Enamel Analysis by 3D Scanning after Three Orthodontic Clean-Up Procedures: An In-Vitro Test of a New Piezoelectric Tool
Abstract
:1. Introduction
- (i)
- leaving adhesive remnants on the tooth surface that may become discoloured over time as a result of environmental exposure to food and lifestyle dyes [7]. The adhesive remnants left on the surfaces of clinical crowns predisposes to the accumulation of the oral cavity biofilm. In both cases, this results in aesthetic discomfort for the patient, as well as an increased risk of carioreceptivity [8].
- (ii)
- iatrogenic enamel damage resulting from an excessively aggressive procedure which, in addition to removing the composite, causes quantitative damage to the enamel surface, such as enamel cracking and fracture, removal of the external layer of enamel rich in fluoride and roughening of the surface [6]. Especially in the anterior sectors, this can cause aesthetic damage as the quaternary anatomy of the tooth morphology is cancelled, as is the perikymata, which defines aesthetic enamel details such as light refraction in the juvenile dentition [9,10,11].
2. Materials and Methods
2.1. Study Design and Setting
2.2. Specimen Preparation (Inclusion Criteria)
2.3. Sample Size Calculation
2.4. Bonding and Debonding Procedures
2.5. Clean-Up Procedures
- Group 1: Tested treatment inserts: ultrasonic scaler (Multipiezo device; Mectron, Italy), with the following inserts:
- Insert diamond grain 30 µm, made of medical grade stainless steel
- Insert made of medical grade stainless steel with an operative part made of modified PEEK.
- Group 2: One step finisher and polisher (Inverted cone One gloss Shofu Dental, Kyoto, Japan)
- Group 3: Twelve-fluted tungsten carbide bur (123-603-00, Dentaurum, Pforzheim, Germany) and Sof-Lex discs Pop-On XT Kit (3M ESPE). The tungsten carbide bur on a low-speed handpiece at 20,000 rpm without water cooling, and a new bur was used after 12 teeth. The Sof-Lex discs system (medium) was used under dry settings with light pressure for 20 s at a handpiece speed of 10,000 rpm.
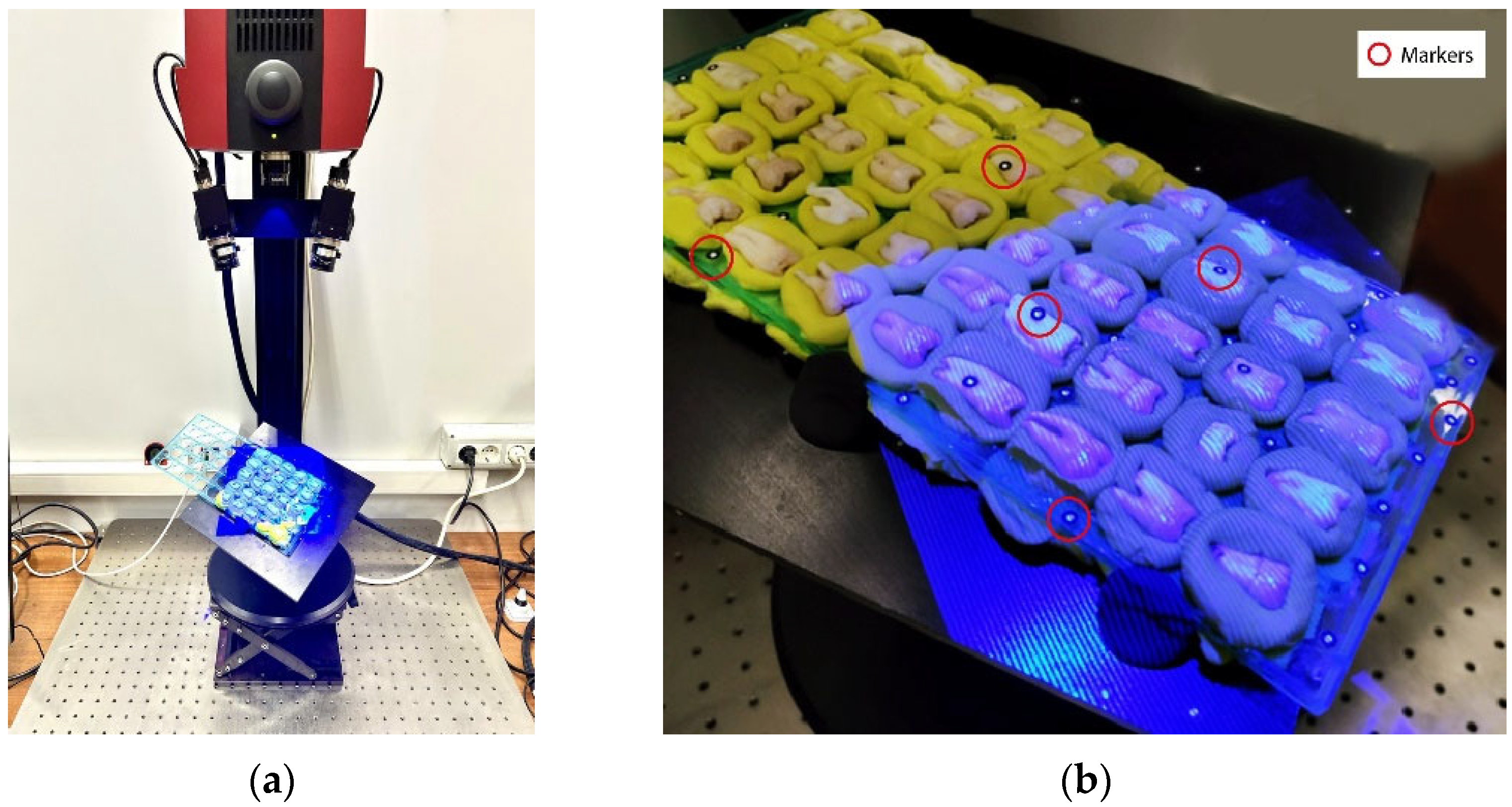
2.6. 3D Optical Analysis
- (i)
- T0 represents the first phase and refers to the original buccal surface of clinical crowns at baseline.
- (ii)
- T1 is the buccal surface condition after mechanical removal of the molar tubes with orthodontic pliers; at this time of follow-up the adhesive remnants were measured.
- (iii)
- T2 represents the last step, and it refers to the cleaned buccal surface, after the three tested procedures for removing adhesive remnants.
2.7. Data Alignment for Comparison
2.8. Data Comparison
- (i)
- Subtraction of T0 closed shell from T1 closed shell to extract the volume of adhesive after bracket removal.
- (ii)
- Subtraction of T0 closed shell from T2 closed shell to extract the volume of the residual adhesive after the clean-up procedure.
- (iii)
- Subtraction of T2 closed shell from T0 closed shell to extract the volume of the enamel loss.
2.9. Statistical Analysis
3. Results
3.1. Results (Difference in Treatment Efficacy)
3.1.1. Residual Adhesive Volume
3.1.2. Enamel Volume Loss
4. Discussion
- (a)
- Insert diamond grain 30 µm, made of medical grade stainless steel.
- (b)
- Insert made of medical grade stainless steel with an operative part made of modified PEEK.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janiszewska-Olszowska, J.; Szatkiewicz, T.; Tomkowski, R.; Tandecka, K.; Grocholewicz, K. Effect of orthodontic debonding and adhesive removal on the enamel–current knowledge and future perspectives–a systematic review. Med. Sci. Monit. 2014, 20, 1991–2001. [Google Scholar] [PubMed] [Green Version]
- Knosel, M.; Mattysek, S.; Jung, K.; Kubein-Meesenburg, D.; Sadat-Khonsari, R.; Ziebolz, D. Suitability of orthodontic brackets for rebonding and reworking following removal by air pressure pulses and conventional debracketing techniques. Angle Orthod. 2010, 80, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fjeld, M.; Øgard, B. Scanning electron microscopic evaluation of enamel surfaces exposed to 3 orthodontic bonding systems. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 575–581. [Google Scholar] [CrossRef]
- Nanjannawar, L.G.; Nanjannawar, G.S. Effects of a self-etching primer and 37% phosphoric Acid etching on enamel: A scanning electron micro- scopic study. J. Contemp. Dent. Pract. 2012, 13, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Bishara, S.E.; Ostby, A.W.; Laffoon, J.F.; Warren, J. Shear bond strength comparison of two adhesive systems following thermocycling. A new self-etch primer and a resin- modified glass ionomer. Angle Orthod. 2007, 77, 337–341. [Google Scholar] [CrossRef] [Green Version]
- Janiszewska-Olszowska, J.; Tandecka, K.; Szatkiewicz, T.; Sporniak-Tutak, K.; Grocholewicz, K. Three-dimensional quantitative analysis of adhesive remnants and enamel loss resulting from debonding orthodontic molar tubes. Head Face Med. 2014, 10, 37. [Google Scholar] [CrossRef] [Green Version]
- Joo, H.J.; Lee, Y.K.; Lee, D.Y.; Kim, Y.J.; Lim, Y.K. Influence of orthodontic adhesives and clean-up procedures on the stain susceptibility of enamel after debonding. Angle Orthod. 2011, 81, 334–340. [Google Scholar] [CrossRef] [Green Version]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Pus, M.D.; Way, D.C. Enamel loss due to orthodontic bonding with filled and unfilled resins using various clean-up techniques. Am. J. Orthod. 1980, 77, 269–283. [Google Scholar] [CrossRef]
- Mazur, M.; Westland, S.; Guerra, F.; Corridore, D.; Vichi, M.; Maruotti, A.; Nardi, G.M.; Ottolenghi, L. Objective and subjective aesthetic performance of icon® treatment for enamel hypomineralization lesions in young adolescents: A retrospective single center study. J. Dent. 2018, 68, 104–108. [Google Scholar] [CrossRef]
- Guerra, F.; Mazur, M.; Corridore, D.; Pasqualotto, D.; Nardi, G.M.; Ottolenghi, L. Evaluation of the esthetic properties of developmental defects of enamel: A spectrophotometric clinical study. Sci. World J. 2015, 2015, 878235. [Google Scholar] [CrossRef]
- Santana, R.M.; Rached, R.N.; Souza, E.M.; Guariza-Filho, O.; Camargo, E.S.; Pithon, M.M.; Tanaka, O.M. Effect of organic solvents and ultrasound on the removal of orthodontic brackets. Orthod. Craniofac. Res. 2016, 19, 137–144. [Google Scholar] [CrossRef]
- Knosel, M.; Mattysek, S.; Jung, K.; Sadat-Khonsari, R.; Kubein-Meesenburg, D.; Bauss, O.; Ziebolz, D. Impulse debracketing compared to conventional debonding. Angle Orthod. 2010, 80, 1036–1044. [Google Scholar] [CrossRef] [Green Version]
- Krell, K.V.; Courey, J.M.; Bishara, S.E. Orthodontic bracket removal using conventional and ultrasonic debond- ing techniques, enamel loss, and time requirements. Am. J. Orthod. Dentofacial. Orthop. 1993, 103, 258–266. [Google Scholar] [CrossRef]
- Al Shamsi, A.; Cunningham, J.L.; Lamey, P.J.; Lynch, E. Shear bond strength and residual adhesive after orthodontic bracket debonding. Angle Orthod. 2006, 76, 694–699. [Google Scholar]
- Dumbryte, I.; Vebriene, J.; Linkeviciene, L.; Malinauskas, M. Enamel microcracks in the form of tooth damage during orthodontic debonding: A systematic review and meta-analysis of in vitro studies. Eur. J. Orthod. 2018, 40, 636–648. [Google Scholar] [CrossRef]
- Mitwally, R.A.; Bakhsh, Z.T.; Feteih, R.M.; Bakry, A.S.; Abbassy, M.A. Orthodontic Bracket Bonding Using Self-adhesive Cement to Facilitate Bracket Debonding. J. Adhes. Dent. 2019, 21, 551–556. [Google Scholar]
- Fitzpatrick, D.A.; Way, D. The effects of wear, acid etching and bond removal on human enamel. Am. J. Orthod. 1997, 72, 671–681. [Google Scholar] [CrossRef]
- Jedliński, M.; Mazur, M.; Grocholewicz, K.; Janiszewska-Olszowska, J. 3D Scanners in Orthodontics-Current Knowledge and Future Perspectives-A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1121. [Google Scholar] [CrossRef]
- Janiszewska-Olszowska, J.; Tandecka, K.; Szatkiewicz, T.; Stępień, P.; Sporniak-Tutak, K.; Grocholewicz, K. Three-dimensional analysis of enamel surface alteration resulting from orthodontic clean-up -comparison of three different tools. BMC Oral. Health 2015, 15, 146. [Google Scholar] [CrossRef] [Green Version]
- Chow, S.; Shao, J.; Wang, H. Sample Size Calculations in Clinical Research, 2nd ed.; Chapman & Hall/CRC Biostatistics Series; Taylor & Francis Group: London, UK, 2008. [Google Scholar]
- Suliman, S.N.; Trojan, T.M.; Tantbirojn, D.; Versluis, A. Enamel loss following ceramic bracket debonding: A quantitative analysis in vitro. Angle Orthod. 2015, 85, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Bishara, S.E.; Trulove, T.S. Comparisons of different debonding techniques for ceramic brackets: An in vitro study. Part II. Findings and clinical implications. Am. J. Orthod. Dentofacial. Orthop. 1990, 98, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.U.; Yap, S.H.; Teo, C.K.; Nq, J.J. Finishing/polishing of composite and compomer restoratives: Effectiveness of one-step systems. Oper. Dent. 2004, 29, 275–279. [Google Scholar] [PubMed]
- Fan, X.C.; Chen, L.; Huang, X.F. Effects of various debonding and adhesive clearance methods on enamel surface: An in vitro study. BMC Oral. Health 2017, 17, 58. [Google Scholar] [CrossRef]
- Sfondrini, M.F.; Scribante, A.; Fraticelli, D.; Roncallo, S.; Gandini, P. Epidemiological survey of different clinical techniques of orthodontic bracket debonding and enamel polishing. J. Orthod. Sci. 2015, 4, 123–127. [Google Scholar]










| Treatment | n | Min | Max | Mean | SD | Median | 95% Conf. Interval |
|---|---|---|---|---|---|---|---|
| All | 75 | <0.001 | 1.491 | 0.143 | 0.243 | 0.043 | (0.087, 0.198) |
| 1 | 25 | <0.001 | 1.491 | 0.239 | 0.337 | 0.121 | (0.100, 0.378) |
| 2 | 25 | <0.001 | 0.511 | 0.069 | 0.124 | 0.02 | (0.018, 0.120) |
| 3 | 25 | <0.001 | 0.766 | 0.120 | 0.193 | 0.024 | (0.040, 0.200) |
| Treatments | Difference * | p |
|---|---|---|
| 2-1 | −0.123 | 0.026 |
| 3-1 | −0.073 | 0.259 |
| 3-2 | 0.050 | 0.535 |
| Treatments | Difference * | p |
|---|---|---|
| 2-1 | −0.184 | 0.001 |
| 3-1 | −0.125 | 0.041 |
| 3-2 | 0.059 | 0.479 |
| Treatment | n | Min | Max | Mean | SD | Median | 95% Conf. Interval |
|---|---|---|---|---|---|---|---|
| All | 75 | 0.001 | 1.104 | 0.219 | 0.235 | 0.143 | (0.165, 0.273) |
| 1 | 25 | 0.001 | 0.772 | 0.180 | 0.177 | 0.122 | (0.107, 0.252) |
| 2 | 25 | 0.002 | 1.104 | 0.187 | 0.299 | 0.065 | (0.064, 0.311) |
| 3 | 25 | 0.017 | 0.79 | 0.290 | 0.205 | 0.211 | (0.206, 0.375) |
| Treatments | Difference * | p |
|---|---|---|
| 2-1 | −0.043 | 0.665 |
| 3-1 | 0.105 | 0.098 |
| 3-2 | 0.148 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardi, G.M.; Mazur, M.; Grassi, R.; Rifuggiato, S.; Stiuso, V.; Janiszewska-Olszowska, J.; Ottolenghi, L.; Barbato, E.; Minetola, P.; Iuliano, L. Enamel Analysis by 3D Scanning after Three Orthodontic Clean-Up Procedures: An In-Vitro Test of a New Piezoelectric Tool. Int. J. Environ. Res. Public Health 2023, 20, 2516. https://doi.org/10.3390/ijerph20032516
Nardi GM, Mazur M, Grassi R, Rifuggiato S, Stiuso V, Janiszewska-Olszowska J, Ottolenghi L, Barbato E, Minetola P, Iuliano L. Enamel Analysis by 3D Scanning after Three Orthodontic Clean-Up Procedures: An In-Vitro Test of a New Piezoelectric Tool. International Journal of Environmental Research and Public Health. 2023; 20(3):2516. https://doi.org/10.3390/ijerph20032516
Chicago/Turabian StyleNardi, Gianna Maria, Marta Mazur, Roberta Grassi, Serena Rifuggiato, Vito Stiuso, Joanna Janiszewska-Olszowska, Livia Ottolenghi, Ersilia Barbato, Paolo Minetola, and Luca Iuliano. 2023. "Enamel Analysis by 3D Scanning after Three Orthodontic Clean-Up Procedures: An In-Vitro Test of a New Piezoelectric Tool" International Journal of Environmental Research and Public Health 20, no. 3: 2516. https://doi.org/10.3390/ijerph20032516












