The Application of Lignocellulosic Biomass Waste in the Iron and Steel Industry in the Context of Challenges Related to the Energy Crisis
Abstract
:1. Introduction
2. The Role of Lignocellulosic Biomass in the Era of Energy Crisis and Decarbonization
2.1. Biomass Characteristics
2.2. Methods of Biomass Conversion
2.3. Biomass Conversion Products
- For wet biomass (or sludge), hydrothermal carbonization is normally carried out in pressured autoclaves at temperatures within the range of 200 and 300 °C. The process purges the solid mass of inorganic chemicals that are water soluble. The product possesses heating properties that are comparable to those of torrefied biomass and a relatively low carbon-to-oxygen ratio [48,66,85,86].
- Slow pyrolysis optimizes the yield of biochar, which may be utilized either directly or after additional processing in a variety of applications, such as replacing coal in combustion, acting as a bio-reducer in the production of metals, enhancing soil, treating water, etc. Condensable and non-condensable hot pyrolysis gases are also produced, and these gases may be used as fuel to substitute fossil-based combustibles in a variety of applications. Bio-oil may be made by collecting the condensable portion [87,88,89,90].
- Fast pyrolysis enhances the output of condensable gas-derived bio-oil. Additionally, a small quantity of biochar as well as some NCG are produced. When compared to those produced by slow pyrolysis, the characteristics of biochar are somewhat different, which might limit its potential applications, such as a bio-reducer [91,92,93].
- In the process of gasification, biomass hydrocarbons completely devolatilize into syngas when enough oxygen is present. CO, CH4, H2, CO2, and smaller quantities of other gases are the main constituents. Syngas can be utilized in combustion to produce hydrogen, Fischer–Tropsch gasoline, Fischer–Tropsch diesel, alcohols, olefins, oxo compounds, synthetic natural gas (SNG), and ammonia, or as an intermediate product for those processes [44,94,95].
3. Lignocellulosic Waste and Energy Management in the Steel Industry
- A drop in the energy intensity per ton of crude steel;
- The adoption of good energy-use practices;
- Employing effective procedures for the recovery of heat and gas energy;
- Allowing plant management to create strategies to reduce the facility’s energy intensity;
- Allowing plant management to prioritize investments that will have the most impact on energy efficiency.
- Reduced company risks and susceptibility to variable energy costs;
- Enhanced productivity;
- Improved product quality and a shift to market sectors with higher added value;
- Decreased environmental compliance costs.
3.1. Replacing Fossil Energy Carriers with Biomass in the Steel Industry
3.2. Advantages of Using Biomass in the Steel/Iron Industry
3.3. The Challenges and Future Prospects in the Application of Lignocellulosic Biomass in the Steel and Iron Industry
- The use of biomass in the iron and steel sector is currently rather restricted, and it faces stiff competition from traditional fossil fuels. Technical and financial issues that call for cooperation between the steel industry and the bioenergy sector are among the difficulties associated with using biomass in the steel industry. Although a significant effort has been made up to this point, there is still long way to industrial application of biomass in iron and steel sector on the wide scale. A peculiarity of Brazil in comparison to other countries consists of the fact that its main energy sources for steel production are coal, electricity, and charcoal obtained from biomass. The example of Brazil, which is one of the biggest steel producers in the world, shows that application of lignocellulosic biomass in steel sector can be possible not only on a pilot scale but also on a wider, industrial scale [138,139].
- According to a report presented by ArcelorMittal [140] in 2018, the company started a EUR 40 million Torero demonstration project in Belgium (Ghent) using 120,000 tonnes of waste wood to create bio-coal that may be used in place of fossil fuels to reduce iron ore. The technique may be able to handle a range of waste streams (such as bio-based and plastic waste) produced by society.
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blenkinsop, P. Steel Makers Fear Deepening Crisis from Energy Crunch as Output Halted. Available online: https://www.reuters.com/business/energy/steel-makers-fear-deepening-crisis-energy-crunch-output-halted-2022-09-23/ (accessed on 2 May 2023).
- Mousa, E.; Wang, C.; Riesbeck, J.; Larsson, M. Biomass Applications in Iron and Steel Industry: An Overview of Challenges and Opportunities. Renew. Sustain. Energy Rev. 2016, 65, 1247–1266. [Google Scholar] [CrossRef]
- Lv, W.; Sun, Z.; Su, Z. Life Cycle Energy Consumption and Greenhouse Gas Emissions of Iron Pelletizing Process in China, a Case Study. J. Clean. Prod. 2019, 233, 1314–1321. [Google Scholar] [CrossRef]
- Kieush, L.; Rieger, J.; Schenk, J.; Brondi, C.; Rovelli, D.; Echterhof, T.; Cirilli, F.; Thaler, C.; Jaeger, N.; Snaet, D.; et al. A Comprehensive Review of Secondary Carbon Bio-Carriers for Application in Metallurgical Processes: Utilization of Torrefied Biomass in Steel Production. Metals 2022, 12, 2005. [Google Scholar] [CrossRef]
- Lu, L.; Li, X.; Mahoney, M.; Zhang, Z. Biomass Materials for Metallurgical Applications. Adv. Mater. Sci. Eng. 2018, 2018, 7297136. [Google Scholar] [CrossRef]
- Shukla, I. Potential of Renewable Agricultural Wastes in the Smart and Sustainable Steelmaking Process. J. Clean. Prod. 2022, 370, 133422. [Google Scholar] [CrossRef]
- Basson, E.; World Steel Association. World Steel in Figures; 2022, Volume 2003. Available online: https://worldsteel.org/wp-content/uploads/World-Steel-in-Figures-2022.pdf (accessed on 25 May 2023).
- World Steel Association. Sustainability Indicators 2022 Report; 2022. Available online: https://worldsteel.org/media-centre/press-releases/2022/sustainability-indicators-2022/ (accessed on 27 May 2023).
- What Is the Carbon Footprint of Steel? Available online: https://www.sustainable-ships.org/stories/2022/carbon-footprint-steel (accessed on 2 June 2023).
- Sanchez-Reaza, J.; Ambasz, D.; Djukic, P. Making the European Green Deal Work for People: The Role of Human Development in the Green Transition; World Bank Group: Washington, DC, USA, 2023. [Google Scholar]
- Yadav, A.; Sharma, V.; Tsai, M.L.; Chen, C.W.; Sun, P.P.; Nargotra, P.; Wang, J.X.; Dong, C. Di Development of Lignocellulosic Biorefineries for the Sustainable Production of Biofuels: Towards Circular Bioeconomy. Bioresour. Technol. 2023, 381, 129145. [Google Scholar] [CrossRef] [PubMed]
- Qian, E.W. Pretreatment and Saccharification of Lignocellulosic Biomass. In Research Approaches to Sustainable Biomass Systems; Elsevier: Amsterdam, The Netherlands, 2013; pp. 181–204. ISBN 9780124046092. [Google Scholar]
- Ortiz, I.; Quintero, R. Recent Advancements in Pretreatment Technologies of Biomass to Produce Bioenergy. In Bioenergy Reserach: Advances and Application; Elsevier: Amsterdam, The Netherlands, 2014; pp. 57–69. ISBN 9780444595614. [Google Scholar]
- Zhang, J.; Fu, H.; Liu, Y.; Dang, H.; Ye, L.; Conejio, A.N.; Xu, R. Review on Biomass Metallurgy: Pretreatment Technology, Metallurgical Mechanism and Process Design. Int. J. Miner. Metall. Mater. 2022, 29, 1133–1149. [Google Scholar] [CrossRef]
- Uwaoma, R.C.; Stokes, W.G.; Bunt, J.R.; Strydom, C.A.; Matjie, R.H. A Metallurgical Coke Replacement Derived from Torrefied Wood Chips Pre-Treated by Wet Oxidation. Bioresour. Technol. Rep. 2022, 19, 101141. [Google Scholar] [CrossRef]
- Hu, Z.W.; Zhang, J.L.; Zuo, H.B.; Liu, Z.J.; Yang, T.J. Applications and Prospects of Bio-Energy in Ironmaking Process. In 2010 the Second China Energy Scientist Forum; Scientific Research Publishing: Irvine, CA, USA, 2010; Volume 1–3, pp. 708–713. [Google Scholar]
- Mayyas, M.; Nekouei, R.K.; Sahajwalla, V. Valorization of Lignin Biomass as a Carbon Feedstock in Steel Industry: Iron Oxide Reduction, Steel Carburizing and Slag Foaming. J. Clean. Prod. 2019, 219, 971–980. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Preethi; Kavitha, S.; Tyagi, V.K.; Gunasekaran, M.; Karthikeyan, O.P.; Kumar, G. Lignocellulosic Biomass Based Biorefinery: A Successful Platform towards Circular Bioeconomy. Fuel 2021, 302, 121086. [Google Scholar] [CrossRef]
- Ojha, D.K.; Viju, D.; Vinu, R. Fast Pyrolysis Kinetics of Lignocellulosic Biomass of Varying Compositions. Energy Convers. Manag. X 2021, 10, 100071. [Google Scholar] [CrossRef]
- Poskart, A.; Skrzyniarz, M.; Sajdak, M.; Zajemska, M.; Skibiński, A. Management of Lignocellulosic Waste towards Energy Recovery by Pyrolysis in the Framework of Circular Economy Strategy. Energies 2021, 14, 5864. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Li, S.; Wang, X.; Lin, R. Comparative Study on the Two-Step Pyrolysis of Different Lignocellulosic Biomass: Effects of Components. J. Anal. Appl. Pyrolysis 2020, 152, 104966. [Google Scholar] [CrossRef]
- Kapoor, R.; Ghosh, P.; Kumar, M.; Sengupta, S.; Gupta, A.; Kumar, S.S.; Vijay, V.; Kumar, V.; Kumar Vijay, V.; Pant, D. Valorization of Agricultural Waste for Biogas Based Circular Economy in India: A Research Outlook. Bioresour. Technol. 2020, 304, 123036. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.W.; Lee, S.M.; Jae, J.; Park, Y.K. Overview of the Recent Advances in Lignocellulose Liquefaction for Producing Biofuels, Bio-Based Materials and Chemicals. Bioresour. Technol. 2019, 279, 373–384. [Google Scholar] [CrossRef]
- Uchman, W.; Skorek-Osikowska, A.; Werle, S. Evaluation of the Potential of the Production of Electricity and Heat Using Energy Crops with Phytoremediation Features. Appl. Therm. Eng. 2017, 126, 194–203. [Google Scholar] [CrossRef]
- Mellin, P.; Wei, W.; Yang, W.; Salman, H.; Hultgren, A. Biomass Availability in Sweden for Use in Blast Furnaces. Energy Procedia 2014, 61, 1352–1355. [Google Scholar] [CrossRef]
- Jha, G.; Soren, S. Study on Applicability of Biomass in Iron Ore Sintering Process. Renew. Sustain. Energy Rev. 2017, 80, 399–407. [Google Scholar] [CrossRef]
- Moroń, W.; Rybak, W. NOx and SO2 Emissions of Coals, Biomass and Their Blends under Different Oxy-Fuel Atmospheres. Atmos. Environ. 2015, 116, 65–71. [Google Scholar] [CrossRef]
- Mahanta, B.; Saikia, A.; Gupta, U.N.; Saikia, P.; Saikia, B.K.; Jayaramudu, J.; Sellamuthu, P.S.; Sadiku, E.R. Study of Low-Rank High Sulfur Coal Fine with Biomass. Curr. Res. Green Sustain. Chem. 2020, 3, 100023. [Google Scholar] [CrossRef]
- Dubinin, Y.V.; Yazykov, N.A.; Yeletsky, P.M.; Tabakaev, R.B.; Belyanovskaya, A.I.; Yakovlev, V.A. Catalytic Co-Combustion of Biomass and Brown Coal in a Fluidized Bed: Economic and Environmental Benefits. J. Environ. Sci. 2023; in press. [Google Scholar] [CrossRef]
- Foong, S.Y.; Chan, Y.H.; Chin, B.L.F.; Lock, S.S.M.; Yee, C.Y.; Yiin, C.L.; Peng, W.; Lam, S.S. Production of Biochar from Rice Straw and Its Application for Wastewater Remediation—An Overview. Bioresour. Technol. 2022, 360, 127588. [Google Scholar] [CrossRef] [PubMed]
- Albergaria Campos, A.M.; Khozhanov, N.; Assis, P.S.; Tursunbaev, K.; Masatbayev, M. Economic and Environmental Analyses of Biomass Torrefaction for Injection as Pulverized Material in Blast Furnaces. REM—Int. Eng. J. 2021, 74, 471–482. [Google Scholar] [CrossRef]
- Soh, M.; Khaerudini, D.S.; Yiin, C.L.; Chew, J.J.; Sunarso, J. Physicochemical and Structural Characterisation of Oil Palm Trunks (OPT) Hydrochar Made via Wet Torrefaction. Clean. Eng. Technol. 2022, 8, 100467. [Google Scholar] [CrossRef]
- Raishan Mohd Rashid, S.; Asma Fazli Abdul Samad, N.; Saleh, S. Upgrading Physicochemical Properties Using Torrefaction Process and Anhydrous Weight Loss Modelling for Palm Mesocarp Fiber. Mater. Today Proc. 2019, 19, 1703–1711. [Google Scholar] [CrossRef]
- Yahaya, A.Z.; Somalu, M.R.; Muchtar, A.; Sulaiman, S.A.; Wan Daud, W.R. Effect of Particle Size and Temperature on Gasification Performance of Coconut and Palm Kernel Shells in Downdraft Fixed-Bed Reactor. Energy 2019, 175, 931–940. [Google Scholar] [CrossRef]
- Solís, A.; Rocha, S.; König, M.; Adam, R.; Garcés, H.O.; Candia, O.; Muñoz, R.; Azócar, L. Preliminary Assessment of Hazelnut Shell Biomass as a Raw Material for Pellet Production. Fuel 2023, 333, 126517. [Google Scholar] [CrossRef]
- Kumar Mishra, R. Pyrolysis of Low-Value Waste Switchgrass: Physicochemical Characterization, Kinetic Investigation, and Online Characterization of Hot Pyrolysis Vapours. Bioresour. Technol. 2022, 347, 126720. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Baghel, P.; Anand, A.; Vijay, V.K.; Kaushal, P. A Comparative Study of Physical and Chemical Activation of Rice Straw Derived Biochar to Enhance Zn+2 Adsorption. Bioresour. Technol. Reports 2021, 15, 100774. [Google Scholar] [CrossRef]
- Variny, M.; Varga, A.; Rimár, M.; Janošovský, J.; Kizek, J.; Lukáč, L.; Jablonský, G.; Mierka, O. Advances in Biomass Co-Combustion with Fossil Fuels in the European Context: A Review. Processes 2021, 9, 100. [Google Scholar] [CrossRef]
- Kihedu, J. Torrefaction and Combustion of Ligno-Cellulosic Biomass. Energy Procedia 2015, 75, 162–167. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. A Review on Torrefied Biomass Pellets as a Sustainable Alternative to Coal in Power Generation. Renew. Sustain. Energy Rev. 2014, 40, 153–160. [Google Scholar] [CrossRef]
- Mei, Y.; Che, Q.; Yang, Q.; Draper, C.; Yang, H.; Zhang, S.; Chen, H. Torrefaction of Different Parts from a Corn Stalk and Its Effect on the Characterization of Products. Ind. Crops Prod. 2016, 92, 26–33. [Google Scholar] [CrossRef]
- Williams, P.T.; Besler, S. The Influence of Temperature and Heating Rate on the Slow Pyrolysis of Biomass. Renew. Energy 1996, 7, 233–250. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K. Mechanism of Waste Biomass Pyrolysis: Effect of Physical and Chemical Pre-Treatments. Sci. Total Environ. 2015, 537, 323–334. [Google Scholar] [CrossRef]
- Kuo, P.; Wu, W.; Chen, W. Gasification Performances of Raw and Torrefied Biomass in a Downdraft Fixed Bed Gasifier Using Thermodynamic Analysis. Fuel 2014, 117, 1231–1241. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, X. Recent Progress in the Direct Liquefaction of Typical Biomass. Prog. Energy Combust. Sci. 2015, 49, 59–80. [Google Scholar] [CrossRef]
- Singh, R.; Prakash, A.; Balagurumurthy, B.; Bhaskar, T. Hydrothermal Liquefaction of Biomass; Elsevier: Amsterdam, The Netherlands, 2015; pp. 269–291. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, E.; Lan, X. Influence of Pre-Treatment on Torrefaction of Phyllostachys Edulis. Bioresour. Technol. 2017, 239, 97–104. [Google Scholar] [CrossRef]
- Erlach, B.; Harder, B.; Tsatsaronis, G. Combined Hydrothermal Carbonization and Gasification of Biomass with Carbon Capture. Energy 2012, 45, 329–338. [Google Scholar] [CrossRef]
- Magdziarz, A. Hydrothermal Carbonization, Torrefaction and Slow Pyrolysis of Miscanthus Giganteus. Energy 2017, 140, 1292–1304. [Google Scholar] [CrossRef]
- Szwaja, S.; Magdziarz, A.; Zajemska, M.; Poskart, A.; Musiał, D. Virginia Mallow as an Energy Crop—Current Status and Energy Perspectives. In Proceedings of the SEED 2017: International Conference on the Sustainable Energy and Environment Development, Krakow, Poland, 14–17 November 2017; p. 224. [Google Scholar]
- Poskart, A.; Szwaja, S.; Zajemska, M.; Musial, D.; Magdziarz, A.; Kurtyka, M. Continuous Torrefaction of Virginia Mallow under Carbon Dioxide Atmosphere in a Screw Conveyor Reactor. In Proceedings of the European Biomass Conference and Exhibition Proceedings, Copenhagen, Denmark, 14–18 May 2018; Volume 2018. [Google Scholar]
- Szwaja, S.; Magdziarz, A.; Zajemska, M.; Poskart, A. A Torrefaction of Sida Hermaphrodita to Improve Fuel Properties. Advanced Analysis of Torrefied Products. Renew. Energy 2019, 141, 894–902. [Google Scholar] [CrossRef]
- Sajdak, M.; Muzyka, R.; Gałko, G.; Ksepko, E.; Zajemska, M.; Sobek, S.; Tercki, D. Actual Trends in the Usability of Biochar as a High-Value Product of Biomass Obtained through Pyrolysis. Energies 2023, 16, 355. [Google Scholar] [CrossRef]
- Szwaja, S.; Poskart, A.; Zajemska, M. A New Approach for Evaluating Biochar Quality from Virginia Mallow Biomass Thermal Processing. J. Clean. Prod. 2019, 214, 356–364. [Google Scholar] [CrossRef]
- Szwaja, S.; Poskart, A.; Szwaja, M.; Zajemska, M. Gasification of Sewage Sludge Enriched with Plant Biomass—Modeling and Tests. In Proceedings of the 2019 10th International Renewable Energy Congress, IREC 2019, Sousse, Tunisia, 26–28 March 2019. [Google Scholar]
- Szwaja, S.; Poskart, A.; Zajemska, M.; Szwaja, M.; Chwist, M. Co-Gasification of Sewage Sludge and Virginia Mallow. Przem. Chem. 2019, 98, 278–282. [Google Scholar] [CrossRef]
- Szwaja, S.; Poskart, A.; Zajemska, M.; Szwaja, M. Theoretical and Experimental Analysis on Co-Gasification of Sewage Sludge with Energetic Crops. Energies 2019, 12, 1750. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, Activation, and Applications of Biochar in Recent Times; Springer: Singapore, 2020; Volume 2, ISBN 0123456789. [Google Scholar]
- Ong, H.C.; Chen, W.H.; Singh, Y.; Gan, Y.Y.; Chen, C.Y.; Show, P.L. A State-of-the-Art Review on Thermochemical Conversion of Biomass for Biofuel Production: A TG-FTIR Approach. Energy Convers. Manag. 2020, 209, 112634. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Radziemska, E.; Ryms, M.; Ostrowski, P. Modern Methods of Thermochemical Biomass Conversion into Gas, Liquid and Solid Fuels. Ecol. Chem. Eng. S 2011, 18, 39–47. [Google Scholar]
- Batista, R.M.; Converti, A.; Pappalardo, J.; Benachour, M.; Sarubbo, L.A. Tools for Optimization of Biomass-to-Energy Conversion Processes. Processes 2023, 11, 854. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Conversion of Biomass to Biofuels and Life Cycle Assessment: A Review. Environ. Chem. Lett. 2021, 19, 4075–4118. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Liu, L.; Yu, H. Application of Co-Pyrolysis Biochar for the Adsorption and Immobilization of Heavy Metals in Contaminated Environmental Substrates. J. Hazard. Mater. 2021, 420, 126655. [Google Scholar] [CrossRef]
- Shi, X.; Ronsse, F.; Pieters, J.G. Finite Element Modeling of Intraparticle Heterogeneous Tar Conversion during Pyrolysis of Woody Biomass Particles. Fuel Process. Technol. 2016, 148, 302–316. [Google Scholar] [CrossRef]
- Cai, N.; Zhang, H.; Nie, J.; Deng, Y.; Baeyens, J. Biochar from Biomass Slow Pyrolysis. IOP Conf. Ser. Earth Environ. Sci. 2020, 586, 012001. [Google Scholar] [CrossRef]
- Garlapalli, R.K.; Wirth, B.; Reza, M.T. Pyrolysis of Hydrochar from Digestate: Effect of Hydrothermal Carbonization and Pyrolysis Temperatures on Pyrochar Formation. Bioresour. Technol. 2016, 220, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Hakala, J.; Kangas, P.; Penttilä, K.; Alarotu, M.; Björnström, M.; Koukkari, P. Replacing Coal Used in Steelmaking with Biocarbon from Forest Industry Side Streams; JULKAISIJA: Melbourne, Australia, 2019. [Google Scholar]
- Kruse, A.; Dahmen, N. Water—A Magic Solvent for Biomass Conversion. J. Supercrit. Fluids 2015, 96, 36–45. [Google Scholar] [CrossRef]
- Garcia-nunez, J.A.; Ramirez-contreras, N.E.; Tatiana, D.; Silva-lora, E.; Stuart, C.; Stockle, C.; Garcia-perez, M. Evolution of Palm Oil Mills into Bio-Refineries: Literature Review on Current and Potential Uses of Residual Biomass and Effluents. Resour. Conserv. Recycl. 2016, 110, 99–114. [Google Scholar] [CrossRef]
- Klemetsrud, B.; Ukaew, S.; Thompson, V.S.; Thompson, D.N.; Klinger, J.; Li, L.; Eatherton, D.; Puengprasert, P.; Shonnard, D. Characterization of Products from Fast Micropyrolysis of Municipal Solid Waste Biomass. ACS Sustain. Chem. Eng. 2016, 4, 5415–5423. [Google Scholar] [CrossRef]
- Valin, S.; Cances, J.; Castelli, P.; Thiery, S.; Dufour, A.; Boissonnet, G.; Spindler, B. Upgrading Biomass Pyrolysis Gas by Conversion of Methane at High Temperature: Experiments and Modelling. Fuel 2009, 88, 834–842. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Umeki, K.; Mousa, E.; Hedayati, A.; Romar, H.; Kemppainen, A.; Wang, C.; Phounglamcheik, A.; Tuomikoski, S.; Norberg, N.; et al. Use of Biomass in Integrated Steelmaking—Status Quo, Future Needs and Comparison to Other Low-CO2 Steel Production Technologies. Appl. Energy 2018, 213, 384–407. [Google Scholar] [CrossRef]
- Singh, L.; Kalia, V.C. Waste Biomass Management—A Holistic Approach; Springer: Cham, Switzerland, 2017; ISBN 9783319495958. [Google Scholar]
- Babich, A.; Arnsfeld, S.; Kowitwarangkul, P.; Senk, D. Biomass Use in Ironmaking: Options and Limits. In Proceedings of the 6th Int. Congr. Sci. Technol. Ironmak. 2012, ICSTI 2012—Incl. Proc. from 42nd Ironmak. Raw Mater. Semin. 13th Brazilian Symp. Iron Ore, Rio de Janeiro, Brazil, 14–18 October 2012; Volume 2, pp. 1166–1178. [Google Scholar]
- Chen, W. Chapter 10—Torrefaction. In Pretreatment of Biomass. Processes and Technologies; Elsevier B.V.: Amsterdam, The Netherlands, 2014; ISBN 9780128000809. [Google Scholar]
- Pauline, A.L.; Joseph, K. Hydrothermal Carbonization of Organic Wastes to Carbonaceous Solid Fuel—A Review of Mechanisms and Process Parameters. Fuel 2020, 279, 118472. [Google Scholar] [CrossRef]
- Dastjerdi, B.; Strezov, V.; Rajaeifar, M.A.; Kumar, R.; Behnia, M. A Systematic Review on Life Cycle Assessment of Different Waste to Energy Valorization Technologies. J. Clean. Prod. 2021, 290, 125747. [Google Scholar] [CrossRef]
- Lee, Y.; Eum, P.R.B.; Ryu, C.; Park, Y.K.; Jung, J.H.; Hyun, S. Characteristics of Biochar Produced from Slow Pyrolysis of Geodae-Uksae 1. Bioresour. Technol. 2013, 130, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Makepa, D.C.; Chihobo, C.H.; Ruziwa, W.R.; Musademba, D. A Systematic Review of the Techno-Economic Assessment and Biomass Supply Chain Uncertainties of Biofuels Production from Fast Pyrolysis of Lignocellulosic Biomass. Fuel Commun. 2023, 14, 100086. [Google Scholar] [CrossRef]
- Yue, Y.; Singh, H.; Singh, B.; Mani, S. Torrefaction of Sorghum Biomass to Improve Fuel Properties. Bioresour. Technol. 2017, 232, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, H.; Yang, Q.; Hao, H.; Zhu, B.; Chen, H. Torrefaction of Agriculture Straws and Its Application on Biomass Pyrolysis Poly-Generation. Bioresour. Technol. 2014, 156, 70–77. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Zhang, L.; Wang, Z.; Zhao, Z.; Zhu, W.; Ma, J.; Shen, B. Effects of Torrefaction Pretreatment on the Structural Features and Combustion Characteristics of Biomass-Based Fuel. Molecules 2023, 28, 4732. [Google Scholar] [CrossRef]
- Puente-urbina, A.; Gait, J.; Moya, R.; Rodríguez-zú, A.; Rica, D.C.; Forestal, E.D.I.; Cartago, P.O.B.; Rica, C.; Rica, C. Study of Light, Middle and Severe Torrefaction and Effects of Extractives and Chemical Compositions on Torrefaction Process by Thermogravimetric Analysis in Five Fast-Growing Plantations of Costa. Energy 2018, 149, 1–10. [Google Scholar] [CrossRef]
- Chen, W.H.; Kuo, P.C. Torrefaction and Co-Torrefaction Characterization of Hemicellulose, Cellulose and Lignin as Well as Torrefaction of Some Basic Constituents in Biomass. Energy 2011, 36, 803–811. [Google Scholar] [CrossRef]
- Lynam, J.G.; Coronella, C.J.; Yan, W.; Reza, M.T.; Vasquez, V.R. Acetic Acid and Lithium Chloride Effects on Hydrothermal Carbonization of Lignocellulosic Biomass. Bioresour. Technol. 2011, 102, 6192–6199. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Perez, S.; Sheng, K. Upgrading Fuel Quality of Moso Bamboo via Low Temperature Thermochemical Treatments: Dry Torrefaction and Hydrothermal Carbonization. Fuel 2017, 196, 473–480. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and Characterization of Slow Pyrolysis Biochar: Influence of Feedstock Type and Pyrolysis Conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Phan, A.N.; Ryu, C.; Sharifi, V.N.; Swithenbank, J. Characterisation of Slow Pyrolysis Products from Segregated Wastes for Energy Production. J. Anal. Appl. Pyrolysis 2008, 81, 65–71. [Google Scholar] [CrossRef]
- Wijayanti, W.; Tanoue, K.I. Char Formation and Gas Products of Woody Biomass Pyrolysis. Energy Procedia 2013, 32, 145–152. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Sánchez, M.E.; Mora, M.; Barrón, V. Slow Pyrolysis of Relevant Biomasses in the Mediterranean Basin. Part 2. Char Characterisation for Carbon Sequestration and Agricultural Uses. J. Clean. Prod. 2016, 120, 191–197. [Google Scholar] [CrossRef]
- Nunez Manzano, M.; Gonzalez Quiroga, A.; Perreault, P.; Madanikashani, S.; Vandewalle, L.A.; Marin, G.B.; Heynderickx, G.J.; Van Geem, K.M. Biomass Fast Pyrolysis in an Innovative Gas-Solid Vortex Reactor: Experimental Proof of Concept. J. Anal. Appl. Pyrolysis 2021, 156, 105165. [Google Scholar] [CrossRef]
- Louwes, A.C.; Basile, L.; Yukananto, R.; Bhagwandas, J.C.; Bramer, E.A.; Brem, G. Torrefied Biomass as Feed for Fast Pyrolysis: An Experimental Study and Chain Analysis. Biomass Bioenergy 2017, 105, 116–126. [Google Scholar] [CrossRef]
- Garcia-Perez, M.; Shen, J.; Wang, X.S.; Li, C.Z. Production and Fuel Properties of Fast Pyrolysis Oil/Bio-Diesel Blends. Fuel Process. Technol. 2010, 91, 296–305. [Google Scholar] [CrossRef]
- Rollinson, A.N.; Williams, O. Experiments on Torrefied Wood Pellet: Study by Gasification and Characterization for Waste Biomass to Energy Applications. R. Soc. Open Sci. 2016, 3, 150578. [Google Scholar] [CrossRef]
- Smoliński, A.; Howaniec, N. Hydrogen Production in the Process of Steam Gasification of Biomass. Res. Rep. Min. Environ. 2008, 3, 67–78. [Google Scholar]
- Krička, T.; Matin, A.; Bilandžija, N.; Jurišić, V.; Antonović, A.; Voća, N.; Grubor, M. Biomass Valorisation of Arundo donax L., Miscanthus × Giganteus and Sida Hermaphrodita for Biofuel Production. Int. Agrophys. 2017, 31, 575–581. [Google Scholar] [CrossRef]
- Grigiante, M.; Antolini, D. Mass Yield as Guide Parameter of the Torrefaction Process. An Experimental Study of the Solid Fuel Properties Referred to Two Types of Biomass. Fuel 2015, 153, 499–509. [Google Scholar] [CrossRef]
- Jensen, P.A.; Trinh, T.N. Production of Pyrolysis Oil Based on Different Biomass Types. Available online: https://www.teknologisk.dk/_/media/52115_Energy%20production%20from%20marine%20biomass%20%28Ulva%20lactuca%29_annex%203.pdf (accessed on 6 June 2023).
- Khanna, R.; Li, K.; Wang, Z.; Sun, M.; Zhang, J.; Mukherjee, P.S. Biochars in Iron and Steel Industries; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 2017, ISBN 9780128148945. [Google Scholar]
- Bazaluk, O.; Kieush, L.; Koveria, A.; Schenk, J.; Pfeiffer, A.; Zheng, H.; Lozynskyi, V. Metallurgical Coke Production with Biomass Additives: Study of Biocoke Properties for Blast Furnace and Submerged Arc Furnace Purposes. Materials 2022, 15, 1147. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, J.G.; Somerville, M.A.; Deev, A.; Jahanshahi, S. Utilization of Biomass as an Alternative Fuel in Ironmaking; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9781782421597. [Google Scholar]
- Chen, W.H.; Hsu, H.J.; Kumar, G.; Budzianowski, W.M.; Ong, H.C. Predictions of Biochar Production and Torrefaction Performance from Sugarcane Bagasse Using Interpolation and Regression Analysis. Bioresour. Technol. 2017, 246, 12–19. [Google Scholar] [CrossRef]
- Arteaga-Pérez, L.E.; Segura, C.; Espinoza, D.; Radovic, L.R.; Jiménez, R. Torrefaction of Pinus Radiata and Eucalyptus Globulus: A Combined Experimental and Modeling Approach to Process Synthesis. Energy Sustain. Dev. 2015, 29, 13–23. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Chen, W.H.; Kamyab, H.; Kumar, G.; Al-Muhtaseb, A.H.; Ngamcharussrivichai, C. Cultivation of Microalgae Chlorella Sp. in Municipal Sewage for Biofuel Production and Utilization of Biochar Derived from Residue for the Conversion of Hematite Iron Ore (Fe2O3) to Iron (Fe)—Integrated Algal Biorefinery. Energy 2019, 189, 116128. [Google Scholar] [CrossRef]
- Feliciano-Bruzual, C. Charcoal Injection in Blast Furnaces (Bio-PCI): CO2 Reduction Potential and Economic Prospects. J. Mater. Res. Technol. 2014, 3, 233–243. [Google Scholar] [CrossRef]
- Narzari, R.; Bordoloi, N.; Chutia, R.S.; Borkotoki, B. Chapter 2-Biochar: An Overview on Its Production, Properties and Potential Benefits. Biol. Biotechnol. Sustain. Dev. 2015, 1, 13–40. [Google Scholar] [CrossRef]
- Mac Nulty, H. An Introduction to Energy Management Systems: Energy Savings and Increased Industrial Productivity for the Iron and Steel Sector; 2014; Volume 14. Available online: https://www.oecd.org/sti/ind/DSTI-SU-SC(2014)14-FINAL-ENG.pdf (accessed on 2 June 2023).
- Medarac, H.; Moya Rivera, J.A.; Somers, J. Production Costs from Iron and Steel Industry in the EU and Third Countries; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Shannon Bouton; Creyts, J.; Kiely, T.; Livingston, J.; Nauclér, T. Energy Efficiency: A Compelling Global Resource; McKinsey Sustainability & Resource Productivity: New York, NY, USA, 2010. [Google Scholar]
- Danish Energy Agency. Energy Policy Toolkit on Energy Efficiency in Industries—Experiences from Denmark; 2016. Available online: https://ens.dk/sites/ens.dk/files/Globalcooperation/ee_in_industries_toolkit.pdf (accessed on 4 June 2023).
- Mac Nulty, H. Energy Efficiency in the Steel Sector: Why It Works Well, but Not Always; 2015. Available online: https://www.oecd.org/sti/ind/Energy-efficiency-steel-sector-1.pdf (accessed on 5 June 2023).
- Rojas-Cardenas, J.C.; Hasanbeigi, A.; Sheinbaum-Pardo, C.; Price, L. Energy Efficiency in the Mexican Iron and Steel Industry from an International Perspective. J. Clean. Prod. 2017, 158, 335–348. [Google Scholar] [CrossRef]
- Johansson, M.T. Improved Energy Efficiency within the Swedish Steel Industry—The Importance of Energy Management and Networking. Energy Effic. 2015, 8, 713–744. [Google Scholar] [CrossRef]
- Grewal, G.S.; Rajpurohit, B.S. Efficient Energy Management Measures in Steel Industry for Economic Utilization. Energy Rep. 2016, 2, 267–273. [Google Scholar] [CrossRef]
- Quader, M.A.; Ahmed, S.; Ghazilla, R.A.R.; Ahmed, S.; Dahari, M. A Comprehensive Review on Energy Efficient CO2 Breakthrough Technologies for Sustainable Green Iron and Steel Manufacturing. Renew. Sustain. Energy Rev. 2015, 50, 594–614. [Google Scholar] [CrossRef]
- Pardo, N.; Moya Rivera, J.A.; Vatopoulos, K. Prospective Scenarios on Energy Efficiency and CO; Publications Office of the European Union: Luxembourg, 2013; ISBN 9789279541919. [Google Scholar]
- ABB Motion. Energy Efficiency in Iron and Steel Making. Available online: https://www.energyefficiencymovement.com/wp-content/uploads/2022/04/ABB_EE_WhitePaper_Metals_250422.pdf (accessed on 3 June 2023).
- Mathieson, J.G.; Rogers, H.; Somerville, M.A.; Jahanshahi, S.; Ridgeway, P. Potential for the Use of Biomass in the Iron and Steel Industry. In Proceedings of the Chemeca 2011: Engineering a Better World, Sydney, Australia, 18–21 September 2011. [Google Scholar]
- Mathieson, J.; Rogers, H.; Somerville, M.; Ridgeway, P.; Jahanshahi, S. Use of Biomass in the Iron and Steel Industry—An Australian Perspective. In Proceedings of the 1st International Conference on Energy Efficiency and CO2 Reduction in the Steel Industry (EECR Steel 2011)–incorporated in METEC InSteelCon 1st Int. Conf. Energy Effic. CO2 Reduct. Steel Ind. (EECR Steel 2011)—Inc. METEC InSteelCon 2011, Dusseldorf, Germany, 27 June–1 July 2011; pp. 1–10. [Google Scholar]
- De Castro, J.A.; Araújo, G.D.M.; Da Mota, I.D.O.; Sasaki, Y.; Yagi, J.I. Analysis of the Combined Injection of Pulverized Coal and Charcoal into Large Blast Furnaces. J. Mater. Res. Technol. 2013, 2, 308–314. [Google Scholar] [CrossRef]
- World Steel Association. Biomass in Steelmaking; 2021. Available online: https://worldsteel.org/wp-content/uploads/Biomass-in-steelmaking.pdf (accessed on 28 May 2023).
- Pinto, R.G.D.; Szklo, A.S.; Rathmann, R. CO2 Emissions Mitigation Strategy in the Brazilian Iron and Steel Sector–From Structural to Intensity Effects. Energy Policy 2018, 114, 380–393. [Google Scholar] [CrossRef]
- Rousset, P.; Figueiredo, C.; De Souza, M.; Quirino, W. Pressure Effect on the Quality of Eucalyptus Wood Charcoal for the Steel Industry: A Statistical Analysis Approach. Fuel Process. Technol. 2011, 92, 1890–1897. [Google Scholar] [CrossRef]
- Purwanto, H.; Zakiyuddin, A.M.; Rozhan, A.N.; Mohamad, A.S.; Salleh, H.M. Effect of Charcoal Derived from Oil Palm Empty Fruit Bunch on the Sinter Characteristics of Low Grade Iron Ore. J. Clean. Prod. 2018, 200, 954–959. [Google Scholar] [CrossRef]
- Ng, K.W.; Giroux, L.; Todoschuk, T. Value-in-Use of Biocarbon Fuel for Direct Injection in Blast Furnace Ironmaking. Ironmak. Steelmak. 2018, 45, 406–411. [Google Scholar] [CrossRef]
- Fan, Z.; Friedmann, S.J. Low-Carbon Production of Iron and Steel: Technology Options, Economic Assessment, and Policy. Joule 2021, 5, 829–862. [Google Scholar] [CrossRef]
- Brodin, I.; Sjöholm, E.; Gellerstedt, G. The Behavior of Kraft Lignin during Thermal Treatment. J. Anal. Appl. Pyrolysis 2010, 87, 70–77. [Google Scholar] [CrossRef]
- Montiano, M.G.; Díaz-Faes, E.; Barriocanal, C.; Alvarez, R. Influence of Biomass on Metallurgical Coke Quality. Fuel 2014, 116, 175–182. [Google Scholar] [CrossRef]
- Gavel, D.J. A Review on Nut Coke Utilisation in the Ironmaking Blast Furnaces. Mater. Sci. Technol. 2017, 33, 381–387. [Google Scholar] [CrossRef]
- Wang, L.; Maziarka, P.; Skreiberg, O.; Løvås, T.; Wadrzyk, M.; Sevault, A. Study of CO2 Gasification Reactivity of Biocarbon Produced at Different Conditions. Energy Procedia 2017, 142, 991–996. [Google Scholar] [CrossRef]
- Koskela, A.; Heikkilä, A.; Bergna, D.; Salminen, J.; Fabritius, T. Effects of Briquetting and High Pyrolysis Temperature on Hydrolysis Lignin Char Properties and Reactivity in Co-Co2-N2 Conditions. Minerals 2021, 11, 187. [Google Scholar] [CrossRef]
- Wiklund, C.M.; Pettersson, F.; Saxén, H. Optimal Resource Allocation in Integrated Steelmaking with Biomass as Auxiliary Reductant in the Blast Furnace. ISIJ Int. 2012, 52, 35–44. [Google Scholar] [CrossRef]
- Helle, H.; Helle, M.; Saxén, H.; Frank, P. Mathematical Optimization of Ironmaking with Biomass as Auxiliary Reductant in the Blast Furnace. ISIJ Int. 2009, 49, 1316–1324. [Google Scholar] [CrossRef]
- Safarian, S. To What Extent Could Biochar Replace Coal and Coke in Steel Industries? Fuel 2023, 339, 127401. [Google Scholar] [CrossRef]
- Kumar, D.; Saxena, V.K.; Tiwari, H.P.; Nandi, B.K.; Verma, A.; Tiwary, V.K. Variability in Metallurgical Coke Reactivity Index (CRI) and Coke Strength after Reaction (CSR): An Experimental Study. ACS Omega 2022, 7, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Yustanti, E.; Wardhono, E.Y.; Mursito, A.T.; Alhamidi, A. Types and Composition of Biomass in Biocoke Synthesis with the Coal Types and Composition of Biomass in Biocoke Synthesis with the Coal Blending Method. Energies 2021, 14, 6570. [Google Scholar] [CrossRef]
- Gul, E.; Riva, L.; Nielsen, H.K.; Yang, H.; Zhou, H.; Yang, Q.; Skreiberg, Ø.; Wang, L.; Barbanera, M.; Zampilli, M.; et al. Substitution of Coke with Pelletized Biocarbon in the European and Chinese Steel Industries: An LCA Analysis. Appl. Energy 2021, 304, 117644. [Google Scholar] [CrossRef]
- Lèbre La Rovere, E.; Gesteira, C.; Grottera, C.; Wills, W. Pathways to Deep Decarbonization in Brazil BR 2015 Report; Sustainable Development Solutions Network (SDSN) and Institute for Sustainable Development and International Relations (IDDRI). 2015. Available online: https://ddpinitiative.org/publications-and/?_sfm_country=Brazil (accessed on 1 June 2023).
- Hebeda, O.; Guimarães, B.S.; Cretton-Souza, G.; La Rovere, E.L.; Pereira, A.O. Pathways for Deep Decarbonization of the Brazilian Iron and Steel Industry. J. Clean. Prod. 2023, 401, 136675. [Google Scholar] [CrossRef]
- ArcelorMittal. ArcelorMittal Climate Action Report; ArcelorMittal: Singapore, 2019. [Google Scholar]
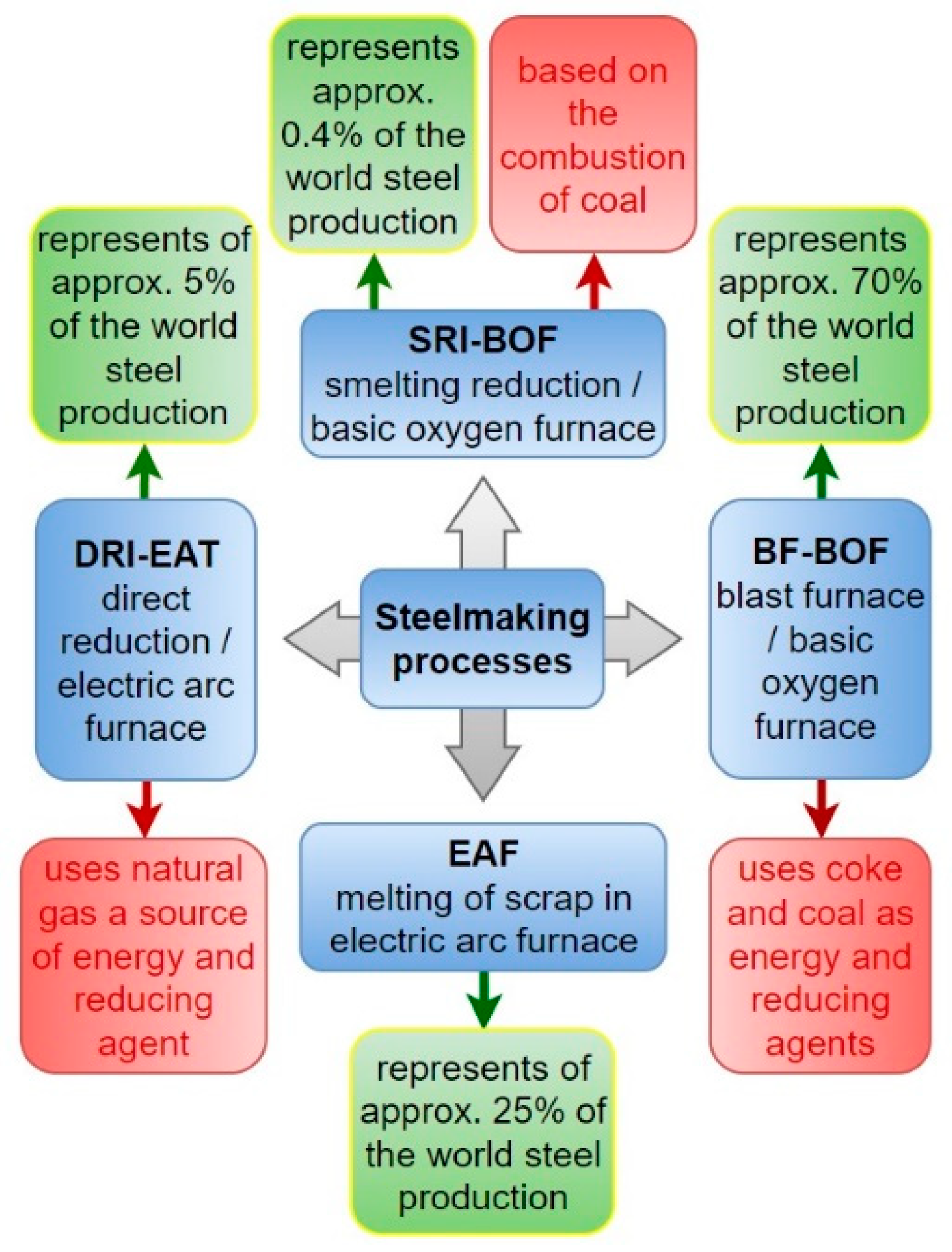
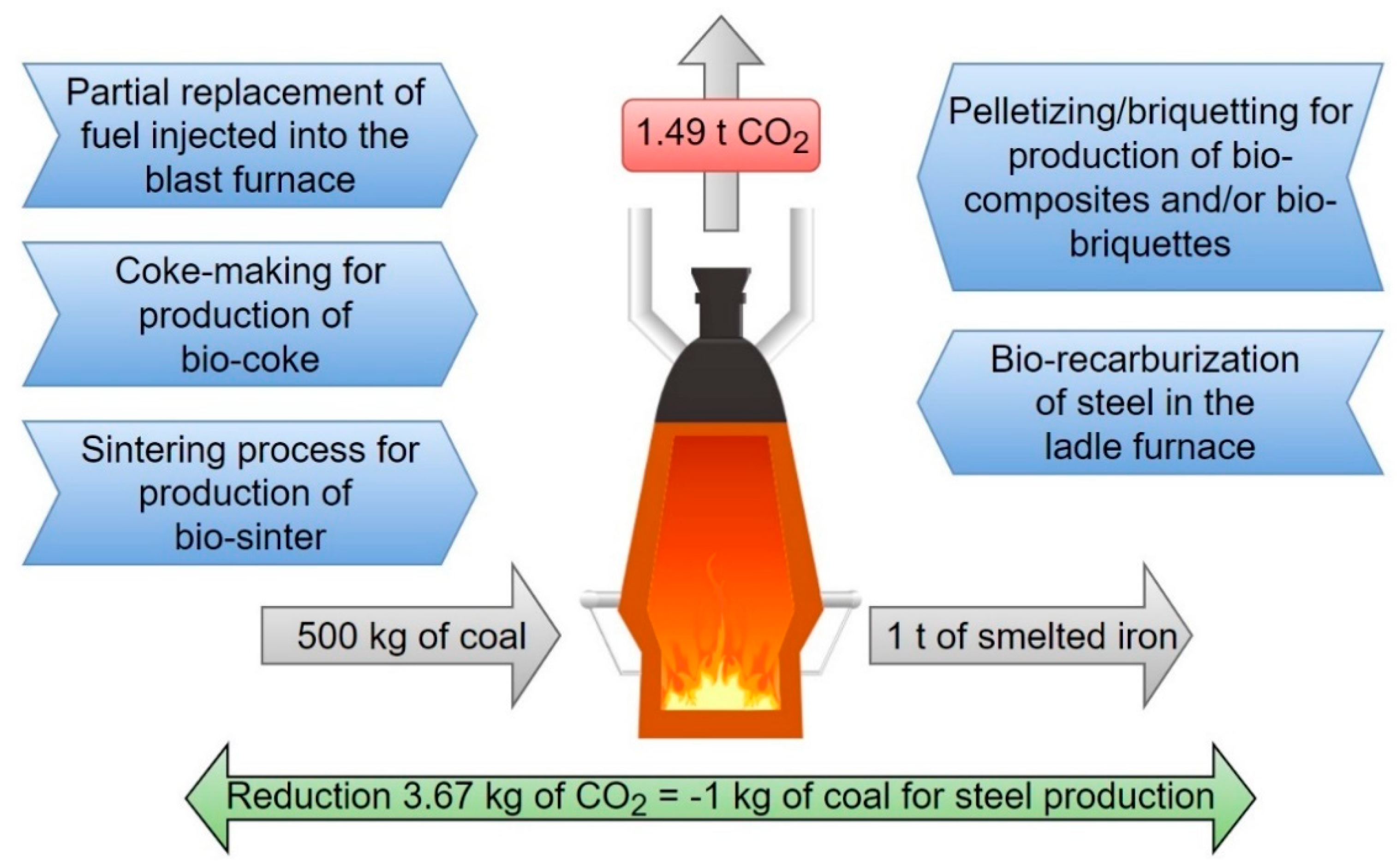
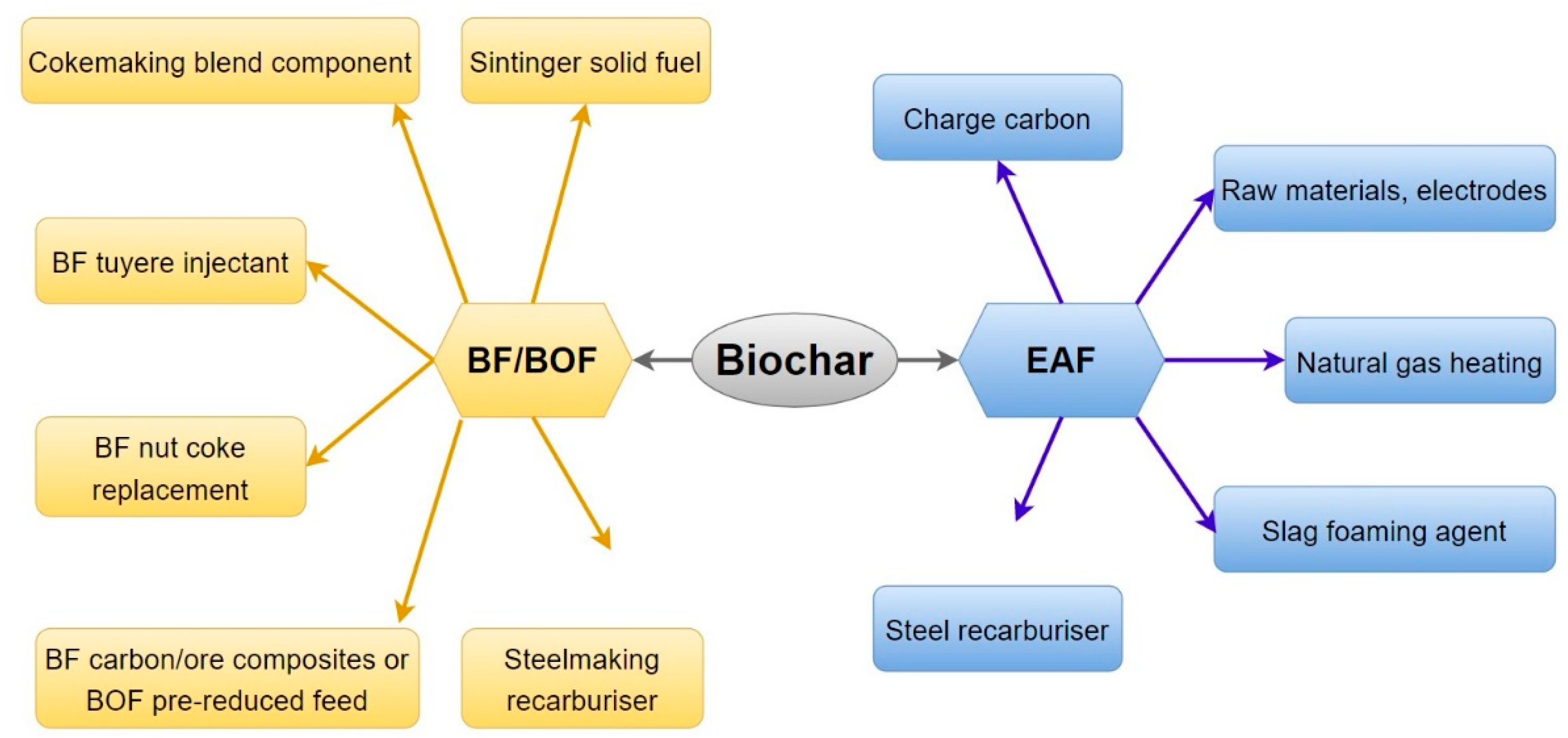
| Biomass | Ultimate Analysis (%) | Proximate Analysis (%) | Source | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | M 1 | VM 2 | FC 3 | A 4 | ||
| Rice straw | 36.2–47.46 | 5.2–6.44 | 0.7–0.83 | - | 40.3–45.15 | 4.98 | 81.54 | 16.46 | 10.82–17.6 | [30] |
| Wheat straw | 41.8–45.5 | 5.5–5.7 | 0.7–1.0 | - | 35.5–47.9 | 7.1 | 76.7 | 9.2 | 7.0 | [30] |
| Barley straw | 45.41 | 6.1 | 1.18 | - | 46.21 | 4.90 | 78.8 | 11.83 | 6.43 | [30] |
| Corn straw | 45.75 | 5.93 | 0.94 | 0.11 | 43.69 | 4.21 | - | - | 5.91 | [30] |
| Sugarcane straw | 41.88 | 5.87 | 0.47 | - | 41.72 | 3.12 | 87.61 | 3.22 | 9.17 | [30] |
| Rape straw | 42.21 | 5.54 | 0.42 | 0.07 | 51.76 | - | - | - | 3.69 | [30] |
| Mustard straw | 54.46 | 6.29 | 0.5 | - | 38.75 | 3.99 | 75.55 | 15.44 | 5.02 | [30] |
| Moringa husk | 48.84 | 6.53 | - | - | - | 1.47 | 76.6 | - | 2.36 | [31] |
| Eucalyptus husk | 50.1 | 5.42 | 5.77 | 68.73 | 2.43 | [31] | ||||
| Sugarcane bagasse | 46.4 | 4.68 | - | - | - | 7.03 | 75.03 | - | 4.33 | [31] |
| Elephant grass | 40.0 | 5.36 | - | - | - | 0.1 | 69.95 | - | 13.5 | [31] |
| Rice husk | 43.4 | 4.33 | - | - | - | 0.1 | 73.18 | - | 9.55 | [31] |
| Corn cob | 45.5 | 6.7 | - | - | - | 0.79 | 81.31 | - | 1.16 | [31] |
| Corn straw | 44.8 | 6.8 | - | - | - | 0.31 | 81.68 | - | 1.58 | [31] |
| Oil palm tree trunk | 40.75 | 6.47 | 0.51 | - | - | - | - | - | - | [32] |
| Mesocarp fibers | 45.2 | 9.04 | 3.12 | 0.1 | 42.53 | - | 75.23 | 18.42 | 5.35 | [33] |
| Palm kernel shells | 46.3 | 5.72 | 0.7 | 0.64 | 47.6 | 9.4 | 69.8 | 16.8 | 4.0 | [34] |
| Hazelnut shells | 50.3 | 6.3 | 0.7 | - | 43.2 | 9.0 | 76.7 | 22.5 | 0.8 | [35] |
| Switch grass | 43.2 | 5.89 | 0.52 | 0.16 | 50.23 | 6.25 | 71.21 | 19.14 | 3.4 | [36] |
| Rice straw | 37.18 | 5.81 | 0.62 | - | 56.39 | 9.8 | 76.32 | 9.08 | 13.91 | [37] |
| Coconut shell | 48.6 | 5.97 | 0.62 | 1.09 | 43.8 | 10.5 | 71.1 | 17.6 | 0.8 | [34] |
| Pine Sawdust | 49.5 | 7.1 | 0.5 | - | 42.8 | 5.0 | 84.5 | 15.4 | 0.1 | [35] |
| Process | Reaction Temperature °C | Conditions | Product | Composition of Product (m-%) | Source | ||
|---|---|---|---|---|---|---|---|
| Solid | Liquid | Gas | |||||
| Pyrolysis, slow | 180–480 | Residence time: 15 min to even several hours; absence of air (oxygen) | Biochar | >60 | 25–30 | 10–15 | [64,65,66,67] |
| Pyrolysis, fast | Above 500 | High heating rate [68]; for a few seconds; absence of air | Bio oil | 20–30 | 30–70 | 20–30 | [68,69,70,71] |
| Gasification | 600–1400 | With the use of oxidizing agent; under higher pressure (1–5 MPa) | Synthesis gas | 10 | 5 | 85 | [66,72,73] |
| Torrefaction | 200–350 | For 15–30 min without presence of air | Biochar | 80 | 15 | 5 | [52,74,75] |
| Hydrothermal Carbonization | 160–250 | Mixed with saturated water-steam; from a few minutes to even few hours | HTC Carbon | 50–80 | 5–20 | 2–5 | [66,72,76] |
| Combustion | Wide range depending on the fuel type | With air excess | Heat | - | - | - | [72] |
| Component | Proximate Analysis, wt.% | Ultimate Analysis, wt.% | Source | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M 1 | A 2 | VM 3 | FC 4 | S | C | H | N | O | P | |||
| Coal A | 1.5 | 11.0 | 30.3 | 58.7 | 0.68 | 75.38 | 4.77 | 1.54 | 6.63 | - | [100] | |
| Coal B | Subbituminous | 16 | 6.5 | 29.5 | - | 0.65 | - | - | - | - | 0.06 | [101] |
| Coal C | High-VM | 2.5 | 9.0 | 34.5 | - | 0.40 | - | - | - | - | 0.01 | [101] |
| Coal D | Semianthracite | 1.5 | 7.5 | 12.5 | - | 0.60 | - | - | - | - | 0.07 | [101] |
| Coal E | Anthracite | 2.0 | 9.0 | 6.5 | - | 0.50 | - | - | - | 0.01 | [101] | |
| Coal F | 1.4 | 11.0 | 33.6 | 55.4 | 0.82 | 74.49 | 5.09 | 1.61 | 6.99 | - | [100] | |
| Coal G | 3.8 | 8.4 | 23.2 | 68.4 | 0.32 | 82.61 | 4.78 | 1.43 | 2.46 | - | [100] | |
| Biochar A | Torrefied softwood | 2.0 | 0.5 | 69.1 | - | 0.02 | - | - | - | - | 0.01 | [101] |
| Biochar B | Semicharcoal (hardwood) | 0.7 | 2.3 | 33.1 | - | 0.05 | - | - | - | - | 0.07 | [101] |
| Biochar C | Charcoal (softwood) | 1.0 | 0.9 | 6.2 | - | 0.05 | - | - | - | - | 0.02 | [101] |
| Biochar D | Charcoal (hardwood) | 1.8 | 3.4 | 7.6 | - | 0.09 | - | - | - | - | 0.10 | [101] |
| Biochar E | Charcoal (mallee) | 1.6 | 3.4 | 0.3 | - | 0.04 | - | - | - | - | 0.0 | [101] |
| Biochar F | Biocoke | 0.65–1.35 | 5.8–10.8 | 1.4–2.7 | 87.8–92.4 | 0.22–0.2 | 86.38–91.65 | - | - | - | - | [4] |
| Biochar G | Charcoal | 0.63 | 7.73 | 25.8 | - | - | 69.7 | 3.2 | - | - | - | [31] |
| Biochar H | Torrefied sugarcane bagasse | 1.41 | 2.52 | 78.74 | 17.33 | - | 40.0 | 6.74 | 0.72 | 52.54 | - | [102] |
| Biochar I | Torrefied eucalyptus | - | - | - | - | - | 56.01 | 5.99 | 0.05 | 36.18 | - | [103] |
| Biochar J | Chlorella sp. | 2 | 12.8 | 32.4 | - | 2 | 35 | 9.2 | 3 | 2.5 | - | [104] |
| Biochar K | Sargassum sp. | 2.5 | 14.2 | 35 | - | 2.6 | 61.5 | 4.2 | 2 | 28 | - | [104] |
| Biochar L | 2.30 | 0.57 | 19.10 | 91.6 | 0.02 | 2.27 | 0.38 | 1.95 | - | [105] | ||
| Biochar M | Safflower seed cake | - | 8.20 | 20.00 | 7180 | - | 70.43 | 3.43 | 3.36 | 22.39 | - | [106] |
| Biochar N | Concarpus waste | - | 5.27 | - | - | - | 76.83 | 2.83 | 0.87 | 14.16 | - | [106] |
| Biochar O | Rice straw | 7.20 | 15.40 | 62.40 | 14.90 | - | 44.80 | 5.10 | 0.90 | 49.20 | - | [106] |
| Biochar P | Pitch pine | - | 7.90 | - | - | - | 70.70 | 3.40 | 0.60 | 25.50 | - | [106] |
| Biochar R | Pine sawdust | 5.00 | 0.30 | 77.70 | 16.90 | - | 50.30 | 6.70 | 0.20 | 42.70 | - | [106] |
| Biochar S | Spruce woodchips | - | 31.00 | - | - | - | 74.80 | 0.14 | 0.15 | 4.20 | - | [106] |
| Biochar T | Corn stovers | 2.3 | 58.00 | 12.70 | 28.70 | - | 33.20 | 1.40 | 0.81 | 8.60 | - | [106] |
| Biochar U | Coconut shell | 4.4 | 0.70 | 80.20 | 22.00 | - | 50.20 | 5.70 | 0.00 | 43.40 | - | [106] |
| Biochar W | Peanut shell | 1.90 | 7.80 | 8.10 | 82.20 | - | 93.61 | 1.99 | 1.05 | 3.35 | - | [106] |
| Biochar X | Pine cone | 1.20 | 4.70 | 6.70 | 87.40 | - | 95.16 | 2.63 | 1.61 | 0.60 | - | [106] |
| Biochar Y | Peanut hull | - | 9.30 | 18.10 | - | - | 81.80 | 2.90 | 2.70 | 3.30 | - | [106] |
| Biochar Z | Switch grass | - | 7.80 | 13.40 | - | - | 84.40 | 2.40 | 1.07 | 4.30 | - | [106] |
| Biochar AA | Pongamia glabra deoiled cake | 4.30 | 11.60 | 14.60 | 69.50 | - | 75.00 | 3.26 | 5.00 | 12.58 | - | [106] |
| Biochar AB | Jute dust | 9.44 | 10.78 | 15.07 | 64.71 | - | 70.25 | 2.78 | 4.04 | 22.93 | - | [106] |
| Biochar AC | Sugarcane bagasse | 1.30 | 8.57 | 9.17 | 80.97 | - | 85.59 | 2.82 | 1.11 | 10.48 | - | [106] |
| Biochar AD | Coco peat | 2.55 | 15.90 | 14.30 | 67.25 | - | 84.44 | 2.88 | 1.02 | 11.67 | - | [106] |
| Biochar AE | Palm kernel shell | 0.00 | 6.86 | 12.29 | 80.85 | - | 87.85 | 2.91 | 1.11 | 8.14 | - | [106] |
| Biochar AF | Cotton seed hull | 6.53 | 7.90 | 18.60 | 67.00 | - | 87.50 | 2.85 | 1.50 | 7.60 | - | [106] |
| Biochar AG | Soybean cake | 1.50 | 16.80 | 10.10 | 71.60 | - | 83.95 | 1.48 | 8.32 | 6.25 | - | [106] |
| Biochar AH | Sesame | 3.40 | 36.80 | 22.00 | 37.80 | - | 86.64 | 3.10 | 6.93 | 3.09 | - | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biniek-Poskart, A.; Sajdak, M.; Skrzyniarz, M.; Rzącki, J.; Skibiński, A.; Zajemska, M. The Application of Lignocellulosic Biomass Waste in the Iron and Steel Industry in the Context of Challenges Related to the Energy Crisis. Energies 2023, 16, 6662. https://doi.org/10.3390/en16186662
Biniek-Poskart A, Sajdak M, Skrzyniarz M, Rzącki J, Skibiński A, Zajemska M. The Application of Lignocellulosic Biomass Waste in the Iron and Steel Industry in the Context of Challenges Related to the Energy Crisis. Energies. 2023; 16(18):6662. https://doi.org/10.3390/en16186662
Chicago/Turabian StyleBiniek-Poskart, Anna, Marcin Sajdak, Magdalena Skrzyniarz, Jakub Rzącki, Andrzej Skibiński, and Monika Zajemska. 2023. "The Application of Lignocellulosic Biomass Waste in the Iron and Steel Industry in the Context of Challenges Related to the Energy Crisis" Energies 16, no. 18: 6662. https://doi.org/10.3390/en16186662
APA StyleBiniek-Poskart, A., Sajdak, M., Skrzyniarz, M., Rzącki, J., Skibiński, A., & Zajemska, M. (2023). The Application of Lignocellulosic Biomass Waste in the Iron and Steel Industry in the Context of Challenges Related to the Energy Crisis. Energies, 16(18), 6662. https://doi.org/10.3390/en16186662










