Analysis of the Possibility of Applying Biochars from Biowaste as Adsorbents to Eliminate Odors from Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
- cI2—iodine concentration [mol/dm3],
- 2.2—dilution factor for 10 cm3 of hydrochloric acid and filtrate,
- cNa2S2O3—sodium thiosulfate concentration [mol/dm3],
- V—volume of sodium thiosulfate solution used for titration [cm3],
- 126.9—the relative atomic mass of iodine,
- m—mass of adsorbent used for testing [g].
- C0—initial odor concentration value [ouE/m3],
- C—final value of odor concentration [ouE/m3].
- E—odorant adsorption efficiency [%],
- H—height of the activated carbon bed [mm],
- a0, a1—coefficients of the equation [-].
3. Results and Discussion
4. Conclusions
- The selected biowaste, which would otherwise find little use, is suited to the problem of developing adsorbents for odor elimination.
- Biochars from beekeeping waste and coffee spent grounds are good alternatives to commercial adsorbents, such as Organosorb 200-1 Wi activated carbon.
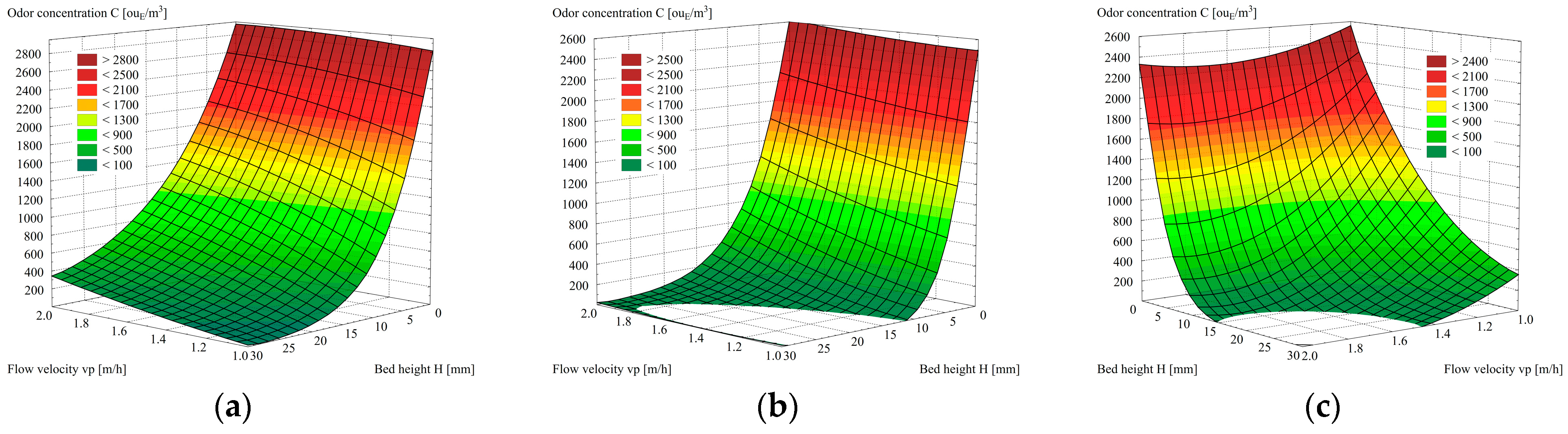
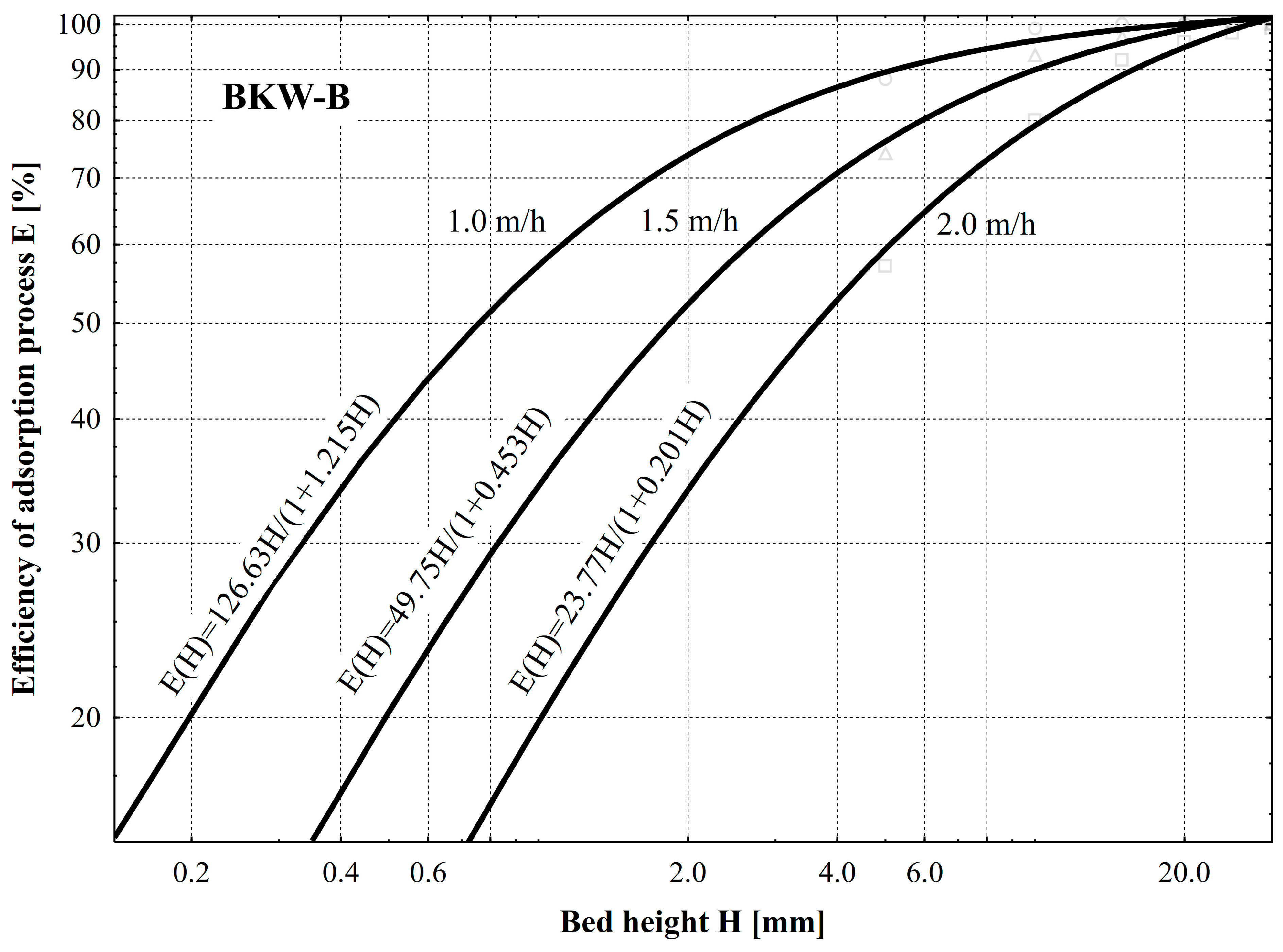
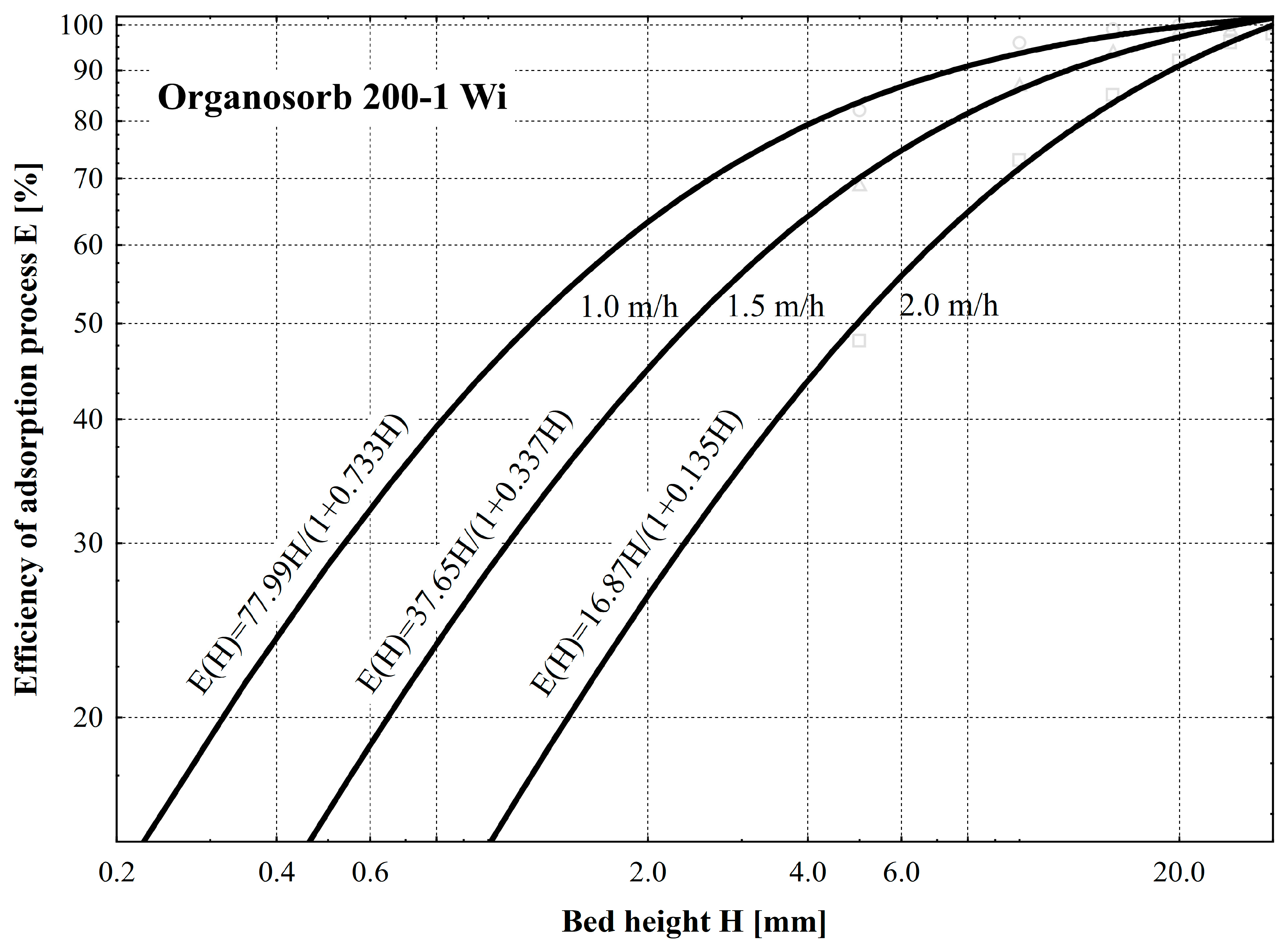
- The biochar from beekeeping waste showed the highest efficiency of odor neutralization. At the height of the bed H = 15 mm and odorant flow rate vp = 1.0 m/h, it is 100% efficient. Biochar from SCGs prepared identically and maintaining the same values of the parameters of the independent variables does not guarantee 100% efficiency since the minimum value of odorant concentration was C = 15 ouE/m3. This is due to the developed macroporous structure, as under the same conditions, the efficiency of commercially produced Organosorb 200-1 Wi activated carbon is slightly higher than that of SCG-B and, simultaneously, lower than that of BKW-B. While maintaining the flow velocity vp =1.0 m/h, total adsorption of odorants occurs in a bed with a minimum height of H = 30 mm.
- The efficiency of the adsorption process E [%] depending on the change in the height of the bed H [mm] is sufficiently described by the mathematical Formula (2), as evidenced by the value of the correlation coefficient ranging from 0.98 to 0.99. Based on the values of the coefficients of Equation (2), the efficiency of the process of adsorption of odorants can be estimated. As a result of decreasing the value of the coefficient a1 and increasing the value of the coefficient a2, an increase in adsorption efficiency was found. Based on the averaged values of the coefficients, an adsorption efficiency series of odor elimination was obtained: SCG-B (a1 = 131; a2 = 0.12) < commercial Organosorb 200-1 Wi (a1 = 114; a2 = 0.40) < BKW-B (a = 111; a12 = 0.62).
- After selecting the most optimal biochars in terms of the adsorption process efficiency results, in the next cycle of research, it is planned to perform a detailed structural analysis and studies on the content of the main functional groups on the surface of adsorbents obtained from biowaste to indicate methods for modifying textural features and specific surface area using different reagents.
- In the next stage of the research, it is necessary to introduce an additional independent variable parameter, i.e., the duration of the adsorption process, and on this basis, estimate the migration rate of the adsorption front, which can enable the possible practical application of modified waste materials.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shusterman, D. Critical Review: The Health Significance of Environmental Odor Pollution. Arch. Environ. Health Int. J. 1992, 47, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Brancher, M.; Griffiths, K.D.; Franco, D.; De Melo Lisboa, H. A Review of Odour Impact Criteria in Selected Countries around the World. Chemosphere 2017, 168, 1531–1570. [Google Scholar] [CrossRef]
- Sówka, I.; Grzelka, A. Application of National and European Solutions in the Assessment of Odour Air Quality in the Vicinity of Waste Facilities. Rocz. Ochr. Śr. 2016, 18, 794–802. [Google Scholar]
- Sobczyński, P.; Miller, U.; Sówka, I. Variability of Odour Emissions from Selected Passive Area Source: Preliminary Analysis for a One-Year Study at a Municipal Wastewater Treatment Plant in Poland. Rocz. Ochr. Śr. 2023, 25, 228–234. [Google Scholar] [CrossRef]
- Lebrero, R.; Bouchy, L.; Stuetz, R.; Muñoz, R. Odor Assessment and Management in Wastewater Treatment Plants: A Review. Crit. Rev. Environ. Sci. Technol. 2011, 41, 915–950. [Google Scholar] [CrossRef]
- Hvitved-Jacobsen, T.; Vollertsen, J.; Yongsiri, C.; Nielsen, A.H.; Abdul-Talib, S. Sewer Microbial Processes, Emissions and Impacts. In Proceedings of the 3rd International Conference on Sewer Processes and Networks, Paris, France, 15–17 April 2002; pp. 1–13. [Google Scholar]
- Milik, J.; Pasela, R.; Szymczak, M.; Chalamonski, M. Evaluation of the Physico-Chemical Composition of Sludge from Municipal Sewage Treatment Plan. Rocz. Ochr. Śr. 2016, 18, 579–590. [Google Scholar]
- Sava, C.; Iluţiu-Varvara, D.-A.; Mare, R.; Roman, M.D.; Rada, S.; Pică, E.M.; Jäntschi, L. Physico-Chemical Characterization and Possible Uses of Sludge Processed from an Urban Sewage Treatment Plant. Heliyon 2024, 10, e29576. [Google Scholar] [CrossRef]
- Kwarciak-Kozłowska, A.; Bańka, B. Neutralization of Odors Generated during Composting of Biodegradable Fraction of Municipal and Industrial Waste by Biofiltration. Inż. Ochr. Śr. 2014, 17, 631–645. [Google Scholar]
- Gatwa-Widera, M.; Kwarciak-Kozlowska, K.-K. Anna Methods for Elimination of Odor in the Composting Process. Rocz. Ochr. Śr. 2016, 18, 850–860. [Google Scholar]
- Frechen, F.-B. Odour Emission Inventory of German Wastewater Treatment Plants–Odour Flow Rates and Odour Emission Capacity. Water Sci. Technol. 2004, 50, 139–146. [Google Scholar] [CrossRef]
- Jiang, G.; Melder, D.; Keller, J.; Yuan, Z. Odor Emissions from Domestic Wastewater: A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1581–1611. [Google Scholar] [CrossRef]
- Suffet, I.H.; Decottignies, V.; Zhou, Y.; Bian, Y.; Vitko, T.G. An Evaluation of Masking Nuisance Odors from a Source by Chemical and Sensory Analyses. Water Environ. Res. 2023, 95, e10901. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Piecuch, T.; Andriyevska, L. The Use of Pine Essential Oil for Masking the Odors Emitted in the Process of Mechanical Dewatering of Municipal Sewage Sludge. Rocz. Ochr. Śr. 2013, 15, 807–822. [Google Scholar]
- Kowalczyk, A.; Piecuch, T. Application of Essential Oil as a Substitute of Flocculant in the Process of Mechanical Dewatering of Municipal Sewage Sludge. Rocz. Ochr. Śr. 2016, 18, 414–444. [Google Scholar]
- Fulazzaky, M.A.; Talaiekhozani, A.; Ponraj, M.; Abd Majid, M.Z.; Hadibarata, T.; Goli, A. Biofiltration Process as an Ideal Approach to Remove Pollutants from Polluted Air. Desalin. Water Treat. 2014, 52, 3600–3615. [Google Scholar] [CrossRef]
- Wani, A.H.; Branion, R.M.R.; Lau, A.K. Biofiltration: A Promising and Cost-effective Control Technology for Odors, VOCs and Air Toxics. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1997, 32, 2027–2055. [Google Scholar] [CrossRef]
- Galwa-Widera, M.; Kwarciak-Kozlowska, A. Biofiltration–An Ecological Method of Removing Odors Generated during Drying Sewage Sludge-Case Study. Desalin. Water Treat. 2021, 232, 236–243. [Google Scholar] [CrossRef]
- Pecorini, I.; Rossi, E.; Iannelli, R. Mitigation of Methane, NMVOCs and Odor Emissions in Active and Passive Biofiltration Systems at Municipal Solid Waste Landfills. Sustainability 2020, 12, 3203. [Google Scholar] [CrossRef]
- Estrada, J.M.; Kraakman, N.J.R.B.; Muñoz, R.; Lebrero, R. A Comparative Analysis of Odour Treatment Technologies in Wastewater Treatment Plants. Environ. Sci. Technol. 2011, 45, 1100–1106. [Google Scholar] [CrossRef]
- Morales-Corts, R.; Gomez-Sanchez, M.A.; Perez-Sanchez, R.; Prieto-Calvo, C. Characterization of Beekeeping Wastes for Using in Seedling Production. Span. J. Agric. Res. 2010, 8, 493–500. [Google Scholar] [CrossRef]
- Piekarski, J.; Dąbrowski, T.; Ignatowicz, K. Effect of Bed Height on Efficiency of Adsorption of Odors from Sewage Sludge Using Modified Biochars from Organic Waste Materials as an Adsorbent. Desalin. Water Treat. 2021, 218, 252–259. [Google Scholar] [CrossRef]
- Piekarski, J.; Dąbrowski, T.; Dąbrowski, J.; Ignatowicz, K. Preliminary studies on odor removal in the adsorption process on biochars produced form sewage sludge and beekeeping waste. Arch. Environ. Prot. 2021, 47, 20–28. [Google Scholar] [CrossRef]
- International Coffee Organization. Coffee Report and Outlook; International Coffee Organization: London, UK, 2023. [Google Scholar]
- Pfluger, R.A. Soluble Coffee Processing. In Solid Wastes: Origin, Collection, Processing, and Disposal; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.À.; Fiol, N.; Villaescusa, I.; Pereira, H. The Chemical Composition of Exhausted Coffee Waste. Ind. Crops Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Singh, T.A.; Pal, N.; Sharma, P.; Passari, A.K. Spent Coffee Ground: Transformation from Environmental Burden into Valuable Bioactive Metabolites. Rev. Environ. Sci. Biotechnol. 2023, 22, 887–898. [Google Scholar] [CrossRef]
- Tun, M.M.; Raclavská, H.; Juchelková, D.; Růžičková, J.; Šafář, M.; Štrbová, K.; Gikas, P. Spent Coffee Ground as Renewable Energy Source: Evaluation of the Drying Processes. J. Environ. Manag. 2020, 275, 111204. [Google Scholar] [CrossRef] [PubMed]
- Sidełko, R.; Janowska, B.; Szymański, K.; Mostowik, N.; Głowacka, A. Advanced Methods to Calculation of Pressure Drop during Aeration in Composting Process. Sci. Total Environ. 2019, 674, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sidełko, R.; Walendzik, B.; Smuga-Kogut, M.; Janowska, B.; Szymański, K.; Głowacka, A.; Leśniańska, A. Impact of reduced straw content on the sewage sludge composting process. Arch. Environ. Prot. 2020, 46, 70–77. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Molinero-García, A.; Martín-García, J.M.; Delgado, G. Sequential Effects of Spent Coffee Grounds on Soil Physical Properties. Soil Use Manag. 2023, 39, 286–297. [Google Scholar] [CrossRef]
- Massaya, J.; Chan, K.H.; Mills-Lamptey, B.; Chuck, C.J. Developing a Biorefinery from Spent Coffee Grounds Using Subcritical Water and Hydrothermal Carbonisation. Biomass Convers. Biorefin. 2023, 13, 1279–1295. [Google Scholar] [CrossRef]
- Kim, H.-J.; Oh, S.-C. Hydrothermal Carbonization of Spent Coffee Grounds. Appl. Sci. 2021, 11, 6542. [Google Scholar] [CrossRef]
- Kang, S.B.; Oh, H.Y.; Kim, J.J.; Choi, K.S. Characteristics of Spent Coffee Ground as a Fuel and Combustion Test in a Small Boiler (6.5 kW). Renew. Energy 2017, 113, 1208–1214. [Google Scholar] [CrossRef]
- Bomfim, A.; Oliveira, D.; Voorwald, H.; Benini, K.; Dumont, M.-J.; Rodrigue, D. Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production. Polymers 2022, 14, 437. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.; Bairwan, R.D.; Rizg, W.Y.; Khalil, H.P.S.A.; Murshid, S.S.A.; Sindi, A.M.; Alissa, M.; Saharudin, N.I.; Abdullah, C.K. Enhancement of Spent Coffee Grounds as Biofiller in Biodegradable Polymer Composite for Sustainable Packaging. Polym. Compos. 2024, 45, 9317–9334. [Google Scholar] [CrossRef]
- Figueroa Campos, G.A.; Perez, J.P.H.; Block, I.; Sagu, S.T.; Saravia Celis, P.; Taubert, A.; Rawel, H.M. Preparation of Activated Carbons from Spent Coffee Grounds and Coffee Parchment and Assessment of Their Adsorbent Efficiency. Processes 2021, 9, 1396. [Google Scholar] [CrossRef]
- Castro, C.S.; Abreu, A.L.; Silva, C.L.T.; Guerreiro, M.C. Phenol Adsorption by Activated Carbon Produced from Spent Coffee Grounds. Water Sci. Technol. 2011, 64, 2059–2065. [Google Scholar] [CrossRef]
- Le, V.T.; Pham, T.M.; Doan, V.D.; Lebedeva, O.E.; Nguyen, H.T. Removal of Pb(ii) Ions from Aqueous Solution Using a Novel Composite Adsorbent of Fe3O4/PVA/Spent Coffee Grounds. Sep. Sci. Technol. 2019, 54, 3070–3081. [Google Scholar] [CrossRef]
- Milanković, V.; Tasić, T.; Pejčić, M.; Pašti, I.; Lazarević-Pašti, T. Spent Coffee Grounds as an Adsorbent for Malathion and Chlorpyrifos—Kinetics, Thermodynamics, and Eco-Neurotoxicity. Foods 2023, 12, 2397. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Nguyen, T.-B.; Huang, C.P.; Chen, C.-W.; Bui, X.-T.; Dong, C.-D. Alkaline Modified Biochar Derived from Spent Coffee Ground for Removal of Tetracycline from Aqueous Solutions. J. Water Process Eng. 2021, 40, 101908. [Google Scholar] [CrossRef]
- Andrade, T.S.; Vakros, J.; Mantzavinos, D.; Lianos, P. Biochar Obtained by Carbonization of Spent Coffee Grounds and Its Application in the Construction of an Energy Storage Device. Chem. Eng. J. Adv. 2020, 4, 100061. [Google Scholar] [CrossRef]
- PN-80/G-04511; Solid Fuels—Determination of Moisture Content. Polish Committee for Standardization: Warsaw, Poland, 1980.
- PN-80/G-04512; Solid Fuels—Determination of Ash by Gravimetric Method. Polish Committee for Standardization: Warsaw, Poland, 1980.
- PN-G-04516:1998; Solid Fuels—Determination of Volatile Matter by Gravimetric Method. Polish Committee for Standardization: Warsaw, Poland, 1998.
- PN-EN 12902:2005; Drinking Water Treatment Products—Inorganic Support and Filter Materials—Test methods. Polish Committee for Standardization: Warsaw, Poland, 2005.
- PN-EN 12915-1:2009; Drinking Water Treatment Products—Granular Activated Carbon—Part 1: Primary Granular Activated Carbon. Polish Committee for Standardization: Warsaw, Poland, 2009.
- PN-EN 13725:2022-07; Emission from Stationary Sources—Determination of Odor Concentration Using Dynamic Olfactometry and Odor Emission Rate. Polish Committee for Standardization: Warsaw, Poland, 2022.
- Yang, J.; Zhao, Z.; Hu, Y.; Abbey, L.; Cesarino, I.; Goonetilleke, A.; He, Q. Exploring the Properties and Potential Uses of Biocarbon from Spent Coffee Grounds: A Comparative Look at Dry and Wet Processing Methods. Processes 2023, 11, 2099. [Google Scholar] [CrossRef]
- Tangmankongworakoon, N. An Approach to Produce Biochar from Coffee Residue for Fuel and Soil Amendment Purpose. Int. J. Recycl. Org. Waste Agric. 2019, 8, 37–44. [Google Scholar] [CrossRef]
- Kang, J.-H.; Song, J.; Yoo, S.S.; Lee, B.-J.; Ji, H.W. Prediction of Odor Concentration Emitted from Wastewater Treatment Plant Using an Artificial Neural Network (ANN). Atmosphere 2020, 11, 784. [Google Scholar] [CrossRef]
- Anfruns, A.; Martin, M.J.; Montes-Morán, M.A. Removal of Odourous VOCs Using Sludge-Based Adsorbents. Chem. Eng. J. 2011, 166, 1022–1031. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Z.; Wu, S.; Cai, Y.; Zhang, J.; Lou, C.; Zhao, W. Modification of the Adsorption Model for the Mixture of Odor Compounds and VOCs on Activated Carbon: Insights from Pore Size Distribution. Sep. Purif. Technol. 2024, 339, 126669. [Google Scholar] [CrossRef]
- Ruthven, D.M. Adsorption, Fundamentals. In Kirk-Othmer Encyclopedia of Chemical Technology; Kirk-Othmer, Ed.; Wiley: Hoboken, NJ, USA, 2001; ISBN 978-0-471-48494-3. [Google Scholar]
- Bastos-Neto, M.; De Azevedo, D.C.S.; De Lucena, S.M.P. Adsorption. In Kirk-Othmer Encyclopedia of Chemical Technology; Kirk-Othmer, Ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 1–59. ISBN 978-0-471-48494-3. [Google Scholar]

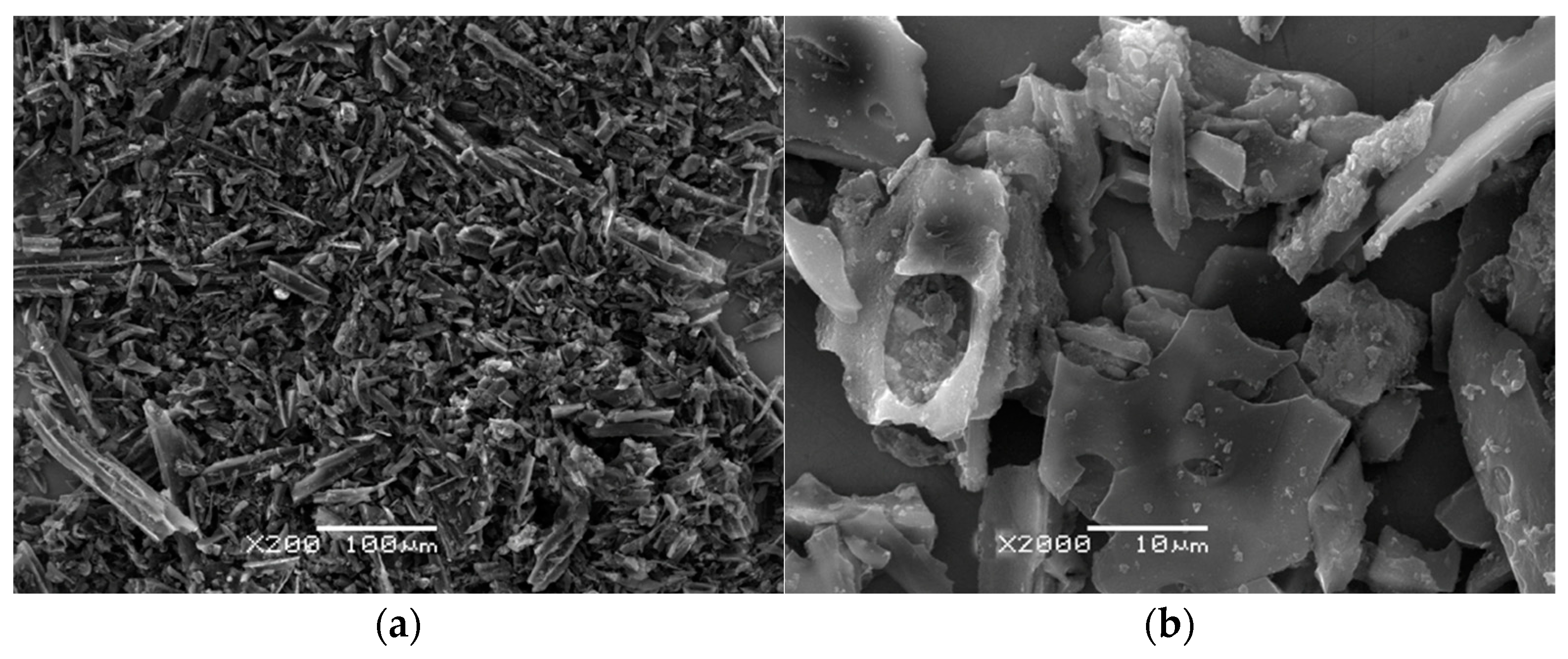



| Lp. | Parameter | Unit | Adsorbent | ||
|---|---|---|---|---|---|
| SCG-B | BKW-B | Organosorb 200-1 Wi | |||
| 1 | Moisture | [%] | 4.0 | 3.1 | 7.8 |
| 2 | Ash | [%] | 8.7 | 12.5 | 5.3 |
| 3 | Volatile parts | [%] | 10.9 | 6.6 | 10 |
| 4 | Fixed carbon | [%] | 76.4 | 77.8 | 76.9 |
| 5 | Iodine number | [mg/g] | 86 | 95 | 900 |
| 6 | Bulk density | [kg/m3] | 269 | 462 | 500 |
| No. | Flow Velocity | Height of the Deposit | Adsorbent | ||
|---|---|---|---|---|---|
| SCG-B | BKW-B | Organosorb 200-1 Wi | |||
| Odor Concentration | |||||
| vp [m/h] | H [mm] | C [ouE/m3] | |||
| 1 | 1.00 | 0 | 2890 | 2590 | 2410 |
| 2 | 5 | 1234 | 306 | 443 | |
| 3 | 10 | 519 | 36 | 93 | |
| 4 | 15 | 234 | 0 | 16 | |
| 5 | 20 | 102 | 0 | 0 | |
| 6 | 25 | 48 | 0 | 0 | |
| 7 | 30 | 15 | 0 | 0 | |
| 8 | 1.50 | 0 | 2882 | 2579 | 2417 |
| 9 | 5 | 1760 | 682 | 756 | |
| 10 | 10 | 1054 | 174 | 310 | |
| 11 | 15 | 650 | 69 | 149 | |
| 12 | 20 | 410 | 12 | 21 | |
| 13 | 25 | 236 | 0 | 15 | |
| 14 | 30 | 148 | 0 | 0 | |
| 15 | 2.00 | 0 | 2898 | 2599 | 2415 |
| 16 | 5 | 2048 | 1119 | 2048 | |
| 17 | 10 | 1410 | 511 | 1410 | |
| 18 | 15 | 992 | 200 | 992 | |
| 19 | 20 | 684 | 94 | 684 | |
| 20 | 25 | 476 | 48 | 476 | |
| 21 | 30 | 341 | 16 | 341 | |
| Adsorbent | Flow Velocity | Factor | Value | R2 |
|---|---|---|---|---|
| SCG-B | 1.0 | a0 | 117.88 | 0.99 |
| a1 | 0.21 | |||
| 1.5 | a0 | 132.04 | 0.99 | |
| a1 | 0.09 | |||
| 2.0 | a0 | 144.78 | 0.99 | |
| a1 | 0.05 | |||
| BKW-B | 1.0 | a0 | 104.22 | 0.99 |
| a1 | 1.22 | |||
| 1.5 | a0 | 109.85 | 0.99 | |
| a1 | 0.45 | |||
| 2.0 | a0 | 118.40 | 0.98 | |
| a1 | 0.20 | |||
| Organosorb 200-1 Wi | 1.0 | a0 | 106.40 | 0.99 |
| a1 | 0.73 | |||
| 1.5 | a0 | 111.65 | 0.98 | |
| a1 | 0.34 | |||
| 2.0 | a0 | 124.54 | 0.99 | |
| a1 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piekarski, J.; Ignatowicz, K.; Dąbrowski, T.; Dąbrowski, B. Analysis of the Possibility of Applying Biochars from Biowaste as Adsorbents to Eliminate Odors from Wastewater Treatment. Energies 2024, 17, 4129. https://doi.org/10.3390/en17164129
Piekarski J, Ignatowicz K, Dąbrowski T, Dąbrowski B. Analysis of the Possibility of Applying Biochars from Biowaste as Adsorbents to Eliminate Odors from Wastewater Treatment. Energies. 2024; 17(16):4129. https://doi.org/10.3390/en17164129
Chicago/Turabian StylePiekarski, Jacek, Katarzyna Ignatowicz, Tomasz Dąbrowski, and Bartosz Dąbrowski. 2024. "Analysis of the Possibility of Applying Biochars from Biowaste as Adsorbents to Eliminate Odors from Wastewater Treatment" Energies 17, no. 16: 4129. https://doi.org/10.3390/en17164129






