Responses of Soil Collembolans to Land Degradation in a Black Soil Region in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sampling Design
2.3. Soil Collembolan Extraction and Identification
2.4. Soil Analysis
2.5. Statistical Analysis
3. Results
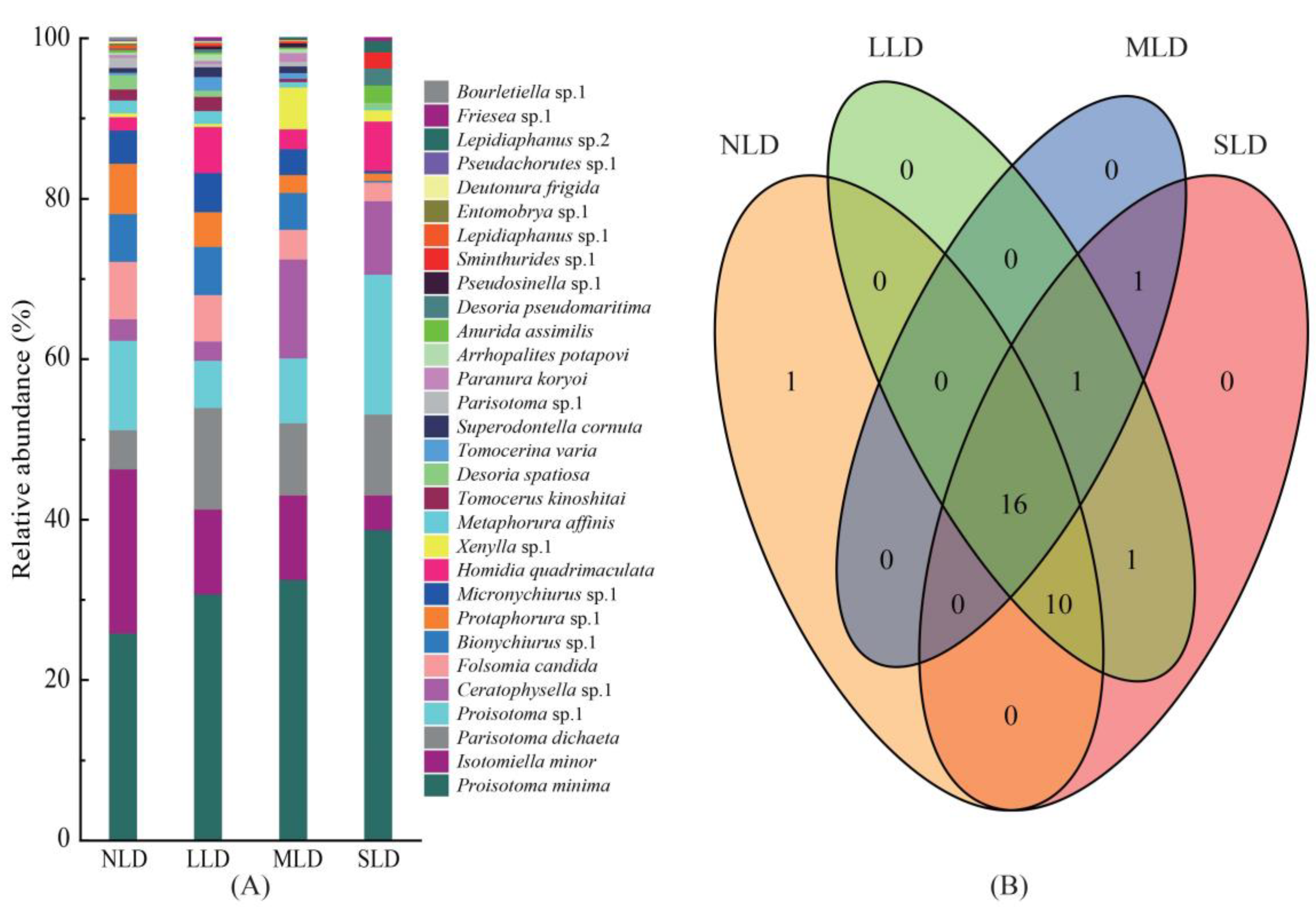
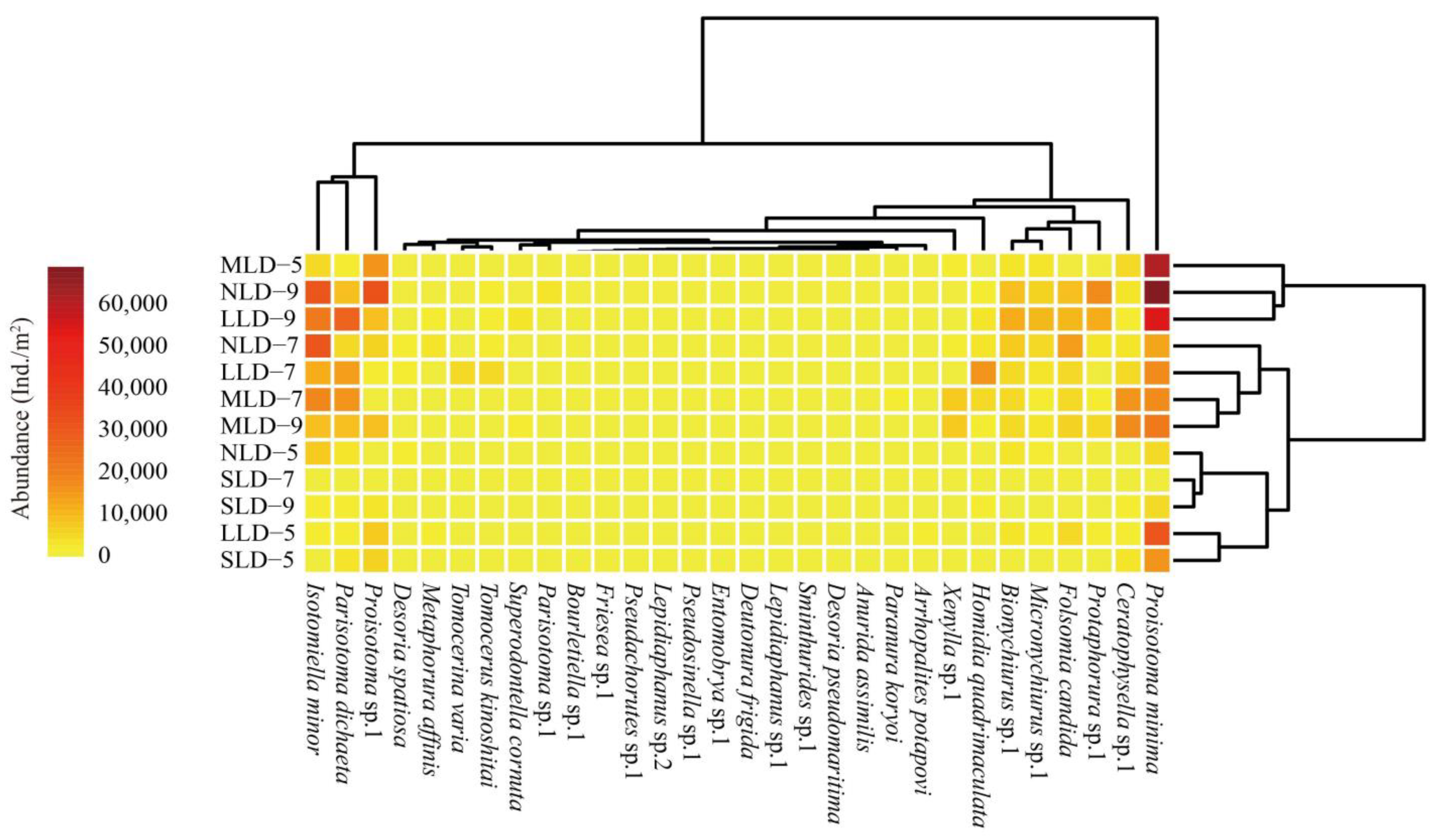
3.1. Taxonomic Composition and Community Structure
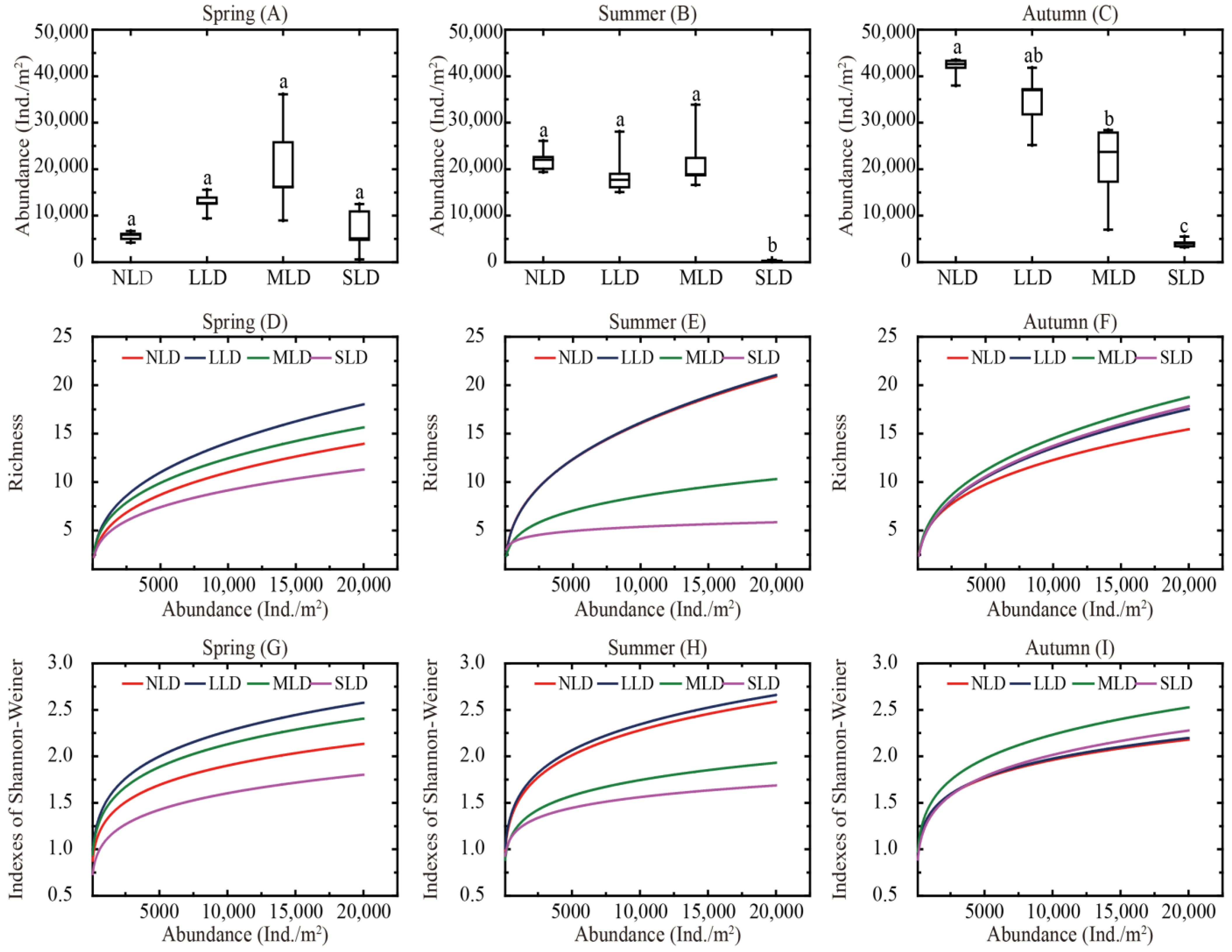
3.2. Diversity Characteristics and Distribution Patterns
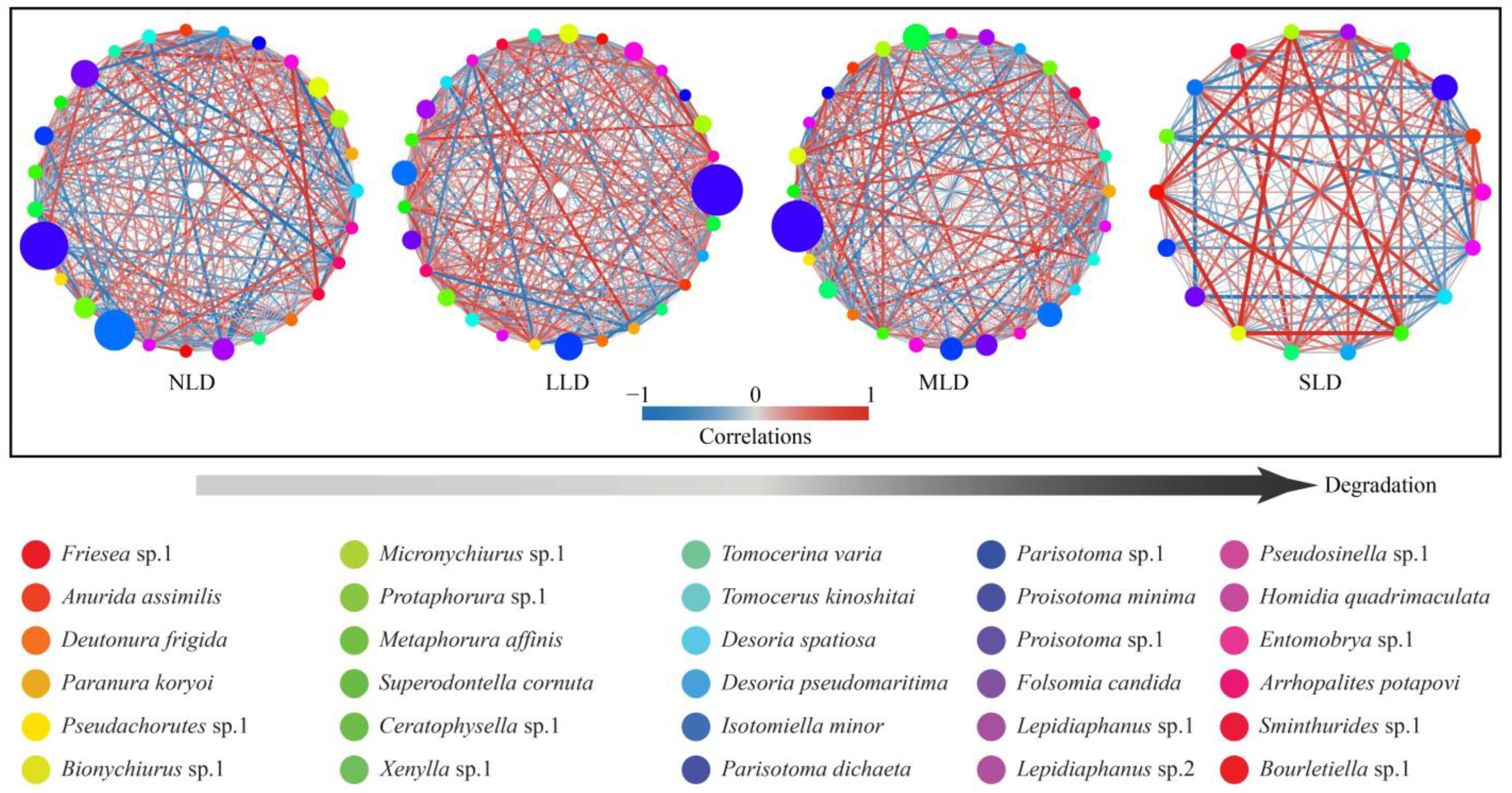
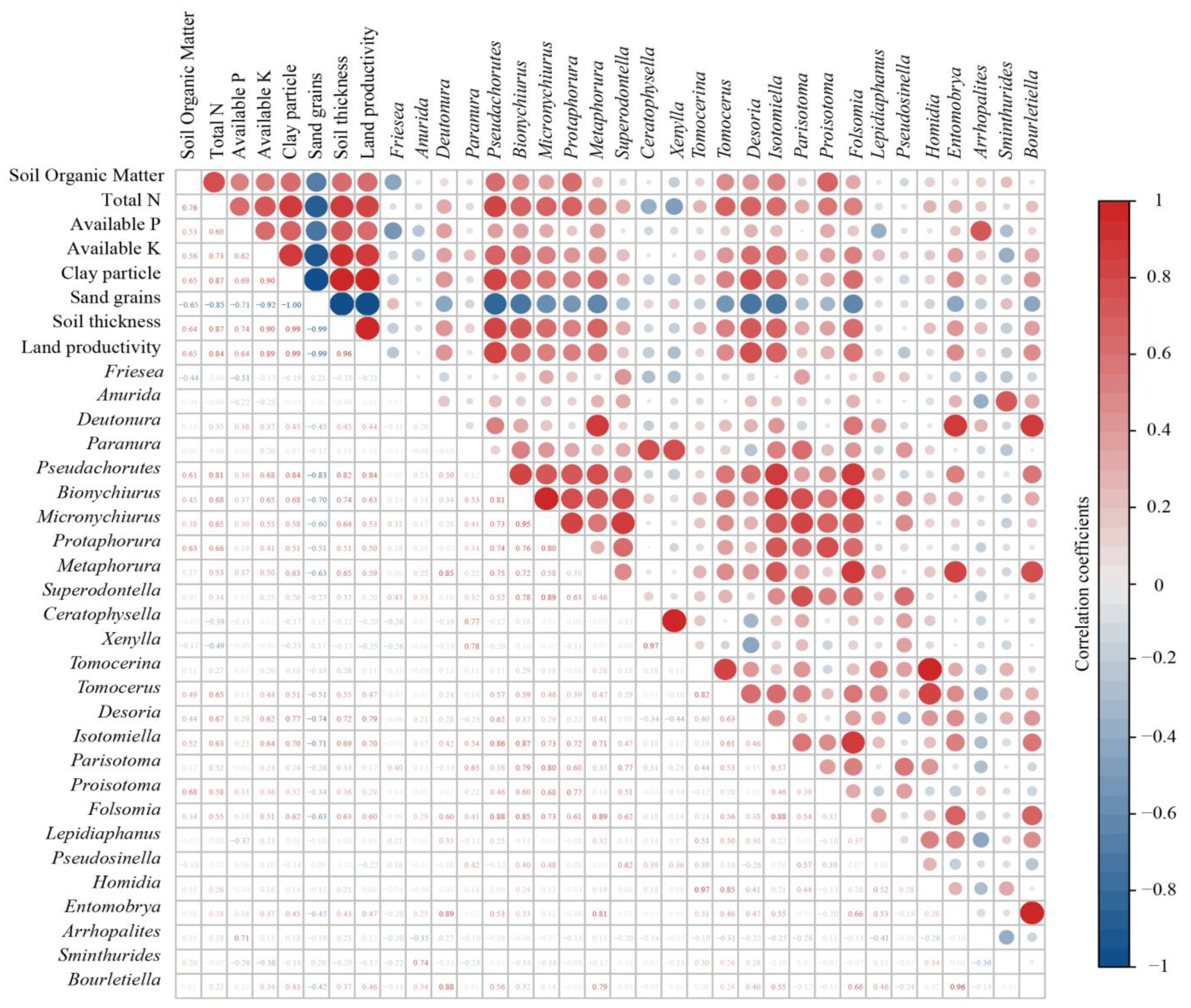
3.3. Correlations of Soil Collembolans with Land Degradation
4. Discussion
4.1. Effects of Land Degradation on Soil Collembolan Communities
4.2. Responses of Soil Collembolans to Land Degradation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. IPCC Special Report: Climate Change and Land; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- UNCCD. Global Land Outlook; UNCCD: Bonn, Germany, 2017. [Google Scholar]
- IPBES. Land Degradation and Restoration Assessment; IPBES: Bonn, Germany, 2018. [Google Scholar]
- Song, X.P.; Hansen, M.C.; Stehman, S.V.; Potapov, P.V.; Tyukavina, A.; Vermote, E.F.; Townshend, J.R. Global land change from 1982 to 2016. Nature 2018, 560, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Ries, J.B. Methodologies for soil erosion and land degradation assessment in mediterranean-type ecosystems. Land Degrad. Dev. 2010, 21, 171–187. [Google Scholar] [CrossRef]
- Saygin, S.D.; Erpul, G.; Basaran, M. Comparison of Aggregate Stability Measurement Methods for Clay-rich Soils in Asartepe Catchment of Turkey. Land Degrad. Dev. 2015, 28, 199–206. [Google Scholar] [CrossRef]
- Dlamini, P.; Chivenge, P.; Manson, A.; Chaplot, V. Land degradation impact on soil organic carbon and nitrogen stocks of sub-tropical humid grasslands in South Africa. Geoderma 2014, 235–236, 372–381. [Google Scholar] [CrossRef]
- Traoré, S.; Ouattara, K.; Ilstedt, U.; Schmidt, M.; Thiombiano, A.; Malmer, A.; Nyberg, G. Effect of land degradation on carbon and nitrogen pools in two soil types of a semi-arid landscape in West Africa. Geoderma 2015, 241–242, 330–338. [Google Scholar] [CrossRef]
- Jin, Q.; Peñuelas, J.; Sardans, J.; Romero, E.; Chen, S.; Liu, X.; Lin, S.; Wang, W. Changes in soil carbon, nitrogen, and phosphorus contents, storages, and stoichiometry during land degradation in jasmine croplands in subtropical China. Exp. Agric. 2021, 57, 113–125. [Google Scholar] [CrossRef]
- Ferreira, A.C.C.; Leite, L.F.C.; de Araújo, A.S.F.; Eisenhauer, N. Land-Use Type Effects on Soil Organic Carbon and Microbial Properties in a Semi-arid Region of Northeast Brazil. Land Degrad. Dev. 2014, 27, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Teng, Y.; Zhou, Q. Variation in soil geochemical properties and microbial communities in areas under land developed for educational use (university and other campuses). Land Degrad. Dev. 2021, 32, 173–182. [Google Scholar] [CrossRef]
- De Vries, F.T.; Thebault, E.; Liiri, M.; Birkhofer, K.; Tsiafouli, M.A.; Bjornlund, L.; Jørgensen, H.B.; Brady, M.V.; Christensen, S.; de Ruiter, P.C.; et al. Soil food web properties explain ecosystem services across European land use systems. Proc. Natl. Acad. Sci. USA 2013, 110, 14296–14301. [Google Scholar] [CrossRef] [Green Version]
- Hopkin, S.P. Biology of the Springtails (Insecta: Collembola); Oxford University Press: New York, NY, USA, 1997; pp. 10–15. [Google Scholar]
- Rusek, J. Biodiversity of Collembola and their functional role in the ecosystem. Biodivers. Conserv. 1998, 7, 1207–1219. [Google Scholar] [CrossRef]
- Makkonen, M.; Berg, M.P.; van Hal, J.R.; Callaghan, T.V.; Press, M.C.; Aerts, R. Traits explain the responses of a sub-arctic Collembola community to climate manipulation. Soil Biol. Biochem. 2011, 43, 377–384. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Winck, B.R.; Rigotti, V.M.; Saccol de Sá, E.L. Effects of different grazing intensities on the composition and diversity of Collembola communities in southern Brazilian grassland. Appl. Soil Ecol. 2019, 144, 98–106. [Google Scholar] [CrossRef]
- Ma, C.; Yin, X.; Xu, H.; Tao, Y. Responses of soil Collembolans to vegetation restoration in temperate coniferous and broad-leaved mixed forests. J. For. Res. 2020, 31, 2333–2345. [Google Scholar] [CrossRef]
- Pollet, S.; Chabert, A.; Burgeon, V.; Cornélis, J.; Fouché, J.; Gers, C.; Hardy, B. Limited effects of century-old biochar on taxonomic and functional diversities of collembolan communities across land-uses. Soil Biol. Biochem. 2022, 164, 108484. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Survey Staff. Soil taxonomy, a basic system of soil classification for making and interpreting soil surveys. In USDA Natural Resource Conservation Service Agriculture Handbook, USDA; The Superintendent of Documents; U.S. Government Printing Office: Washington, DC, USA, 2001; p. 886. [Google Scholar]
- Zhou, Z.; Li, Z.; Zhang, Z.; You, L.; Xu, L.; Huang, H.; Wang, X.; Gao, Y.; Cui, X. Treatment of the saline-alkali soil with acidic corn stalk biochar and its effect on the sorghum yield in western Songnen Plain. Sci. Total Environ. 2021, 797, 149190. [Google Scholar] [CrossRef]
- Shen, W.; Cao, X.; Shen, F. Classification and Grading of Land Degradation in China. J. Ecol. Rural Environ. 2006, 22, 88–93. [Google Scholar]
- Christiansen, K.A.; Bellinger, P.F. The Collembola of North America North of Rio Grande, 2nd ed.; Grinnell College: Grinnell, IA, USA, 1998. [Google Scholar]
- Dunger, W. Synopses on Palearctic Collembola; State Museum of the Natural History Museum of Görlitz: Gorlitz, Germany, 2012. [Google Scholar]
- Bellinger, P.F.; Christiansen, K.A.; Janssens, F. Checklist of the Collembola of the World. Available online: https://www.collembola.org/ (accessed on 6 February 2023).
- Bao, S. Soil Agro-Chemistrical Analysis; China Agricultural Press: Beijing, China, 2000; pp. 25–151. [Google Scholar]
- Chen, H. VennDiagram: Generate High-Resolution Venn and Euler Plots. R Package Version 1.7.1. Available online: https://CRAN.R-project.org/package=VennDiagram (accessed on 12 April 2022).
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; de Hollander, M.; Soto, R.L.; Bouffaud, M.L.; Buée, M.; Dimmers, W.; et al. Soil networks become more connected and take up more carbon as nature restoration progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 17 May 2020).
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples, Version 50. Available online: http://purl.oclc.org/estimates (accessed on 1 December 2018).
- Kolde, R. pheatmap: Pretty Heatmaps. R Package, Version 1.0.12. Available online: https://CRAN.R-roject.org/package=pheatmap (accessed on 4 January 2019).
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 6 February 2023).
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix, Version 0.92. Available online: https://github.com/taiyun/corrplot (accessed on 31 August 2022).
- Rosseel, Y. lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.; Tang, H.; Liu, Q.; Cheng, F.; Zhang, L.; Sang, L.; Huang, Y.; Shen, C.; Gao, B.; Niu, Z. Determinants of Soil Bacterial Diversity in a Black Soil Region in a Large-Scale Area. Land 2022, 11, 731. [Google Scholar] [CrossRef]
- Ferlian, O.; Klarner, B.; Langeneckert, A.E.; Scheu, S. Trophic niche differentiation and utilisation of food resources in collembolans based on complementary analyses of fatty acids and stable isotopes. Soil Biol. Biochem. 2015, 82, 28–35. [Google Scholar] [CrossRef]
- Potapov, A.M.; Klarner, B.; Sandmann, D.; Widyastuti, R.; Scheu, S. Linking size spectrum, energy flux and trophic multifunctionality in soil food webs of tropical land-use systems. J. Anim. Ecol. 2019, 88, 1845–1859. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.H. Intermediate-disturbance hypothesis. Science 1979, 204, 1345. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, T.; Potapov, M.; Zhang, F.; Wu, D.; Scheu, S.; Sun, X. Ecological and evolutionary processes shape below-ground springtail communities along an elevational gradient. J. Biogeogr. 2022, 49, 469–482. [Google Scholar] [CrossRef]
- Sengupta, S.; Ergon, T.; Leinaas, H.P. Thermal plasticity in postembryonic life history traits of a widely distributed Collembola: Effects of macroclimate and microhabitat on genotypic differences. Ecol Evol. 2017, 7, 8100–8112. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, S.M.; Borgå, K.; Rundberget, J.T.; Leinaas, H.P. Effects on life-history traits of Hypogastrura viatica (Collembola) exposed to imidacloprid through soil or diet. Environ. Toxicol. Chem. 2021, 40, 3111–3122. [Google Scholar] [CrossRef]
- Salmon, S.; Ponge, J.F.; Gachet, S.; Deharveng, L.; Lefebvre, N.; Delabrosse, F. Linking species, traits and habitat characteristics of Collembola at European scale. Soil Biol. Biochem. 2014, 75, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Potapov, A.M.; Semenina, E.E.; Korotkevich, A.Y.; Kuznetsova, N.A.; Tiunov, A.V. Connecting taxonomy and ecology: Trophic niches of collembolans as related to taxonomic identity and life forms. Soil Biol. Biochem. 2016, 101, 20–31. [Google Scholar] [CrossRef]
- Saifutdinov, R.A.; Gongalsky, K.B.; Zaitsev, A.S. Evidence of a trait-specific response to burning in springtails (Hexapoda: Collembola) in the boreal forests of European Russia. Geoderma 2018, 332, 173–179. [Google Scholar] [CrossRef]
- Sterzyńska, M.; Shrubovych, J.; Kaprus, I. Effect of hydrologic regime and forest age on Collembola in riparian forests. Appl. Soil Ecol. 2014, 75, 199–209. [Google Scholar] [CrossRef]
- Potapov, A.M.; Pollierer, M.M.; Salmon, S.; Šustr, V.; Chen, T. Multidimensional trophic niche revealed by complementary approaches: Gut content, digestive enzymes, fatty acids and stable isotopes in Collembola. J. Anim. Ecol. 2021, 90, 1919–1933. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Liang, C.; He, B.; Bush, R.T. Erosion vulnerability of sandy clay loam soil in Southwest China: Modeling soil detachment capacity by flume simulation. Catena 2019, 178, 90–99. [Google Scholar] [CrossRef]
- Pi, H.; Huggins, D.R.; Sharratt, B. Wind erosion of soil influenced by clay amendment in the inland Pacific Northwest, USA. Land Degrad. Dev. 2021, 32, 241–255. [Google Scholar] [CrossRef]
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Weber, P.K.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Change 2015, 5, 588–595. [Google Scholar] [CrossRef] [Green Version]
- Kooch, Y.; Ehsani, S.; Akbarinia, M. Stratification of soil organic matter and biota dynamics in natural and anthropogenic ecosystems. Soil Till. Res. 2020, 200, 104621. [Google Scholar] [CrossRef]
- Korboulewsky, N.; Heiniger, C.; De Danieli, S.; Brun, J.J. Effect of tree mixture on Collembola diversity and community structure in temperate broadleaf and coniferous forests. Forest Ecol. Manag. 2021, 482, 118876. [Google Scholar] [CrossRef]
- Widenfalk, L.A.; Malmström, A.; Berg, M.P.; Bengtsson, J. Small-scale Collembola community composition in a pine forest soil –Overdispersion in functional traits indicates the importance of species interactions. Soil Biol. Biochem. 2016, 103, 52–62. [Google Scholar] [CrossRef] [Green Version]

| SOM (g/kg) | TN (g/kg) | AP (mg/kg) | AK (mg/kg) | CP (%) | SG (%) | Soil Thickness (cm) | Yield (t/hm2) | |
|---|---|---|---|---|---|---|---|---|
| NLD | 40.67 | 6.90 | 13.43 | 274.01 | 37.76 | 59.63 | 101.05 | 1.25 |
| LLD | 33.11 | 6.43 | 13.24 | 215.32 | 25.96 | 71.12 | 85.88 | 1.00 |
| MLD | 30.01 | 3.40 | 11.10 | 208.62 | 15.33 | 80.37 | 62.57 | 0.85 |
| SLD | 23.57 | 2.96 | 6.34 | 134.91 | 7.23 | 90.55 | 40.33 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Nie, R.; Du, G. Responses of Soil Collembolans to Land Degradation in a Black Soil Region in China. Int. J. Environ. Res. Public Health 2023, 20, 4820. https://doi.org/10.3390/ijerph20064820
Ma C, Nie R, Du G. Responses of Soil Collembolans to Land Degradation in a Black Soil Region in China. International Journal of Environmental Research and Public Health. 2023; 20(6):4820. https://doi.org/10.3390/ijerph20064820
Chicago/Turabian StyleMa, Chen, Runze Nie, and Guoming Du. 2023. "Responses of Soil Collembolans to Land Degradation in a Black Soil Region in China" International Journal of Environmental Research and Public Health 20, no. 6: 4820. https://doi.org/10.3390/ijerph20064820







