google Scholar] [CrossRef]
- Manyuchi, M.M.; Mbohwa, C.; Muzenda, E. Value addition of coal fines and sawdust to briquettes using molasses as a binder. S. Afr. J. Chem. Eng. 2018, 26, 70–73. [Google Scholar] [CrossRef]
- Shuma, R.; Madyira, D.M. Production of loose biomass briquettes from agricultural and forestry residues. In Proceedings of the International Conference on Sustainable Material Processing and Manufacturing, SMPM, Kruger, South Africa, 23–25 January 2017; Manufacturing, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 7, pp. 98–105. [Google Scholar]
- Anatasya, A.; Umiati, N.A.K.; Subagio, A. The effect of binding types on the biomass briquette calorific value from cow manure as a solid energy source. Proceedings of International Conference on Energy, Environmental, Epidemiology and Information System, ICENIS, Semarang, Indonesia, 7–8 August 2019. [Google Scholar]
- Ugwu, K. Evaluation of binders in the production of briquettes from empty fruit bunches of Elais Guinensis. Int. J. Renew. Sustain. Energy 2013, 2, 176. [Google Scholar] [CrossRef] [Green Version]
- Saeed, A.A.H.; Harun, N.Y.; Bilad, M.R.; Afzal, M.T.; Parvez, A.M.; Roslan, F.A.S.; Rahim, S.A.; Vinayagam, V.D.; Afolabi, H.K. Moisture content impact on properties of briquette produced from rice husk waste. Sustainability 2021, 13, 3069. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, K.; Sun, Y. Pelletizing properties of wheat straw blending with rice straw. Energy Fuels 2017, 31, 5126–5134. [Google Scholar] [CrossRef]
- Wu, S.R. Heating Application of Bio-Pellet to Enhance Utilization of Renewable Energy in the APEC Region. Available online: https://www.apec.org/docs/default-source/Publications/2017/12/Heating-Applications-of-Bio-pellet-to-Enhance-Utilization-of-Renewable-Energy-in-the-APEC-Region/217_EWG_Heating-Applications-of-Bio-pellet.pdf (accessed on 10 January 2022).
- Homdoung, N.; Sasujit, K.; Uttharuan, J.; Wongsiriamnuay, T.; Tippayawong, N. Influence of torrefaction temperature and time on the yields and properties of torrefied biomass. Eng. Appl. Sci. Res. 2019, 46, 170–175. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal carbonization as a valuable tool for energy and environmental applications: A review. Energies 2020, 13, 4098. [Google Scholar] [CrossRef]
- Ahiduzzaman, M.; Islam, A.S. Assessment of rice husk briquette fuel use as an alternative source of woodfuel. Int. J. Renew. Energy Res. 2016, 6, 1–11. [Google Scholar]
- Kaniapan, S.; Hassan, S.; Ya, H.; Nesan, K.P.; Azeem, M. The utilisation of palm oil and oil palm residues and the related challenges as a sustainable alternative in biofuel, bioenergy, and transportation sector: A review. Sustainability 2021, 13, 3110. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Mohammed, T.I. Fuel developed from rice bran briquettes and palm kernel shells. Int. J. Energy Eng. 2015, 9–15. [Google Scholar] [CrossRef]
- Akolgo, G.A.; Awafo, E.A.; Essandoh, E.O.; Owusu, P.A.; Uba, F.; Adu-Poku, K.A. Assessment of the potential of charred briquettes of sawdust, rice and coconut husks: Using water boiling and user acceptability tests. Sci. Afr. 2021, 12, e00789. [Google Scholar] [CrossRef]
- Defonseka, C. Rice hulls pellets as alternate solid fuel for energy generation. Polym. Renew. Resour. 2018, 9, 133–144. [Google Scholar] [CrossRef]
- Yang, I.; Kim, S.H.; Sagong, M.; Han, G.S. Fuel characteristics of agropellets fabricated with rice straw and husk. Korean J. Chem. Eng. 2016, 33, 851–857. [Google Scholar] [CrossRef]
- Bajo, P.O.; Acda, M.N. Fuel pellets from a mixture of rice husk and wood particles. BioResources 2017, 12, 6618–6628. [Google Scholar] [CrossRef] [Green Version]
- Tumuluru, J.S.; Sokhansanj, S.; Hess, J.R.; Wright, C.T.; Boardman, R.D. A review on biomass torrefaction process and product properties for energy applications. Ind. Biotechnol. 2011, 7, 384–401. [Google Scholar] [CrossRef] [Green Version]
- Arcate, J.R. Global markets and technologies for torrefied wood in 2002. Wood Energy 2002, 5, 26–28. [Google Scholar]
- Nhuchhen, D.R.; Basu, P.; Acharya, B. A comprehensive review on biomass torrefaction. Int. J. Renew. Energy Biofuels 2014, 2014, 1–56. [Google Scholar] [CrossRef]
- Garcia, D.P.; Caraschi, J.C.; Ventorim, G.; Vieira, F.H.A.; de Protásio, P.T. Comparative energy properties of torrefied pellets in relation to pine and elephant grass pellets. BioResources 2018, 13, 2898–2906. [Google Scholar] [CrossRef] [Green Version]
- Manouchehrinejad, M.; Yue, Y.; de Morais, R.A.L.; Souza, L.M.O.; Singh, H.; Mani, S. Densification of thermally treated energy cane and napier grass. Bioenergy Res. 2018, 11, 538–550. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, J.; Zhang, Q.; Zhu, X.; Lu, Q. Upgrading of rice husk by torrefaction and its influence on the fuel properties. BioResources 2014, 9, 5893–5905. [Google Scholar] [CrossRef]
- Kwo, W.T.; Saidatul, S.J. The Valorization of rice waste via torrefaction method. Int. J. Chem. Eng. Appl. 2016, 7, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Ji, G.; Mu, L.; Zhang, Y.; Li, A. Comprehensive research on the solid, liquid, and gaseous products of rice husk and rice straw torrefaction. Sustain. Energy Fuels 2021, 5, 687–697. [Google Scholar] [CrossRef]
- Sadaka, S.; Negi, S. Improvements of biomass physical and thermochemical characteristics via torrefaction process. Environ. Prog. Sustain. Energy 2009, 28, 427–434. [Google Scholar] [CrossRef]
- Kai, X.; Meng, Y.; Yang, T.; Li, B.; Xing, W. Effect of torrefaction on rice straw physicochemical characteristics and particulate matter emission behavior during combustion. Bioresour. Technol. 2019, 278, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, R.; Ishii, K.; Sato, M.; Fujiyama, A. Characteristics of wood pellets mixed with torrefied rice straw as a biomass fuel. Int. J. Energy Environ. Eng. 2019, 10, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Homdoung, N.; Uttaruan, J.; Sasujit, K.; Wongsiriumnauy, T.; Tippayawong, N. Characterization of torrefied biomass pellets from agricultural residues for solid fuels. Agric. Eng. Int. CIGR J. 2020, 22, 118–128. [Google Scholar]
- Kumar, S.; Ankaram, S. Waste-to-energy model/tool presentation. In Current Developments in Biotechnology and Bioengineering: Waste Treatment Processes for Energy Generation, 1st ed.; Sunil, K.R.K.A.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 239–258. [Google Scholar]
- Kumar, M.; Olajire Oyedun, A.; Kumar, A. A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; César, T.-M.P.; Abaide, E.R.; Zabot, G.L.; De Castilhos, F. Hydrothermal carbonization and Liquefaction: Differences, progress, challenges, and opportunities. Bioresour. Technol. 2022, 343, 126084. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; An, Z.; Wu, M.; Yuan, A.; Zhao, H.; Zhang, J.; Xu, J. Peony pollen derived nitrogen-doped activated carbon for supercapacitor application. Chin. Chem. Lett. 2020, 31, 1644–1647. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Cheng, Y.; Zhong, H.; Tian, H.; Pan, J.; Pareek, V.K.; Jiang, S.P.; Lamonier, J.F.; Jaroniec, M.; Liu, J. From waste Coca Cola® to activated carbons with impressive capabilities for CO2 adsorption and supercapacitors. Carbon N. Y. 2017, 116, 490–499. [Google Scholar] [CrossRef]
- Li, Y.; Meas, A.; Shan, S.; Yang, R.; Gai, X.; Wang, H.; Tsend, N. Hydrochars from bamboo sawdust through acid assisted and two-stage hydrothermal carbonization for removal of two organics from aqueous solution. Bioresour. Technol. 2018, 261, 257–264. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, Z.; Xiao, X.; Zeng, P.; Xue, Q. Effect of inorganic potassium compounds on the hydrothermal carbonization of Cd-contaminated rice straw for experimental-scale hydrochar. Biomass Bioenergy 2019, 130, 105357. [Google Scholar] [CrossRef]
- Maguyon-Detras, M.C.; Migo, M.V.P.; van Hung, N.V.; Gummert, M. Thermochemical Conversion of Rice Straw. In Sustainable Rice Straw Management; Gummert, M., Hung, N., Chivenge, P., Douthwaite, B., Eds.; Springer: Cham, Switzerland, 2020; pp. 43–64. [Google Scholar]
- Roos, C.J.; Roos, C. Clean Heat and Power Using Biomass Gasification for Industrial and Agricultural Projects; Northwest, Clean Energy Application Center: Washington, DC, USA, 2010. [Google Scholar]
- Westerhof, R.J.M.; Kuipers, N.J.M.; Kersten, S.R.A.; Van Swaaij, W.P.M. Controlling the water content of biomass fast pyrolysis oil. Ind. Eng. Chem. Res. 2007, 46, 9238–9247. [Google Scholar] [CrossRef]
- Demirbas, A. Biorefineries: Current activities and future developments. Energy Convers. Manag. 2009, 50, 2782–2801. [Google Scholar] [CrossRef]
- Tan, Y. Feasibility Study on Solid Waste to Energy Technological Aspects; Fung Institute for Engineering Leadership, UC Berkeley College of Engineering: Berkeley, CA, USA, 2013. [Google Scholar]
- Manuales Sobre Energías Renovables: Biomasa. Primera Edición. San José: BUN-CA Fundación Red de Energía. Available online: http://www.bio-nica.info/biblioteca/BUNCA2002Biomasa.pdf (accessed on 20 January 2021).
- Osadolor, O.O.; James, O.A. Applying conventional combustion science and technology to alternative energy resources in industrial systems. Energy Power Eng. 2013, 2013, 570–576. [Google Scholar] [CrossRef] [Green Version]
- Nussbaumer, T. Combustion and co-combustion of biomass: Fundamentals, technologies, and primary measures for emission reduction. Energy Fuels 2003, 17, 1510–1521. [Google Scholar] [CrossRef]
- Hemalatha, M.; Hemasruthi, P.; Gokila Priya, S.; Gayathri, N.; Kavinilavu, K.S. Formulation of ecofriendly detergent powder using paddy husk ash. Asian J. Appl. Sci. Technol. 2018, 2, 70–78. [Google Scholar]
- Chaloupková, V.; Ivanova, T.; Hutla, P.; Špunarová, M. Ash melting behavior of rice straw and calcium additives. Agriculture 2021, 11, 1282. [Google Scholar] [CrossRef]
- Brand, M.A.; Jacinto, R.C.; Antunes, R.; da Cunha, A.B. Production of briquettes as a tool to optimize the use of waste from rice cultivation and industrial processing. Renew. Energy 2017, 111, 116–123. [Google Scholar] [CrossRef]
- Thy, P.; Jenkins, B.M.; Lesher, C.E.; Grundvig, S. Compositional constraints on slag formation and potassium volatilization from rice straw blended wood fuel. Fuel Process. Technol. 2006, 87, 383–408. [Google Scholar] [CrossRef]
- Fernández, M.J.; Mediavilla, I.; Barro, R.; Borjabad, E.; Ramos, R.; Carrasco, J.E. Sintering reduction of herbaceous biomass when blended with woody biomass: Predictive and combustion tests. Fuel 2019, 239, 1115–1124. [Google Scholar] [CrossRef]
- Reisinger, K.; Haslinger, C.; Herger, M.; Hofbauer, H. Hospodárná energie—BIOBIB—A DATABASE FOR BIOFUELS. Available online: https://infoenergie.azol.cz/energy.php?nav01=121&nav02=343 (accessed on 27 February 2022).
- Armesto, L.; Bahillo, A.; Veijonen, K.; Cabanillas, A.; Otero, J. Combustion behaviour of rice husk in a bubbling fluidised bed. Biomass Bioenergy 2002, 23, 171–179. [Google Scholar] [CrossRef]
- Rozainee, M.; Tan, K.G.; Ariffin, M.; Zainura, Z.N.; Ngo, S.P.; Salema, A.A. Effect of fluidising velocity on the combustion of rice husk in a bench-scale fluidised bed combustor for the production of amorphous rice husk ash. Bioresour. Technol. 2008, 99, 703–713. [Google Scholar] [CrossRef]
- Madhiyanon, T.; Sathitruangsak, P.; Soponronnarit, S. Combustion characteristics of rice-husk in a short-combustion-chamber fluidized-bed combustor (SFBC). Appl. Therm. Eng. 2010, 4, 347–353. [Google Scholar] [CrossRef]
- Duan, F.; Chyang, C.S.; Lin, C.W.; Tso, J. Experimental study on rice husk combustion in a vortexing fluidized-bed with flue gas recirculation (FGR). Bioresour. Technol. 2013, 134, 204–211. [Google Scholar] [CrossRef]
- Zain, M.F.M.; Islam, M.N.; Mahmud, F.; Jamil, M. Production of rice husk ash for use in concrete as a supplementary cementitious material. Constr. Build. Mater. 2011, 25, 798–805. [Google Scholar] [CrossRef]
- Guilemot, A.; Bruant, R.; Pasquiou, V.; Boucher, E. Feasibility Study for the Implementation of Two ORC Power Plants of 1 MWe each using Rice Straw as a Fuel in the Context of a Publicprivate Partnership with the Institutions PhilRice and UPLB; Enertime: Courbevoie, France, 2014. [Google Scholar]
- Yukun, L. Biomass Energy to Provide Heat, Fuel. China Daily. 2021. Available online: http://www.chinadaily.com.cn/a/202104/15/WS60779480a31024ad0bab5a24.html (accessed on 3 December 2021).
- Biomass Power Projects. Available online: https://www.peda.gov.in/biomass-power-projects (accessed on 22 November 2021).
- Arvo, L.; Nguyen, D.C. Development of Biomass Fuel Chains in Vietnam; VTT Technical Research Centre: Espoo, Finland, 2013. [Google Scholar]
- Guy, B. Interlaw Book on Renewable Energies; Primento: Bruxelles, Belgium, 2015. [Google Scholar]
- Energy for Environment Foundation Study on Determination of Promotional Approach for Comprehensive Community Biomass Power Plant; Energy for Environment Foundation: Thailand, 2012.
- Philrice, M. Maligaya Flatbed Dryer. In Pinoy Rice Knowledge Bank Handout Series; Phillippines Rice Research Institute (PRRI): Laguna, Phillippines, 2010–2012; pp. 1–2. [Google Scholar]
- Migo-Sumagang, M.V.P.; Maguyon-Detras, M.C.; Gummert, M.; Alfafara, C.G.; Borines, M.G.; Capunitan, J.A.; Van Hung, N. Rice-straw-based heat generation system compared to open-field burning and soil incorporation of rice straw: An assessment of energy, GHG emissions, and economic impacts. Sustainability 2020, 12, 5327. [Google Scholar] [CrossRef]
- Eri, Q.; Wu, W.; Zhao, X. Numerical investigation of the air-steam biomass gasification process based on thermodynamic equilibrium model. Energies 2017, 10, 2163. [Google Scholar] [CrossRef] [Green Version]
- Umar, H.A.; Sulaiman, S.A.; Meor Said, M.A.; Gungor, A.; Shahbaz, M.; Inayat, M.; Ahmad, R.K. Assessing the implementation levels of oil palm waste conversion methods in Malaysia and the challenges of commercialisation: Towards sustainable energy production. Biomass Bioenergy 2021, 151, 106179. [Google Scholar] [CrossRef]
- Park, S.J.; Son, S.H.; Kook, J.W.; Ra, H.W.; Yoon, S.J.; Mun, T.Y.; Moon, J.H.; Yoon, S.M.; Kim, J.H.; Kim, Y.K.; et al. Gasification operational characteristics of 20-tons-Per-Day rice husk fluidized-bed reactor. Renew. Energy 2021, 169, 788–798. [Google Scholar] [CrossRef]
- Makwana, J.P.; Pandey, J.; Mishra, G. Improving the properties of producer gas using high temperature gasification of rice husk in a pilot scale fluidized bed gasifier (FBG). Renew. Energy 2019, 130, 943–951. [Google Scholar] [CrossRef]
- Korberg, A.D.; Mathiesen, B.V.; Clausen, L.R.; Skov, I.R. The role of biomass gasification in low-carbon energy and transport systems. Smart Energy 2021, 1, 100006. [Google Scholar] [CrossRef]
- Gary, C. Young Introduction to Gasification/Pyrolysis and Combustion Technology(s). In Municipal Solid Waste to Energy Conversion Processes: Economic, Technical and Renewable Comparisons; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–12. [Google Scholar]
- Calvo, L.F.; Gil, M.V.; Otero, M.; Morán, A.; García, A.I. Gasification of rice straw in a fluidized-bed gasifier for syngas application in close-coupled boiler-gasifier systems. Bioresour. Technol. 2012, 109, 206–214. [Google Scholar] [CrossRef]
- Baloch, H.A.; Yang, T.; Sun, H.; Li, J.; Nizamuddin, S.; Li, R.; Kou, Z.; Sun, Y.; Bhutto, A.W. Parametric study of pyrolysis and steam gasification of rice straw in presence of K2CO3. Korean J. Chem. Eng. 2016, 33, 2567–2574. [Google Scholar] [CrossRef]
- Ouda, O.K.M.; Raza, S.A.; Nizami, A.S.; Rehan, M.; Al-Waked, R.; Korres, N.E. Waste to energy potential: A case study of Saudi Arabia. Renew. Sustain. Energy Rev. 2016, 61, 328–340. [Google Scholar] [CrossRef]
- Brandin, J.; Tunér, M.; Odenbrand, I. Small Scale Gasifiction Gas Engine CHP for Biofuels; Linnaeus University: Växjö, Sweden, 2011. [Google Scholar]
- Dimpl, E. Small-Scale Electricity Generation from Biomass Experience with Small-Scale Technologies for Basic Energy Supply, 1st ed.; Blunck, M., Ed.; GTZ-HERA—Poverty-oriented Basic Energy Service: Eschborn, Germany, 2010. [Google Scholar]
- Gajera, Z.R.; Verma, K.; Tekade, S.P.; Sawarkar, A.N. Kinetics of co-gasification of rice husk biomass and high sulphur petroleum coke with oxygen as gasifying medium via TGA. Bioresour. Technol. Rep. 2020, 11. [Google Scholar] [CrossRef]
- Susastriawan, A.A.P.; Saptoadi, H.; Purnomo. Comparison of the gasification performance in the downdraft fixed-bed gasifier fed by different feedstocks: Rice husk, sawdust, and their mixture. Sustain. Energy Technol. Assess. 2019, 34, 27–34. [Google Scholar] [CrossRef]
- Gao, X.; Xu, F.; Bao, F.; Tu, C.; Zhang, Y.; Wang, Y.; Yang, Y.; Li, B. Simulation and optimization of rice husk gasification using intrinsic reaction rate based CFD model. Renew. Energy 2019, 139, 611–620. [Google Scholar] [CrossRef]
- Hoque, M.E.; Rashid, F.; Aziz, M. Gasification and power generation characteristics of rice husk, sawdust, and coconut shell using a fixed-bed downdraft gasifier. Sustainable 2021, 13, 2027. [Google Scholar] [CrossRef]
- Widyawati, M.; Church, T.L.; Florin, N.H.; Harris, A.T. Hydrogen synthesis from biomass pyrolysis with in situ carbon dioxide capture using calcium oxide. Int. J. Hydrogen Energy 2011, 36, 4800–4813. [Google Scholar] [CrossRef]
- Weldekidan, H.; Strezov, V.; Town, G.; Kan, T. Production and analysis of fuels and chemicals obtained from rice husk pyrolysis with concentrated solar radiation. Fuel 2018, 233, 396–403. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z.; Gong, H.; Chen, L.; Yu, H.; Wu, W. Characteristics of dehydration during rice husk pyrolysis and catalytic mechanism of dehydration reaction with NiO/Γ-Al2O3 as catalyst. Fuel 2019, 245, 131–138. [Google Scholar] [CrossRef]
- Biswas, B.; Pandy, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, S.; Zhang, H.; Liu, X.; Zhang, H. Evaluation of pyrolysis behavior and products properties of rice husk after combined pretreatment of washing and torrefaction. Biomass Bioenergy 2019, 127, 105293. [Google Scholar] [CrossRef]
- Lin, B.; Huang, Q.; Chi, Y. Co-pyrolysis of oily sludge and rice husk for improving pyrolysis oil quality. Fuel Process. Technol. 2018, 177, 275–282. [Google Scholar] [CrossRef]
- Vieira, F.R.; Romero Luna, C.M.; Arce, G.L.A.F.; Ávila, I. Optimization of slow pyrolysis process parameters using a fixed bed reactor for biochar yield from rice husk. Biomass Bioenergy 2020, 132, 105412. [Google Scholar] [CrossRef]
- Wang, C.; Bi, H.; Lin, Q.; Jiang, X.; Jiang, C. Co-pyrolysis of sewage sludge and rice husk by TG–FTIR–MS: Pyrolysis behavior, kinetics, and condensable/non-condensable gases characteristics. Renew. Energy 2020, 160, 1048–1066. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Li, J.; Xue, Y.; Liu, J.; Mei, M.; Hou, H.; Chen, S. Co-pyrolysis behavior of sewage sludge and rice husk by TG-MS and residue analysis. J. Clean. Prod. 2020, 250, 119557. [Google Scholar] [CrossRef]
- Capareda, S. Introduction to Biomass Energy Conversions; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2014. [Google Scholar]
- Lohri, C.R.; Sweeney, D.; Rajabu, H.M. Carbonizing Urban Biowaste for Low-Cost Char Production in Developing Countries A Review of Knowledge, Practices and Technologies. Available online: https://www.eawag.ch/fileadmin/Domain1/Abteilungen/sandec/publikationen/SWM/Carbonization_of_Urban_Bio-waste/Carbonization_of_Biowaste_in_DCs_FINALx.pdf (accessed on 10 January 2022).
- Van, D.P.; Fujiwara, T.; Tho, B.L.; Toan, P.P.S.; Minh, G.H. A review of anaerobic digestion systems for biodegradable waste: Configurations, operating parameters, and current trends. Environ. Eng. Res. 2020, 25, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Amani, T.; Nosrati, M.; Sreekrishnan, T.R. Anaerobic digestion from the viewpoint of microbiological, chemical, and operational aspects—A review. Environ. Rev. 2010, 18, 255–278. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wu, J. Enhancement of methane production in anaerobic digestion process: A review. Appl. Energy 2019, 240, 120–137. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A review on anaerobic co-digestion with a focus on the microbial populations and the effect of multi-stage digester configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef] [Green Version]
- Hamzah, M.A.F.; Jahim, J.M.; Abdul, P.M.; Asis, A.J. Investigation of temperature effect on start-up operation from anaerobic digestion of acidified palm oil mill effluent. Energies 2019, 12, 2473. [Google Scholar] [CrossRef] [Green Version]
- Hsu, T.C.; Guo, G.L.; Chen, W.H.; Hwang, W.S. Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol. 2010, 101, 4907–4913. [Google Scholar] [CrossRef]
- Ecem Öner, B.; Akyol, Ç.; Bozan, M.; Ince, O.; Aydin, S.; Ince, B. Bioaugmentation with Clostridium thermocellum to enhance the anaerobic biodegradation of lignocellulosic agricultural residues. Bioresour. Technol. 2018, 249, 620–625. [Google Scholar] [CrossRef]
- Shetty, D.; Joshi, A.; Dagar, S.S.; Kshirsagar, P.; Dhakephalkar, P.K. Bioaugmentation of anaerobic fungus Orpinomyces joyonii boosts sustainable biomethanation of rice straw without pretreatment. Biomass Bioenergy 2020, 138, 105546. [Google Scholar] [CrossRef]
- Li, D.; Liu, S.; Mi, L.; Li, Z.; Yuan, Y.; Yan, Z.; Liu, X. Effects of feedstock ratio and organic loading rate on the anaerobic mesophilic co-digestion of rice straw and cow manure. Bioresour. Technol. 2015, 189, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Ngan, N.V.C.; Viet, L.H.; Cu, N.D.; Phong, N.H. Biogas production of pig manure with water hyacinth juice from batch anaerobic digestion. In Environmental Change and Agricultural Sustainability in the Mekong Delta; Stewart, M.A., Conclanis, P.A., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 45, pp. 355–369. [Google Scholar]
- Baek, G.; Kim, D.; Kim, J.; Kim, H.; Lee, C. Treatment of cattle manure by anaerobic co-digestion with food waste and pig manure: Methane yield and synergistic effect. Int. J. Environ. Res. Public Health 2020, 17, 4737. [Google Scholar] [CrossRef]
- Zhou, J.M. The Effect of Different C/N Ratios on the Composting of pig manure and edible fungus residue with rice bran. Compos. Sci. Util. 2017, 25, 120–129. [Google Scholar] [CrossRef]
- Jha, B.; Isha, A.; Trivedi, A.; Chandra, R.; Subbarao, P.M.V. Anaerobic co-digestion of rice straw and de-oiled rice bran for biomethane production. Energy Rep. 2021, 7, 704–710. [Google Scholar] [CrossRef]
- Saadia, M.; Rabia, L.S.R.N.; Sheikh, Z.; Zainab, A.; Khoja, A.H.; Juchelkova, D.; Atabani, A. Enhanced methane production from anaerobic co-digestion of wheat straw rice straw and sugarcane bagasse. Appl. Sci. 2021, 11, 6069. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, T.; Mei, Z.; Li, J.; Qiu, K.; Ge, Y. Dry anaerobic digestion technologies for agricultural straw and acceptability in China. Sustainability 2018, 10, 4588. [Google Scholar] [CrossRef] [Green Version]
- Syafrudin, S.; Nugraha, W.D.; Matin, H.H.A.; Saputri, E.S.; Budiyono, B. The effectiveness of biogas method from rice husks waste: Liquid anaerobic digestion and solid-state anaerobic digestion. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Semarang, Indonesia, 23–24 October 2019. [Google Scholar]
- Vivek, N.; Nair, L.M.; Mohan, B.; Nair, S.C.; Sindhu, R.; Pandey, A.; Shurpali, N.; Binod, P. Bio-butanol production from rice straw—Recent trends, possibilities, and challenges. Bioresour. Technol. Rep. 2019, 7, 100224. [Google Scholar] [CrossRef]
- Pinu, F.R.; Villas-Boas, S.G. Extracellular microbial metabolomics: The State of the Art. Metabolites 2017, 7, 43. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, X.; Wang, B. A review on the general cheese processing technology, flavor biochemical pathways and the influence of yeasts in cheese. Front. Microbiol. 2021, 12, 2186. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Hao, G.; Yu, H.; Tian, H.; Zhao, G. Role of lactic acid bacteria on the yogurt flavour: A review. Int. J. Food Prop. 2017, 20, S316–S330. [Google Scholar] [CrossRef] [Green Version]
- Maicas, S. The role of yeasts in fermentation processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Kolesinska, B.; Fraczyk, J.; Binczarski, M.; Modelska, M.; Berlowska, J.; Dziugan, P.; Antolak, H.; Kaminski, Z.J.; Witonska, I.A.; Kregiel, D. Butanol synthesis routes for biofuel production: Trends and perspectives. Materials 2019, 12, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feinauer, M.; Ehrenberger, S.; Buchgeister, J. Life cycle assessment of a farmed wood butanol-gasoline blend as an alternative transport fuel for passenger cars. Fuel 2021, 306, 121651. [Google Scholar] [CrossRef]
- Hönig, V.; Kotek, M.; Mařík, J. Use of butanol as a fuel for internal combustion engines. Agron. Res. 2014, 12, 333–340. [Google Scholar]
- Li, Y.; Meng, L.; Nithyanandan, K.; Lee, T.H.; Lin, Y.; Lee, C.; Fon, F.; Liao, S. Combustion, performance and emissions characteristics of a spark-ignition engine fueled with isopropanol-n-butanol-ethanol and gasoline blends. Fuel 2016, 184, 864–872. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Cheng, W.; Yin, Q.; Zuo, R.; Song, A.; Zheng, Q.; Wang, P.; Wang, X.; Liu, J. Effect of steam explosion and microbial fermentation on cellulose and lignin degradation of corn stover. Bioresour. Technol. 2012, 104, 587–592. [Google Scholar] [CrossRef]
- Bellido, C.; Loureiro Pinto, M.; Coca, M.; González-Benito, G.; García-Cubero, M.T. Acetone-butanol-ethanol (ABE) production by Clostridium beijerinckii from wheat straw hydrolysates: Efficient use of penta and hexa carbohydrates. Bioresour. Technol. 2014, 167, 198–205. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Adesanya, Y.; Okonkwo, C.; Zhang, H.; Huhnke, R.L.; Ezeji, T. Feasibility of using biochar as buffer and mineral nutrients replacement for acetone-butanol-ethanol production from non-detoxified switchgrass hydrolysate. Bioresour. Technol. 2020, 298, 122569. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K.; Zilouei, H. Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresour. Technol. 2014, 152, 450–456. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, Y.; Yu, Z.; Liao, J.; Zhang, Y. Pretreatment by microwave/alkali of rice straw and its enzymic hydrolysis. Process Biochem. 2005, 40, 3082–3086. [Google Scholar] [CrossRef]
- Moradi, F.; Amiri, H.; Soleimanian-Zad, S.; Ehsani, M.R.; Karimi, K. Improvement of acetone, butanol and ethanol production from rice straw by acid and alkaline pretreatments. Fuel 2013, 112, 8–13. [Google Scholar] [CrossRef]
- Valles, A.; Capilla, M.; Álvarez-Hornos, F.J.; García-Puchol, M.; San-Valero, P.; Gabaldón, C. Optimization of alkali pretreatment to enhance rice straw conversion to butanol. Biomass Bioenergy 2021, 150, 106131. [Google Scholar] [CrossRef]
- Basaglia, M.; D’Ambra, M.; Piubello, G.; Zanconato, V.; Favaro, L.; Casella, S. Agro-food residues and bioethanol potential: A study for a specific area. Process 2021, 9, 344. [Google Scholar] [CrossRef]
- Dale, B. Time to rethink cellulosic biofuels? Biofuels Bioprod. Biorefining 2018, 12, 5–7. [Google Scholar] [CrossRef]
- Abdelhady, S.; Shalaby, M.A.; Shaban, A. Techno-economic analysis for the optimal design of a national network of agro-energy biomass power plants in Egypt. Energies 2021, 14, 3063. [Google Scholar] [CrossRef]
- Abdelhady, S.; Borello, D.; Shaban, A. Techno-economic assessment of biomass power plant fed with rice straw: Sensitivity and parametric analysis of the performance and the LCOE. Renew. Energy 2018, 115, 1026–1034. [Google Scholar] [CrossRef]
- Short, W.; Packey, D.J.; Holt, T. A Manual for the Economic Evaluation of Energy Efficiency and Renewable Energy Technologies; National Renewable Energy Laboratory: Golden, CO, USA, 1995. [Google Scholar]
- Zamalloa, C.; Vulsteke, E.; Albrecht, J.; Verstraete, W. The techno-economic potential of renewable energy through the anaerobic digestion of microalgae. Bioresour. Technol. 2011, 102, 1149–1158. [Google Scholar] [CrossRef]
- Geoffrey Rothwell, T.G. Electricity economics: Regulation and deregulation. J. Econ. Lit. 2004, 42, 868–869. [Google Scholar]
- Rahman, M.M.; Paatero, J.V. A methodological approach for assessing potential of sustainable agricultural residues for electricity generation: South Asian perspective. Biomass Bioenergy 2012, 47, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Martinot, E.; Mastny, L.; Rosbotham, L.; Suding, P.; Sonntag-O’Brien, V.; Lempp, P. Renewables 2007—Global Status Report; Worldwatch Institute: Washington DC, USA; Paris, France, 2008. [Google Scholar]
- Uzair, M.; Sohail, S.S.; Shaikh, N.U.; Shan, A. Agricultural residue as an alternate energy source: A case study of Punjab province, Pakistan. Renew. Energy 2020, 162, 2066–2074. [Google Scholar] [CrossRef]
- Mana, A.A.; Allouhi, A.; Ouazzani, K.; Jamil, A. Feasibility of agriculture biomass power generation in Morocco: Techno-economic analysis. J. Clean. Prod. 2021, 295, 126293. [Google Scholar] [CrossRef]
- Ezz, H.; Ibrahim, M.G.; Fujii, M.; Nasr, M. Dual biogas and biochar production from rice straw biomass: A techno-economic and sustainable development approach. Biomass Convers. Biorefinery 2021, 1–15. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Naqvi, M.; Ammar Taqvi, S.A.; Iqbal, F.; Inayat, A.; Khoja, A.H.; Mehran, M.T.; Ayoub, M.; Shahbaz, M.; Saidina Amin, N.A. Agro-industrial residue gasification feasibility in captive power plants: A South-Asian case study. Energy 2021, 214, 118952. [Google Scholar] [CrossRef]
- Middelhoff, E.; Andrade Furtado, L.; Peterseim, J.H.; Madden, B.; Ximenes, F.; Florin, N. Hybrid concentrated solar biomass (HCSB) plant for electricity generation in Australia: Design and evaluation of techno-economic and environmental performance. Energy Convers. Manag. 2021, 240, 114244. [Google Scholar] [CrossRef]
- La Picirelli de Souza, L.; Rajabi, H.S.; Silva, L.E.E.; Escobar, P.J.C.; Comodi, G.; Villarini, M.; Colantoni, A. Theoretical and technical assessment of agroforestry residue potential for electricity generation in Brazil towards 2050. Energy Rep. 2021, 7, 2574–2587. [Google Scholar] [CrossRef]
- International Energy Agency (IEA) Global Biofuel Production in 2019 and Forecast to 2025—Charts. Available online: https://www.iea.org/data-and-statistics/charts/global-biofuel-production-in-2019-and-forecast-to-2025 (accessed on 28 November 2021).
- Zhou, Y.; Searle, S.; Anup, S. Techno-Economic Analysis of Cellulosic Ethanol in Industrial Using Agricultural Residues; International Council on Clean Transportation: Washington, DC, USA, 2021. [Google Scholar]
- Hassan, M.K.; Chowdhury, R.; Ghosh, S.; Manna, D.; Pappinen, A.; Kuittinen, S. Energy and environmental impact assessment of Indian rice straw for the production of second-generation bioethanol. Sustain. Energy Technol. Assess. 2021, 47, 101546. [Google Scholar] [CrossRef]
| Agronomy | |||
|---|---|---|---|
| Usage | Industry | Year | References |
| Fertilizer | Agricultural | 2017 | [21] |
| Bio-compost (mushroom cultivation) | Agricultural | 2014 | [22] |
| Material | |||
| Aluminium alloy/clay composite | Construction | 2021 | [23] |
| Supercapacitor | Electronic | 2021 | [24] |
| RH-based nano-silica catalyst | Acid reforming | 2021 | [25] |
| Cement-based composite | Construction | 2021 | [26] |
| Tableware (biodegradable cutlery) | Hotels, restaurants, etc. | 2019 | [27] |
| Thermal Insulation | Power plants | 2019 | [28] |
| Filaments for fused-deposition modelling | 3D Construction | 2019 | [29] |
| Building blocks/bricks | Construction | 2016 | [30] |
| Fuels/Energy/Treatment | |||
| Treatment | Wastewater | 2021 | [31] |
| Bioethanol | Transportation/power generation | 2018 | [32] |
| Biogas | Cooking/power generation | 2018 | [33] |
| Energy feedstock | Power generation | 2011 | [34] |
| Biomass Type | Cellulose (wt.%) | Hemicellulose (wt.%) | Lignin (wt.%) | Silica (SiO) (wt.%) | Ref. |
|---|---|---|---|---|---|
| RH a | 25–35 | 18–21 | 26–31 | 15–17 | [40] |
| RS a | 36.40 | 20.40 | 14.30 | 6.20 | [41] |
| CC a | 45.80 | 39.40 | 11.30 | 1.13 | [42] |
| WH b | 42.58 | 18.54 | 11.21 | NA | [43] |
| SB b | 39.75 | 38.03 | 22.01 | NA | [44] |
| Proximate Analysis (wt.%) | |||||
|---|---|---|---|---|---|
| Biomass Type | MC | VM | AC | FC | Ref. |
| RH b | 4.07–9.50 | 51.98–71.47 | 16.30–17.36 | 3.11–25.10 | [45,46,47] |
| RS b | 8.53–13.06 | 66.75–70.20 | 6.90–9.22 | 10.97–14.57 | [48,49,50] |
| CC a | 7.14–11.02 | 69.31–87.76 | 1.05–5.07 | 11.19–14.60 | [42,51,52] |
| WH a | 4.40–8.45 | 65.59–69.19 | 4.99–12.11 | 12.72–20.97 | [43,53,54] |
| SB a | 8.37–10.3 | 75.72–88.48 | 1.60–2.20 | 9.41–16.30 | [55,56,57] |
| Ultimate Analysis (DRY basis wt.%) | |||||||
|---|---|---|---|---|---|---|---|
| Biomass Type | C | H | O | N | S | HHV (MJ/kg) | Ref. |
| RH | 33.14–41.78 | 5.14–5.50 | 36.31–37.20 | 0.30–0.55 | 0.08–0.20 | 14.61–15.44 | [61,65,66,67] |
| RS | 37.10–39.65 | 4.88–5.20 | 35.80–44.30 | 0.50–0.92 | 0.10–0.12 | 12.10–16.60 | [62,68,69] |
| CC | 41.07–43.81 | 6.49–6.54 | 46.47–50.41 | 0.25–0.77 | 0.15–0.69 | 16.13–16.46 | [42,51,52] |
| WH | 47.14–48.50 | 5.50–5.59 | 39.90–46.03 | 0.30–0.37 | 0.06–0.10 | 18.90–19.22 | [43,68] |
| SB | 41.45–48.81 | 5.51–6.20 | 43.10–50.37 | 0.20–0.51 | 0.02–0.10 | 15.96–19.19 | [55,57,67] |
| Parameters | ||||||
|---|---|---|---|---|---|---|
| Pre-Treatment Technique | Temperature (°C) | Reaction Time (min) | By-Products | Advantages | Disadvantages | Ref. |
| I | 30–700 | 3–10 | CO, CO2, H2O and solid fuel | Higher compressibility strength | Requires additional binding agent | [75,76,77,78,79,80] |
| II | 80–120 | - | CO2, water and other by-products | Sensitive for moisture absorption, swell and breakage | Does not require additional binding agent | [81,82] |
| III | 200–400 | 20–60 | Gaseous, aqueous chemicals and solid fuel (char) | Higher energy content, lower moisture content and hydrophobic | Torrefied fuel does not guarantee less corrosion on boiler tubes | [72,83] |
| IV | 180–280 | >20 | Gases, (aqueous) liquids and solids (hydrochar) | Milder reaction temperature and pressure (autogenous), does not require drying processes, and high solid yield | Corrosion, coke and tar formation, and the process needs high capital investment | [84] |
| Parameters | Pyrolysis | Gasification | Combustion |
|---|---|---|---|
| Process Conditions | |||
| Temperature, (°C) | 300–600 | >600 | >700 |
| Reaction time | 1 s (fast pyrolysis), days (slow pyrolysis) | Several seconds to minutes | - |
| Equivalent ratio (ER) | 0 | 0 < ER < 1 | 1 |
| Products | |||
| Gaseous | CO, CH4, CXHY, CO2, H2O, oils, N- and S-containing compounds | CO, H2, CO2, H2O, CH4, CXHY, tars, NHy, NOx, H2S, COS | CO2, H2O, CO, CXHY, NOX, SOX |
| Solid | C, (N, S), ash | Ash, (N, S) | Fly ash and bottom ash |
| Liquid | Bio-oil/liquid (tar) | - | - |
| Stages of AD | Ref. | |||
|---|---|---|---|---|
| Hydrolysis | ||||
| Substrate | Microbes | End Product | Specification | |
| Cellulose, starch, xylan, etc. | I | Simple sugar/monomers | Exo-enzymes inhibit the environmental fluctuations and toxins in the feedstocks. Work well in pH (6–8). Slow process. Rate limiting. | [164,165] |
| Acidogenesis | ||||
| II | Presence of acid-forming bacteria. Strong and fast growth. Work well in pH (4–8). Inefficient below pH < 4. | [164,165] | ||
| Acetogenesis | ||||
| III | The growth kinetic of acetogenesis is lower than that of acidogenesis. Strict anaerobes which become weaker in acid environment. Work well in pH (6.5–6.2). | [164,165] | ||
| Methanogenesis | ||||
| IV | Methanogens should be maintained at a stable condition with pH (6.5–7.5). Hydrogenotrophic methanogenesis process produces higher energy than that of aceticlastic methonegenesis. | [164,165,166] | ||
| Year | In Billion Litres, (Bl) | |||
|---|---|---|---|---|
| Total | Bioethanol | Biodiesel | Other Biofuels | |
| 2000 | 18.0 | 13.2 | 26.7 | 8.09 |
| 2005 | 38.4 | 26.7 | 3.66 | 8.09 |
| 2010 | 106 | 66.5 | 19.9 | 19.7 |
| 2015 | 128 | 79.4 | 30.0 | 19.0 |
| 2016 | 134 | 82.7 | 33.9 | 17.3 |
| 2017 | 138 | 85.1 | 36.1 | 16.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaniapan, S.; Pasupuleti, J.; Patma Nesan, K.; Abubackar, H.N.; Umar, H.A.; Oladosu, T.L.; Bello, S.R.; Rene, E.R. A Review of the Sustainable Utilization of Rice Residues for Bioenergy Conversion Using Different Valorization Techniques, Their Challenges, and Techno-Economic Assessment. Int. J. Environ. Res. Public Health 2022, 19, 3427. https://doi.org/10.3390/ijerph19063427
Kaniapan S, Pasupuleti J, Patma Nesan K, Abubackar HN, Umar HA, Oladosu TL, Bello SR, Rene ER. A Review of the Sustainable Utilization of Rice Residues for Bioenergy Conversion Using Different Valorization Techniques, Their Challenges, and Techno-Economic Assessment. International Journal of Environmental Research and Public Health. 2022; 19(6):3427. https://doi.org/10.3390/ijerph19063427
Chicago/Turabian StyleKaniapan, Sivabalan, Jagadeesh Pasupuleti, Kartikeyan Patma Nesan, Haris Nalakath Abubackar, Hadiza Aminu Umar, Temidayo Lekan Oladosu, Segun R. Bello, and Eldon R. Rene. 2022. "A Review of the Sustainable Utilization of Rice Residues for Bioenergy Conversion Using Different Valorization Techniques, Their Challenges, and Techno-Economic Assessment" International Journal of Environmental Research and Public Health 19, no. 6: 3427. https://doi.org/10.3390/ijerph19063427
APA StyleKaniapan, S., Pasupuleti, J., Patma Nesan, K., Abubackar, H. N., Umar, H. A., Oladosu, T. L., Bello, S. R., & Rene, E. R. (2022). A Review of the Sustainable Utilization of Rice Residues for Bioenergy Conversion Using Different Valorization Techniques, Their Challenges, and Techno-Economic Assessment. International Journal of Environmental Research and Public Health, 19(6), 3427. https://doi.org/10.3390/ijerph19063427











