Recent Advancements in Pd-Based Membranes for Hydrogen Separation
Abstract
:1. Introduction
- Brown or black: produced by coal (lignite or bituminous coal) gasification;
- Grey: produced via steam reforming of methane;
- Blue: produced via steam reforming or gasification but with the adoption of Carbon Capture Utilization and Storage (CCUS) technologies;
- Turquoise: produced via methane pyrolisis;
- Yellow: produced via electrolysis using the electricity from the grid;
- Pink or red: produced via electrolysis or thermo-catalysis through the energy (electrical or thermal) derived from nuclear plants;
- Orange: produced by biomass [3];
- Green: produced using renewable sources [4].
2. Hydrogen Permeation through Membranes
- Diffusion of molecular hydrogen through the surface of the membrane;
- Dissociation of molecular hydrogen on the palladium surface;
- Dissolution of atomic hydrogen into the bulk metal;
- Diffusion of atomic hydrogen through the bulk metal;
- Association of atomic hydrogen on the palladium surface;
- Desorption of molecular hydrogen from the surface;
- Diffusion of molecular hydrogen from the surface [26].


3. Characteristics of Palladium and Its Alloy Membranes
- Adsorption on the Pd-based membrane surface;
- Dissociation of hydrogen sulfide:
3.1. Palladium Alloys Adopted in Membrane Fabrication
Ternary Alloys
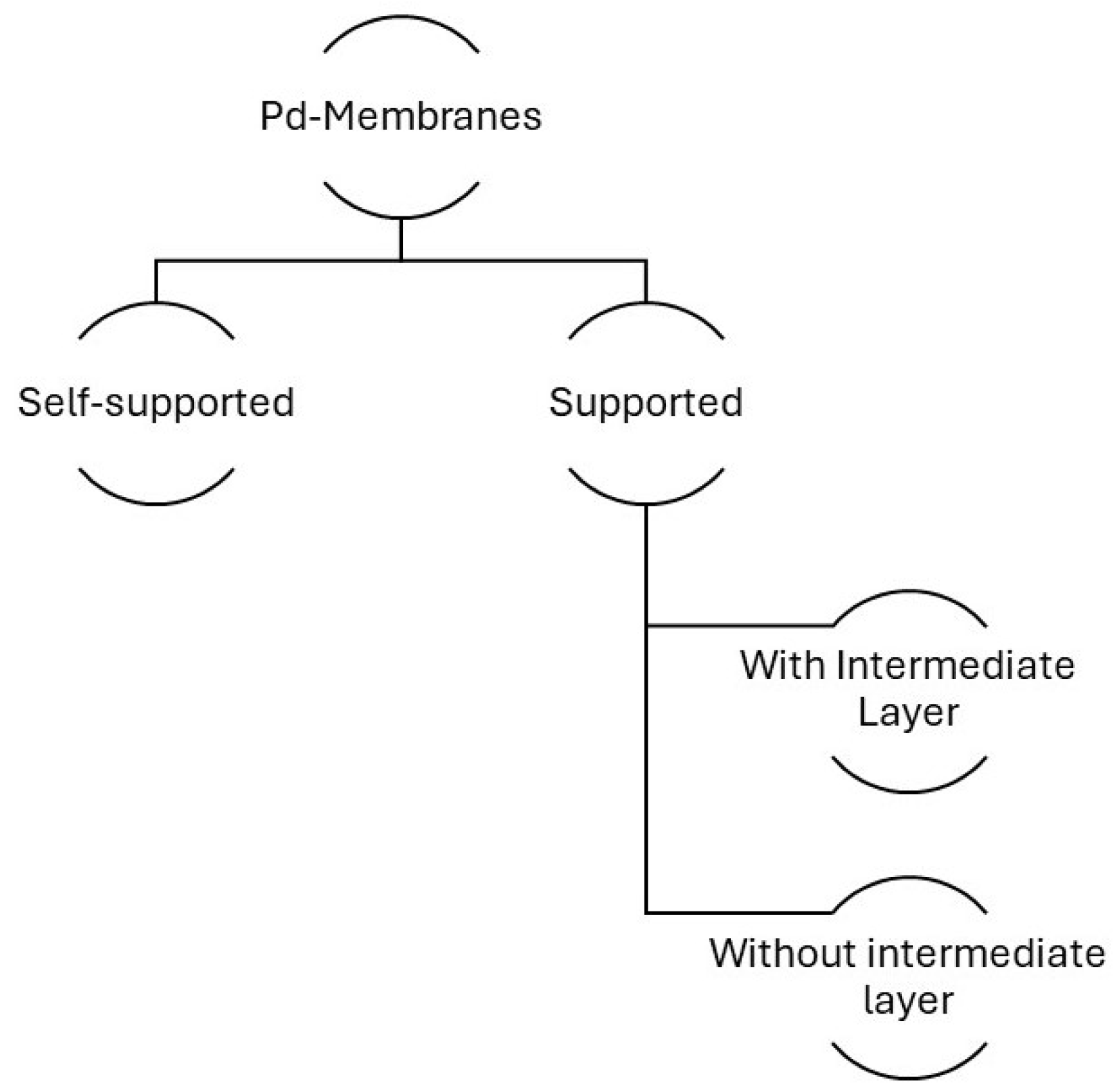
3.2. Membrane Types
3.2.1. Self-Supported Membranes
3.2.2. Supported Membranes
3.2.3. Intermediate Layers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WMO | World Meteorological Organization |
| GHG | Greenhouse Gas |
| CCUS | Carbon Capture Utilization and Storage |
| PEMFC | Proton Exchange Membrane Fuel Cell |
| PSA | Pressure Swing Adsorption |
| BCC | Body-Centered Cubic |
| FCC | Face-Centered Cubic |
| PSS | Porous Steel Support |
| ELP | Electroless Plating |
| ELP-PP | Electroless Pore-Plating |
| MS | Magnetron Sputtering |
References
- World Meterological Organization. WMO Confirms 2023 Smashes Global Temperature Record. 2023. Available online: https://wmo.int/news/media-centre/wmo-confirms-2023-smashes-global-temperature-record (accessed on 26 April 2024).
- Farias, C.B.B.; Barreiros, R.C.S.; da Silva, M.F.; Casazza, A.A.; Converti, A.; Sarubbo, L.A. Use of Hydrogen as Fuel: A Trend of the 21st Century. Energies 2022, 15, 311. [Google Scholar] [CrossRef]
- Osselin, F.; Soulaine, C.; Fauguerolles, C.; Gaucher, E.; Scaillet, B.; Pichavant, M. Orange hydrogen is the new green. Nat. Geosci. 2022, 15, 765–769. [Google Scholar] [CrossRef]
- Arcos, J.M.M.; Santos, D.M.F. The Hydrogen Color Spectrum: Techno-Economic Analysis of the Available Technologies for Hydrogen Production. Gases 2023, 3, 25–46. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, Y.; Williams, P.T.; Yang, H.; Chen, H. Co-production of hydrogen and carbon nanotubes from real-world waste plastics: Influence of catalyst composition and operational parameters. Appl. Catal. Environ. 2018, 221, 584–597. [Google Scholar] [CrossRef]
- IEA. Global Hydrogen Review 2023. 2023. Available online: https://www.iea.org/reports/global-hydrogen-review-2023 (accessed on 26 April 2024).
- Shchegolkov, A.V.; Shchegolkov, A.V.; Zemtsova, N.V.; Stanishevskiy, Y.M.; Vetcher, A.A. Recent Advantages on Waste Management in Hydrogen Industry. Polymers 2022, 14, 4992. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, R.; Wang, J.; Cheng, D.g.; Chen, F.; Xu, L.; Gao, M.; Kang, Y.; Eguchi, M.; Yamauchi, Y. Silica Confinement for Stable and Magnetic Co-Cu Alloy Nanoparticles in Nitrogen-Doped Carbon for Enhanced Hydrogen Evolution. Angew. Chem. Int. Ed. 2024, 63, e202404505. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, Z.; Xu, L.; Qin, H.; Dong, J.; Lv, Q.; Han, J.; Song, F. Ultrafine nickel-rhodium nanoparticles anchored on two-dimensional vanadium carbide for high performance hydrous hydrazine decomposition at mild conditions. J. Colloid Interface Sci. 2024, 669, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Reis, A.; Berry, K. Experimental evaluation of CO poisoning on the performance of a high temperature proton exchange membrane fuel cell. J. Power Sources 2009, 193, 691–698. [Google Scholar] [CrossRef]
- ISO 14687:2019; Hydrogen fuel Quality—Product Specification. International Standard: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/69539.html (accessed on 26 April 2024).
- Du, Z.; Liu, C.; Zhai, J.; Guo, X.; Xiong, Y.; Su, W.; He, G. A Review of Hydrogen Purification Technologies for Fuel Cell Vehicles. Catalysts 2021, 11, 393. [Google Scholar] [CrossRef]
- SIRCAR, S.; GOLDEN, T.C. Purification of Hydrogen by Pressure Swing Adsorption. Sep. Sci. Technol. 2000, 35, 667–687. [Google Scholar] [CrossRef]
- Bang, G.; Moon, D.K.; Kang, J.H.; Han, Y.J.; Kim, K.M.; Lee, C.H. High-purity hydrogen production via a water-gas-shift reaction in a palladium-copper catalytic membrane reactor integrated with pressure swing adsorption. Chem. Eng. J. 2021, 411, 128473. [Google Scholar] [CrossRef]
- Shaposhnik, V.A. Prospects of membrane catalysis in hydrogen energetics. Mini review. Condens. Matter Interphases 2024, 26, 37–44. [Google Scholar] [CrossRef]
- Stenina, I.; Yaroslavtsev, A. Modern Technologies of Hydrogen Production. Processes 2023, 11, 56. [Google Scholar] [CrossRef]
- Jokar, S.; Farokhnia, A.; Tavakolian, M.; Pejman, M.; Parvasi, P.; Javanmardi, J.; Zare, F.; Gonçalves, M.C.; Basile, A. The recent areas of applicability of palladium based membrane technologies for hydrogen production from methane and natural gas: A review. Int. J. Hydrogen Energy 2023, 48, 6451–6476. [Google Scholar] [CrossRef]
- Habib, M.A.; Haque, M.A.; Harale, A.; Paglieri, S.; Alrashed, F.S.; Al-Sayoud, A.; Nemitallah, M.A.; Hossain, S.; Abuelyamen, A.; Mokheimer, E.M.; et al. Palladium-alloy membrane reactors for fuel reforming and hydrogen production: Hydrogen Production Modeling. Case Stud. Therm. Eng. 2023, 49, 103359. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, C.Y. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- Suzuki, A.; Yukawa, H. A Review for Consistent Analysis of Hydrogen Permeability through Dense Metallic Membranes. Membranes 2020, 10, 120. [Google Scholar] [CrossRef]
- Bosko, M.L.; Dalla Fontana, A.; Tarditi, A.; Cornaglia, L. Advances in hydrogen selective membranes based on palladium ternary alloys. Int. J. Hydrogen Energy 2021, 46, 15572–15594. [Google Scholar] [CrossRef]
- Girotto, C.P.; Nippes, R.P.; Macruz, P.D.; Gomes, A.D.; de Souza, M.; Rodriguez, M.T. Effect of physicochemical properties on the performance of palladium-based composite membranes: A review. J. Mater. Res. 2023, 38, 4868–4891. [Google Scholar] [CrossRef]
- Shere, L.; Hill, A.K.; Mays, T.J.; Lawless, R.; Brown, R.; Perera, S.P. The next generation of low tritium hydrogen isotope separation technologies for future fusion power plants. Int. J. Hydrogen Energy 2024, 55, 319–338. [Google Scholar] [CrossRef]
- Graham, T. On the absorption and dialytic separation of gases by colloid septa. J. Frankl. Inst. 1867, 83, 39–41. [Google Scholar] [CrossRef]
- Al-Mufachi, N.; Rees, N.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sustain. Energy Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Yun, S.; Ted Oyama, S. Correlations in palladium membranes for hydrogen separation: A review. J. Membr. Sci. 2011, 375, 28–45. [Google Scholar] [CrossRef]
- Rahimpour, M.; Samimi, F.; Babapoor, A.; Tohidian, T.; Mohebi, S. Palladium membranes applications in reaction systems for hydrogen separation and purification: A review. Chem. Eng. Process. Process. Intensif. 2017, 121, 24–49. [Google Scholar] [CrossRef]
- Morreale, B.D. The Influence of H2S on Palladium and Palladium-Copper Alloy Membranes. Ph.D. Thesis, University of Pittsburgh, Pittsburgh, PA, USA, 2007. [Google Scholar]
- Dolan, M. Non-Pd BCC alloy membranes for industrial hydrogen separation. J. Membr. Sci. 2010, 362, 12–28. [Google Scholar] [CrossRef]
- Cardoso, S.P.; Azenha, I.S.; Lin, Z.; Portugal, I.; Rodrigues, A.E.; Silva, C.M. Inorganic Membranes for Hydrogen Separation. Sep. Purif. Rev. 2018, 47, 229–266. [Google Scholar] [CrossRef]
- Easa, J.; Yan, C.; Schneider, W.F.; O’Brien, C.P. CO and C3H6 poisoning of hydrogen permeation across Pd77Ag23 alloy membranes: A comparative study with pure palladium. Chem. Eng. J. 2022, 430, 133080. [Google Scholar] [CrossRef]
- Gabitto, J.; Tsouris, C. Modeling sulfur poisoning of palladium membranes used for hydrogen separation. Int. J. Chem. Eng. 2019, 2019, 9825280. [Google Scholar] [CrossRef]
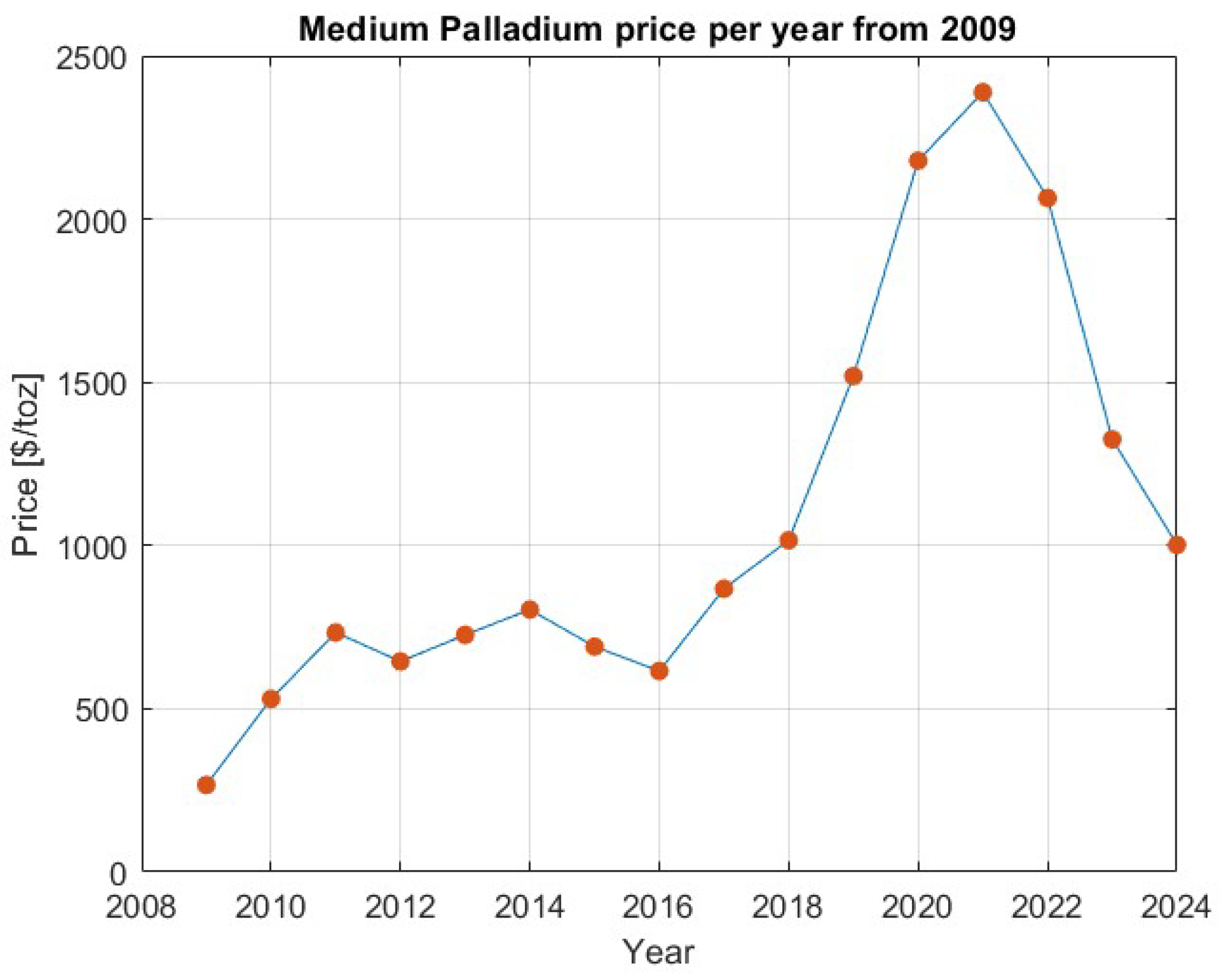
- Macrotrends. 2024. Available online: https://www.macrotrends.net/2542/palladium-prices-historical-chart-data (accessed on 26 April 2024).
- Peters, T.; Carvalho, P.; Stange, M.; Bredesen, R. Formation of hydrogen bubbles in Pd-Ag membranes during H2 permeation. Int. J. Hydrogen Energy 2020, 45, 7488–7496. [Google Scholar] [CrossRef]
- Peters, T.; Kaleta, T.; Stange, M.; Bredesen, R. Hydrogen transport through a selection of thin Pd-alloy membranes: Membrane stability, H2S inhibition, and flux recovery in hydrogen and simulated WGS mixtures. Catal. Today 2012, 193, 8–19. [Google Scholar] [CrossRef]
- Pati, S.; Ashok, J.; Dewangan, N.; Chen, T.; Kawi, S. Ultra-thin (1 μm) Pd-Cu membrane reactor for coupling CO2 hydrogenation and propane dehydrogenation applications. J. Membr. Sci. 2020, 595, 117496. [Google Scholar] [CrossRef]
- Jia, H.; Wu, P.; Zeng, G.; Salas-Colera, E.; Serrano, A.; Castro, G.R.; Xu, H.; Sun, C.; Goldbach, A. High-temperature stability of Pd alloy membranes containing Cu and Au. J. Membr. Sci. 2017, 544, 151–160. [Google Scholar] [CrossRef]
- Acha, E.; van Delft, Y.; Cambra, J.; Arias, P. Thin PdCu membrane for hydrogen purification from in-situ produced methane reforming complex mixtures containing H2S. Chem. Eng. Sci. 2018, 176, 429–438. [Google Scholar] [CrossRef]
- Dalla Fontana, A.; Sirini, N.; Cornaglia, L.M.; Tarditi, A.M. Hydrogen permeation and surface properties of PdAu and PdAgAu membranes in the presence of CO, CO2 and H2S. J. Membr. Sci. 2018, 563, 351–359. [Google Scholar] [CrossRef]
- Conde, J.J.; Maroño, M.; Sánchez-Hervás, J.M. Pd-Based Membranes for Hydrogen Separation: Review of Alloying Elements and Their Influence on Membrane Properties. Sep. Purif. Rev. 2017, 46, 152–177. [Google Scholar] [CrossRef]
- Lee, S.M.; Xu, N.; Kim, S.S.; Li, A.; Grace, J.R.; Lim, C.J.; Boyd, T.; Ryi, S.K.; Susdorf, A.; Schaadt, A. Palladium/ruthenium composite membrane for hydrogen separation from the off-gas of solar cell production via chemical vapor deposition. J. Membr. Sci. 2017, 541, 1–8. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, Z.; Tong, Y.; Du, M.; Mi, J.; Yu, Q.; Li, S. Improved sulfur tolerance of Pd-Ru membranes: Influence of H2S concentration and exposure time on the hydrogen flux. Int. J. Hydrogen Energy 2023, 48, 38335–38343. [Google Scholar] [CrossRef]
- Liu, J.; Bellini, S.; de Nooijer, N.C.; Sun, Y.; Pacheco Tanaka, D.A.; Tang, C.; Li, H.; Gallucci, F.; Caravella, A. Hydrogen permeation and stability in ultra-thin PdRu supported membranes. Int. J. Hydrogen Energy 2020, 45, 7455–7467. [Google Scholar] [CrossRef]
- Omidifar, M.; Akbar Babaluo, A.; Jamshidi, S. H2 permeance and surface characterization of a thin (2 μm) Pd-Ni composite membrane prepared by electroless plating. Chem. Eng. Sci. 2024, 283, 119370. [Google Scholar] [CrossRef]
- O’Brien, C.P.; Lee, I.C. The interaction of CO with PdCu hydrogen separation membranes: An operando infrared spectroscopy study. Catal. Today 2019, 336, 216–222. [Google Scholar] [CrossRef]
- Peng, L.; Rao, Y.; Luo, L.; Chen, C. The poisoning of Pd-Y alloy membranes by carbon monoxide. J. Alloys Compd. 2009, 486, 74–77. [Google Scholar] [CrossRef]
- de Nooijer, N.; Sanchez, J.D.; Melendez, J.; Fernandez, E.; Pacheco Tanaka, D.A.; van Sint Annaland, M.; Gallucci, F. Influence of H2S on the hydrogen flux of thin-film PdAgAu membranes. Int. J. Hydrogen Energy 2020, 45, 7303–7312. [Google Scholar] [CrossRef]
- Escalante, Y.; Tarditi, A.M. Thermally stable membranes based on PdNiAu systems with high nickel content for hydrogen separation. J. Membr. Sci. 2023, 676, 121581. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Z.; Tong, Y.; Yin, Z.; Li, S. High hydrogen permeability of Pd-Ru-In membranes prepared by electroless co-deposition. Sep. Purif. Technol. 2024, 343, 127073. [Google Scholar] [CrossRef]
- Mukaida, M.; Takahashi, N.; Hisamatsu, K.; Ishitsuka, M.; Hara, S.; Suda, H.; Haraya, K. Preparation for defect-free self-supported Pd membranes by an electroless plating method. J. Membr. Sci. 2010, 365, 378–381. [Google Scholar] [CrossRef]
- Wang, X.; Feng, X.; Yang, L.; An, Y.; Yao, Y.; Chen, K.; Song, J.; Shi, Y.; Chen, C.; Luo, W. Highly efficient and direct recovery of low-pressure hydrogen isotopes from tritium extraction gas by PdY alloy membrane permeator. Fusion Eng. Des. 2024, 202, 114348. [Google Scholar] [CrossRef]
- Fuerst, T.F.; Taylor, C.N.; Shimada, M. Deuterium Permeation Through a Self-Supported Palladium-Silver Membrane in Helium Gas Mixtures. IEEE Trans. Plasma Sci. 2024, 1–5. [Google Scholar] [CrossRef]
- Jazani, O.; Bennett, J.; Liguori, S. Effect of temperature, air exposure and gas mixture on Pd82-Ag15-Y3 membrane for hydrogen separation. Int. J. Hydrogen Energy 2024, 51, 624–636. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Chen, F.; Furukawa, M.; Mine, K. The (α + β) hydrogen miscibility gaps in hydrogenated palladium-rich Pd-Y(Gd)-Ag ternary alloys. J. Less Common Met. 1990, 166, 45–56. [Google Scholar] [CrossRef]
- Orakwe, I.; Shehu, H.; Gobina, E. Preparation and characterization of palladium ceramic alumina membrane for hydrogen permeation. Int. J. Hydrogen Energy 2019, 44, 9914–9921. [Google Scholar] [CrossRef]
- Iulianelli, A.; Jansen, J.C.; Esposito, E.; Longo, M.; Dalena, F.; Basile, A. Hydrogen permeation and separation characteristics of a thin Pd-Au/Al2O3 membrane: The effect of the intermediate layer absence. Catal. Today 2019, 330, 32–38. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, S.W.; Chen, C.Y.; Chi, Y.H.; Lin, Y.L. Impact of vacuum operation on hydrogen permeation through a palladium membrane tube. Int. J. Hydrogen Energy 2019, 44, 14434–14444. [Google Scholar] [CrossRef]
- Chen, W.H.; Escalante, J. Influence of vacuum degree on hydrogen permeation through a Pd membrane in different H2/N2 gas mixtures. Renew. Energy 2020, 155, 1245–1263. [Google Scholar] [CrossRef]
- Tosto, E.; Martinez-Diaz, D.; Sanz, R.; Azzato, G.; Calles, J.A.; Medrano, J.A.; Fernandez, E.; Pacheco Tanaka, D.A.; Gallucci, F.; Alique, D.; et al. Systematic experimental assessment of concentration polarization and inhibition in Pd-based membranes for hydrogen purification. Fuel Process. Technol. 2021, 213, 106661. [Google Scholar] [CrossRef]
- Yue, L.; Chen, C.; Li, J.; Xiao, C.; Xia, X.; Ran, G.; Fu, X.; Hou, J.; Gong, Y.; Wang, H. Inhibition Effect of CO on Hydrogen Permeation Through a Pd/Al2O3 Composite Membrane: A Comprehensive Study on Concentration Polarization and Competitive Adsorption Effect. Fusion Sci. Technol. 2020, 76, 680–689. [Google Scholar] [CrossRef]
- Magnone, E.; Lee, S.H.; Park, J.H. Relationships between electroless plating temperature, Pd film thickness and hydrogen separation performance of Pd-coated Al2O3 hollow fibers. Mater. Lett. 2020, 272, 127811. [Google Scholar] [CrossRef]
- Park, Y.; Kwak, Y.; Yu, S.; Badakhsh, A.; Lee, Y.J.; Jeong, H.; Kim, Y.; Sohn, H.; Nam, S.W.; Yoon, C.W.; et al. Degradation mechanism of a Pd/Ta composite membrane: Catalytic surface fouling with inter-diffusion. J. Alloys Compd. 2021, 854, 157196. [Google Scholar] [CrossRef]
- Ryu, S.; Badakhsh, A.; Oh, J.G.; Ham, H.C.; Sohn, H.; Yoon, S.P.; Choi, S.H. Experimental and Numerical Study of Pd/Ta and PdCu/Ta Composites for Thermocatalytic Hydrogen Permeation. Membranes 2023, 13, 23. [Google Scholar] [CrossRef]
- Lundin, S.T.B.; Patki, N.S.; Zhang, Z.; Fuerst, T.F.; Wolden, C.A.; Way, J.D. PdAu/YSZ composite hydrogen separation membranes with enhanced stability in the presence of CO. J. Membr. Sci. 2020, 611, 118371. [Google Scholar] [CrossRef]
- Alique, D.; Martinez-Diaz, D.; Sanz, R.; Calles, J.A. Review of Supported Pd-Based Membranes Preparation by Electroless Plating for Ultra-Pure Hydrogen Production. Membranes 2018, 8, 5. [Google Scholar] [CrossRef]
- Salomé Macedo, M.; Acha Uriarte, N.; Soria, M.; Madeira, L.M.; Calles, J.; Sanz, R.; Alique, D. Effect of ceria particle size as intermediate layer for preparation of composite Pd-membranes by electroless pore-plating onto porous stainless-steel supports. Sep. Purif. Technol. 2023, 327, 124932. [Google Scholar] [CrossRef]
- Martinez-Diaz, D.; Sanz, R.; Calles, J.; Alique, D. H2 permeation increase of electroless pore-plated Pd/PSS membranes with CeO2 intermediate barriers. Sep. Purif. Technol. 2019, 216, 16–24. [Google Scholar] [CrossRef]
- Martinez-Diaz, D.; Alique, D.; Calles, J.; Sanz, R. Pd-thickness reduction in electroless pore-plated membranes by using doped-ceria as interlayer. Int. J. Hydrogen Energy 2020, 45, 7278–7289. [Google Scholar] [CrossRef]
- Martinez-Diaz, D.; Martínez del Monte, D.; García-Rojas, E.; Alique, D.; Calles, J.; Sanz, R. Comprehensive permeation analysis and mechanical resistance of electroless pore-plated Pd-membranes with ordered mesoporous ceria as intermediate layer. Sep. Purif. Technol. 2021, 258, 118066. [Google Scholar] [CrossRef]
- Martinez-Diaz, D.; Sanz, R.; Carrero, A.; Calles, J.A.; Alique, D. Effective H2 Separation through Electroless Pore-Plated Pd Membranes Containing Graphite Lead Barriers. Membranes 2020, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Kiadehi, A.D.; Taghizadeh, M. Fabrication, characterization, and application of palladium composite membrane on porous stainless steel substrate with NaY zeolite as an intermediate layer for hydrogen purification. Int. J. Hydrogen Energy 2019, 44, 2889–2904. [Google Scholar] [CrossRef]
- Dehghani Kiadehi, A.; Taghizadeh, M.; Rami, M.D. Preparation of Pd/SAPO-34/PSS composite membranes for hydrogen separation: Effect of crystallization time on the zeolite growth on PSS support. J. Ind. Eng. Chem. 2020, 81, 206–218. [Google Scholar] [CrossRef]
- Sanz-Villanueva, D.; Alique, D.; Vizcaíno, A.; Sanz, R.; Calles, J. Pre-activation of SBA-15 intermediate barriers with Pd nuclei to increase thermal and mechanical resistances of pore-plated Pd-membranes. Int. J. Hydrogen Energy 2021, 46, 20198–20212. [Google Scholar] [CrossRef]
- de Moura Silva, C.L.; Ribeiro, S.R.F.L.; Terra, N.M.; Cardoso, V.L.; Reis, M.H.M. Improved hydrogen permeation through thin Pd/Al2O3 composite membranes with graphene oxide as intermediate layer. Int. J. Hydrogen Energy 2020, 45, 22990–23005. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jin, X.; Ding, W.; Hu, X.; Li, H. Coating the porous Al2O3 substrate with a natural mineral of Nontronite-15A for fabrication of hydrogen-permeable palladium membranes. Int. J. Hydrogen Energy 2020, 45, 7412–7422. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, Y.; Zou, D.; Pan, Q.; Jiang, C.; Li, Y.; Chen, C. Effect of single atomic layer graphene film on the thermal stability and hydrogen permeation of Pd-coated Nb composite membrane. Int. J. Hydrogen Energy 2022, 47, 8359–8371. [Google Scholar] [CrossRef]
- Liang, X.; Li, X.; Chen, R.; Nagaumi, H.; Guo, J.; Liu, D. Enhancement of hydrogen permeation stability at high temperatures for Pd/Nb30Ti35Co35/Pd composite membranes by HfN intermediate layer. J. Membr. Sci. 2022, 643, 120062. [Google Scholar] [CrossRef]
- Cerone, N.; Zimbardi, F.; Contuzzi, L.; Tosti, S.; Fabbiano, L.; Zito, G.D.; Carnevale, M.O.; Valerio, V. Pre-pilot scale study of hydrogen production from biomass syngas via water-gas shift in Pd-Ag catalytic membrane reactor and dedicated hydrogen permeation unit. Int. J. Hydrogen Energy, 2024; in press. [Google Scholar] [CrossRef]
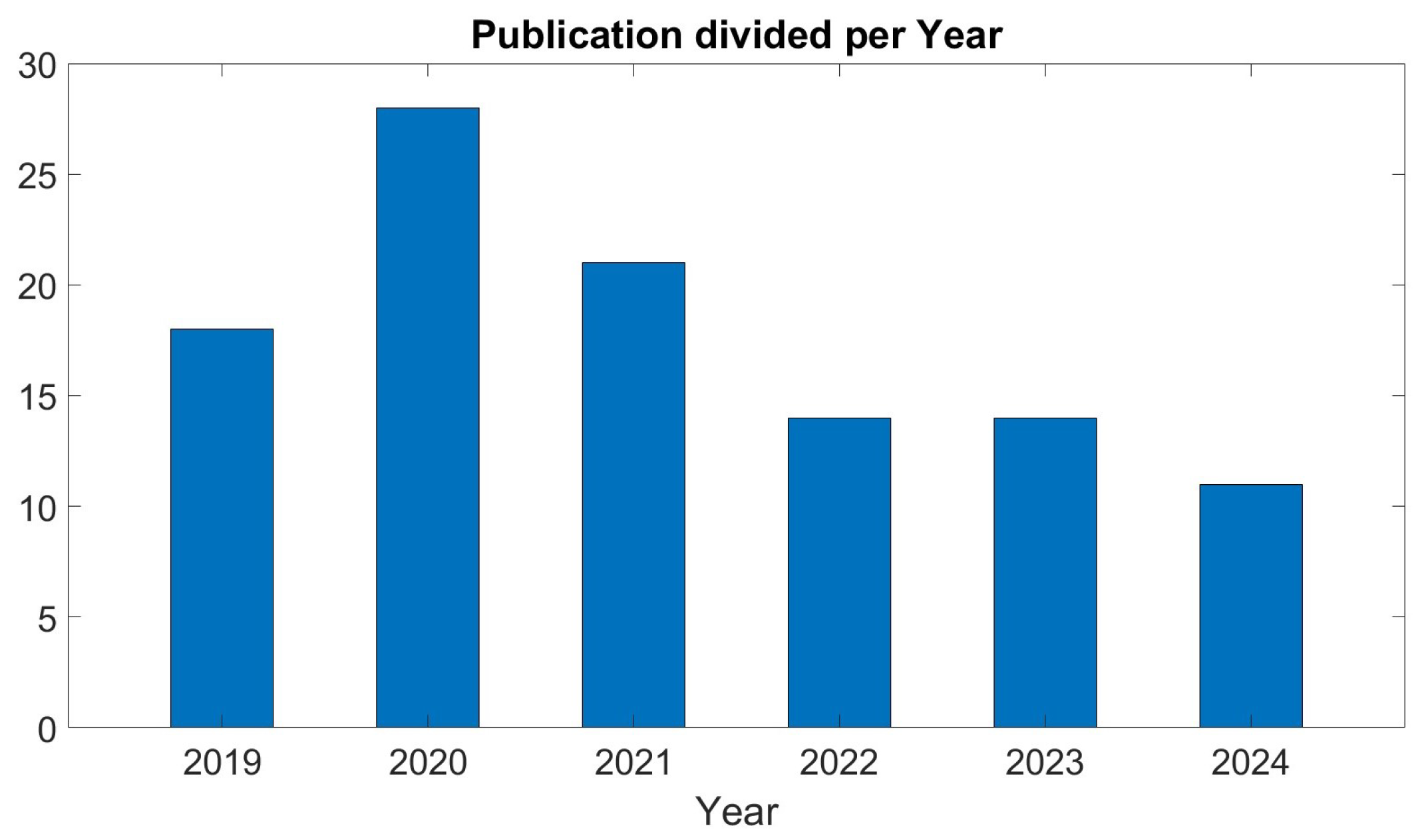

| Year | Number of Publications |
|---|---|
| 2024 | 11 |
| 2023 | 14 |
| 2022 | 14 |
| 2021 | 21 |
| 2020 | 28 |
| 2019 | 18 |
| Researcher | Number of Publications |
|---|---|
| Gallucci, F. | 11 |
| Sanz, R. | 7 |
| Calles, J. A. | 7 |
| Alique, D. | 7 |
| Martinez-Diaz, D. | 5 |
| Chen, W. H. | 5 |
| Element | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Silver (Ag) |
|
| [25,31,34,35] |
| Copper (Cu) |
|
| [25,35,36,37,38,45] |
| Gold (Au) |
|
| [37,39] |
| Yttrium (Y) |
|
| [25,40,46] |
| Ruthenium (Ru) |
|
| [41,42,43] |
| Nickel (Ni) |
|
| [44] |
| Membrane | Preparation Method | [μm] | T [°C] | [kPa] | Permeability/ Permeance | Ref. | |
|---|---|---|---|---|---|---|---|
| Pd-Y | Cold-Rolling | 80 | 400 | 25–300 | (1) | N. D. | [51] |
| Pd-Ag | Cold-Rolling | 76.2 | 400 | ≈90 | ≈ (1) | N.D. | [52] |
| Pd-Ag-Y | Cold-Rolling | 38 | 400 | 200 | (1) | ∞ | [53] |
| Pd/-Al2O3 | ELP | 8.8 | 350 | 100 | ≈ (2) | 3.45 | [55] |
| Pd-Au/-Al2O3 | ELP | 8 | 400 | 50 | (2) | 500 | [56] |
| Pd/Modified-PSS | ELP | 7–8.53 | 320–380 | 202.6 | 1.47–2.07 (2) | 92–∞ | [57,58] |
| Pd/PSS | ELP-PP | 20 | 395 | 200 | ≈ (2) | ≥1000 | [59] |
| Pd-Ag/PSS | MS | 10 | 450 | 400 | (1) | 39,000 | [34] |
| Pd/Al2O3 | ELP | 5 | 350 | 30–100 | 4.9 × 10−9 (1) | 7935–37,640 | [60] |
| Pd/Ta | ELP | 1 | 450 | 250 | 5.9–12.2 × 10−8 (1) | N.D. | [62] |
| Membrane | Preparation Method | [μm] | T [°C] | [kPa] | H2 Permeance/Permeability | Ref. | |
|---|---|---|---|---|---|---|---|
| Pd/CeO2/PSS | ELP-PP | 10 | 400 | 25–300 | 5.98 × 10−4 (1) | ≥10,000 | [66] |
| Pd/CeO2/PSS | ELP-PP | 15.4 | 400 | 100–200 | 5.37 × 10−4 (1) | ≥10,000 | [67] |
| Pd/Doped-CeO2/PSS | ELP-PP | 9.1 | 350–450 | 100–200 | 4.46–6.39 × 10−4 (1) | ≥10,000 | [68] |
| Pd/Mesoporous-CeO2/PSS | ELP-PP | 10 | 400 | 100–200 | 1.03 × 10−3 (1) | ≥24,000 | [69] |
| Pd/Graphite/PSS | ELP-PP | 17 | 400 | 100 | 4.01 × 10−4 (1) | ≥10,000 | [70] |
| Pd/NaY/PSS | ELP | 7 | 450 | 100 | 6.2 × 10−4 (1) | 736 | [71] |
| Pd/SAPO-34/PSS | ELP | 9 | 450 | 100 | 7.1 × 10−7 (2) | 866 | [72] |
| Pd/Doped-SBA-15/PSS | ELP-PP | 7.1 | 400 | 50–250 | 3.81 × 10−4 (1) | ≥2500 | [73] |
| Pd/GO/Al2O3 | ELP | 0.91 | 450 | 100 | 2.4 × 10−6 (2) | ∞ | [74] |
| Pd/NA-15A/Al2O3 | ELP | 2 | 450 | 100 | ≈3.05 × 10−3 (1) | ≈3500 | [75] |
| Pd/G/Nb | MS | 0.1 | 450 | 500 | 1.83 × 10−9 (3) | N.D | [76] |
| Pd/HfN/Nb30Ti35CO35 | MS | 1 | 250–400 | 600 | 2.65 × 10−8 (3) | N.D. | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerone, N.; Zito, G.D.; Florio, C.; Fabbiano, L.; Zimbardi, F. Recent Advancements in Pd-Based Membranes for Hydrogen Separation. Energies 2024, 17, 4095. https://doi.org/10.3390/en17164095
Cerone N, Zito GD, Florio C, Fabbiano L, Zimbardi F. Recent Advancements in Pd-Based Membranes for Hydrogen Separation. Energies. 2024; 17(16):4095. https://doi.org/10.3390/en17164095
Chicago/Turabian StyleCerone, Nadia, Giuseppe Domenico Zito, Carmine Florio, Laura Fabbiano, and Francesco Zimbardi. 2024. "Recent Advancements in Pd-Based Membranes for Hydrogen Separation" Energies 17, no. 16: 4095. https://doi.org/10.3390/en17164095






