Application of Torrefaction for Improved Fuel Properties of Sunflower Husks
Abstract
:1. Introduction
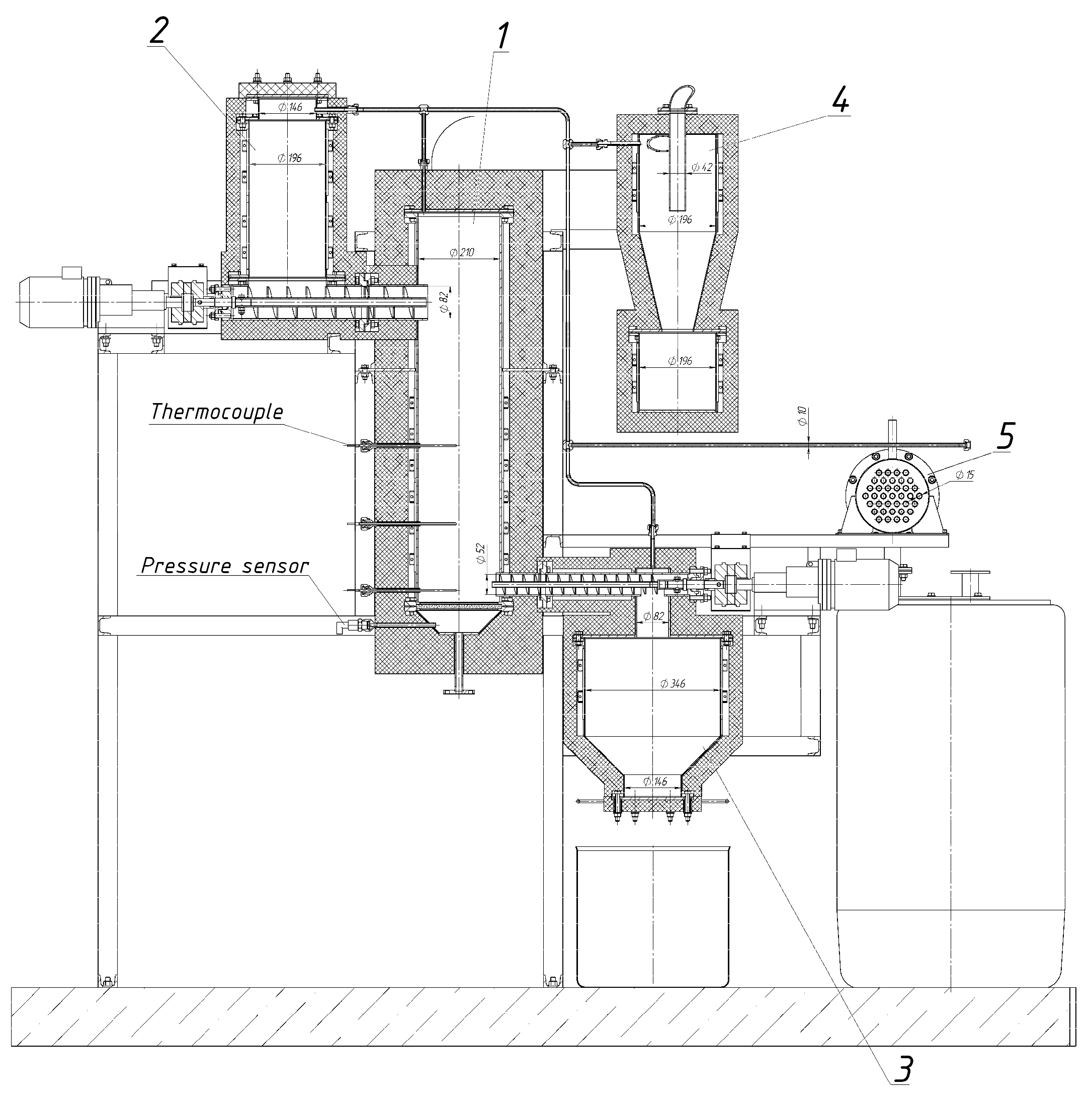
2. Materials and Methods
2.1. Sample Characterization
2.2. Torrefaction
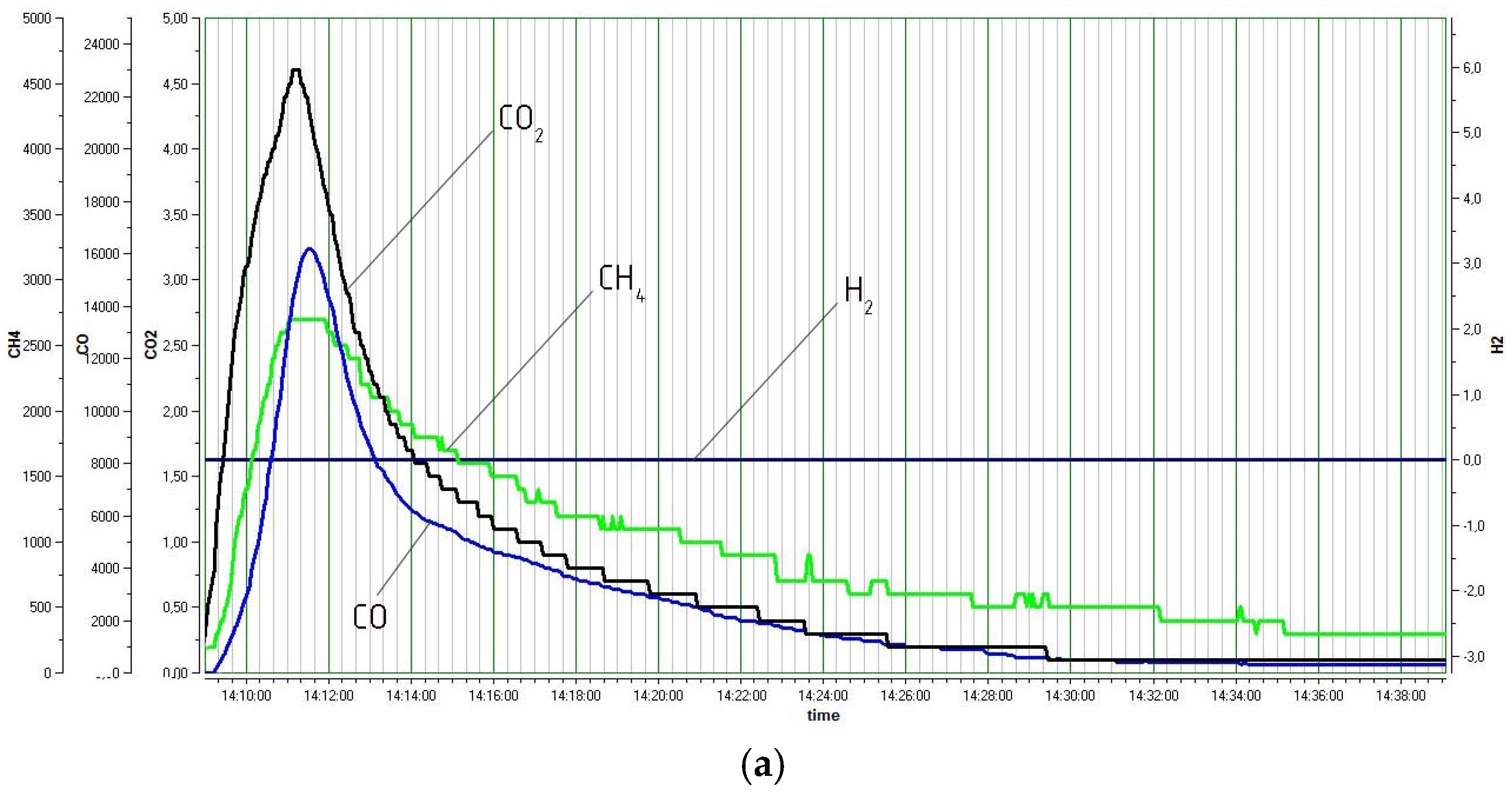
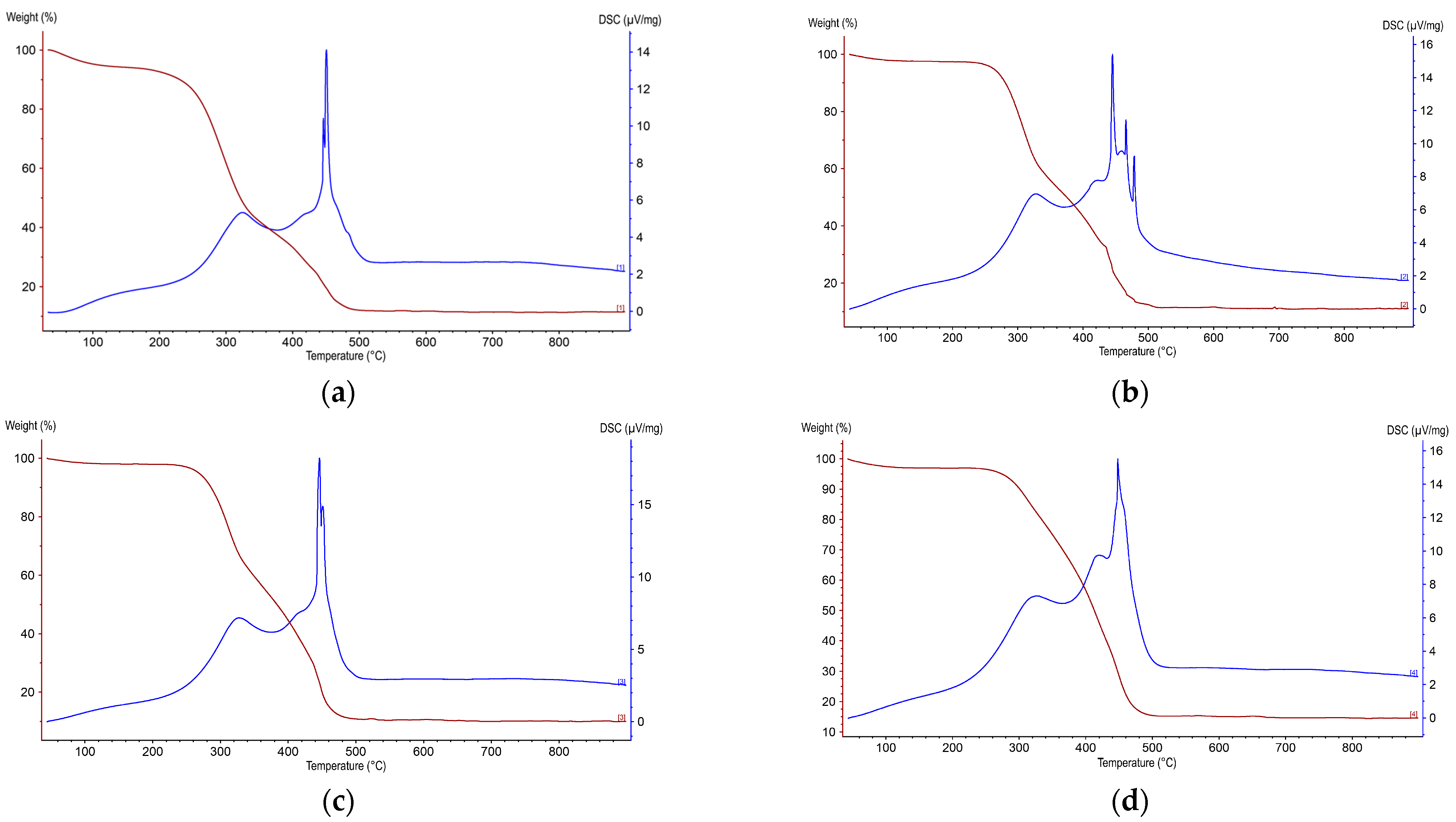
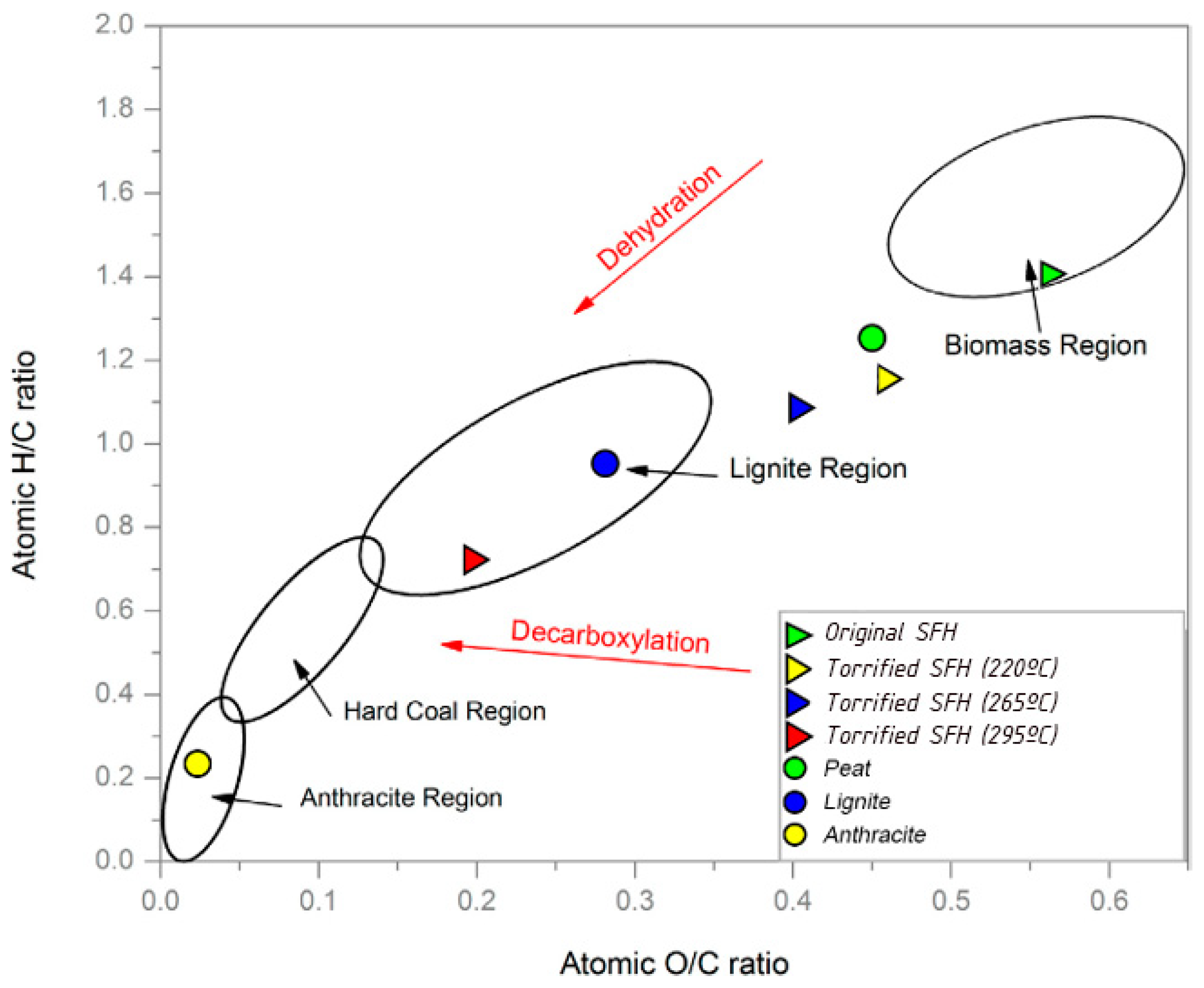
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kanwal, F.; Ahmed, A.; Jamil, F.; Rafiq, S.; Ayub, H.M.U.; Ghauri, M.; Khurram, M.S.; Munir, S.; Inayat, A.; Abu Bakar, M.S.; et al. Co-Combustion of Blends of Coal and Underutilised Biomass Residues for Environmental Friendly Electrical Energy Production. Sustainability 2021, 13, 4881. [Google Scholar] [CrossRef]
- Ayub, H.M.U.; Park, S.J.; Binns, M. Biomass to Syngas: Modified Non-Stoichiometric Thermodynamic Models for the Downdraft Biomass Gasification. Energies 2020, 13, 5668. [Google Scholar] [CrossRef]
- World Bioenergy Association. Global Bioenergy Statistics 2020; World Bioenergy Association: Stockholm, Sweden, 2020. [Google Scholar]
- World Energy Outlook 2021; International Energy Agency: Paris, France, 2021; p. 386. Available online: https://www.iea.org/reports/world-energy-outlook-2021 (accessed on 2 August 2024).
- Renewables 2021: Analysis and Forecast to 2026. International Energy Agency. Available online: https://www.iea.org/renewables2021 (accessed on 2 August 2024).
- Shackley, S.; Hammond, J.; Gaunt, J.; Ibarrola, R. The Feasibility and Costs of Biochar Deployment in the UK. Carbon Manag. 2011, 2, 335–356. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Wright, C.T.; Hess, J.R.; Kenney, K.L. A Review of Biomass Densification Systems to Develop Uniform Feedstock Commodities for Bioenergy Application. Biofuels Bioprod. Biorefin. 2011, 5, 683–707. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. A Review on Torrefied Biomass Pellets as a Sustainable Alternative to Coal in Power Generation. Renew. Sustain. Energy Rev. 2014, 40, 153–160. [Google Scholar] [CrossRef]
- Wang, L.; Riva, L.; Skreiberg, O.; Khalil, R.; Bartocci, P.; Yang, Q.; Yang, H.; Wang, X.; Chen, D.; Rudolfsson, M.; et al. Effect of Torrefaction on Properties of Pellets Produced from Woody Biomass. Energy Fuels 2020, 34, 15343–15354. [Google Scholar] [CrossRef]
- Wannapeera, J.; Worasuwannarak, N. Upgrading of woody biomass by torrefaction under pressure. J. Anal. Appl. Pyrolysis 2012, 96, 173–180. [Google Scholar] [CrossRef]
- Matyjewicz, B.; Świechowski, K.; Koziel, J.A.; Białowiec, A. Proof-of-Concept of High-Pressure Torrefaction for Improvement of Pelletized Biomass Fuel Properties and Process Cost Reduction. Energies 2020, 13, 4790. [Google Scholar] [CrossRef]
- Brachi, P.; Miccio, F.; Ruoppolo, G.; Miccio, M. Pressurized steam torrefaction of biomass: Focus on solid, liquid, and gas phase distributions. Ind. Eng. Chem. Res. 2017, 56, 12163–12173. [Google Scholar] [CrossRef]
- Tu, R.; Sun, Y.; Wu, Y.; Fan, X.; Cheng, S.; Jiang, E.; Xu, X. The fuel properties and adsorption capacities of torrefied camellia shell obtained via different steam-torrefaction reactors. Energy 2022, 238, 121969. [Google Scholar] [CrossRef]
- Tong, S.; Xiao, L.; Li, X.; Zhu, X.; Liu, H.; Luo, G.; Worasuwannarak, N.; Kerdsuwan, S.; Fungtammasan, B.; Yao, H. A gas-pressurized torrefaction method for biomass wastes. Energy Convers. Manag. 2018, 173, 29–36. [Google Scholar] [CrossRef]
- Sun, Y.; Tong, S.; Li, X.; Wang, F.; Hu, Z.; Dacres, O.D.; Edreis, E.M.A.; Worasuwannarak, N.; Sun, M.; Liu, H.; et al. Gas-pressurized torrefaction of biomass wastes: The optimization of pressurization condition and the pyrolysis of torrefied biomass. Bioresour. Technol. 2021, 319, 124216. [Google Scholar] [CrossRef] [PubMed]
- Bach, Q.V.; Tran, K.Q.; Khalil, R.A.; Skreiberg, Ø.; Seisenbaeva, G. Comparative assessment of wet torrefaction. Energy Fuels 2013, 27, 6743–6753. [Google Scholar] [CrossRef]
- Funke, A.; Felix Reebs, F.; Kruse, A. Experimental comparison of hydrothermal and vapothermal carbonization. Fuel Process. Technol. 2013, 115, 261–269. [Google Scholar] [CrossRef]
- Roy, B.; Kleine-Möllhoff, P.; Dalibard, A. Superheated Steam Torrefaction of Biomass Residues with Valorisation of Platform Chemicals—Part 1: Ecological Assessment. Sustainability 2022, 14, 1212. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, X.; Qi, Z.; Wang, H.; Yang, R.; Lin, W.; Li, J.; Zhou, W.; Ronsse, F. Superheated steam as carrier gas and the sole heat source to enhance biomass torrefaction. Bioresour. Technol. 2021, 331, 124955. [Google Scholar] [CrossRef]
- Mobini, M.; Meyer, J.C.; Trippe, F.; Sowlati, T.; Fröhling, M.; Schultmann, F. Assessing the integration of torrefaction into wood pellet production. J. Clean. Prod. 2014, 78, 216–225. [Google Scholar] [CrossRef]
- Couhert, C.; Salvador, S.; Commandré, J.M. Impact of torrefaction on syngas production from wood. Fuel 2009, 88, 2286–2290. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, J.S.; Liu, B.J.; Mei, Q.F.; Luo, Z.Y. Influence of torrefaction pretreatment on biomass gasification technology. Chin. Sci. Bull. 2011, 56, 1449–1456. [Google Scholar] [CrossRef]
- Pinto, F.; Gominho, J.; André, R.; Gonçalves, D.; Miranda, M.; Telles Varela, F.; Neves, D.; Santos, J.; Lourenço, A.; Pereira, H. Improvement of gasification performance of Eucalyptus globulus stumps with torrefaction and densification pre-treatments. Fuel 2017, 206, 289–299. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, C.J.; Hung, C.I.; Shen, C.H.; Hsu, H.W. A comparison of gasification phenomena among raw biomass, torrefied biomass and coal in an entrained-flow reactor. Appl. Energy 2013, 112, 421–430. [Google Scholar] [CrossRef]
- Rousset, P.; Aguiar, C.; Labbé, N.; Commandré, J.M. Enhancing the combustible properties of bamboo by torrefaction. Bioresour. Technol. 2011, 102, 8225–8231. [Google Scholar] [CrossRef] [PubMed]
- Pimchuai, A.; Dutta, A.; Basu, P. Torrefaction of agriculture residue to enhance combustible properties. Energy Fuels 2010, 24, 4638–4645. [Google Scholar] [CrossRef]
- Li, J.; Brzdekiewicz, A.; Yang, W.; Blasiak, W. Co-firing based on biomass torrefaction in a pulverized coal boiler with aim of 100% fuel switching. Appl. Energy 2012, 99, 344–354. [Google Scholar] [CrossRef]
- Perea-Moreno, M.-A.; Manzano-Agugliaro, F.; Perea-Moreno, A.-J. Sustainable Energy Based on Sunflower Seed Husk Boiler for Residential Buildings. Sustainability 2018, 10, 3407. [Google Scholar] [CrossRef]
- De Fusco, L.; Boucquey, A.; Blondeau, J.; Jeanmart, H.; Contino, F. Fouling propensity of high-phosphorus solid fuels: Predictive criteria and ash deposits characterization of sunflower hulls with P/Ca-additives in a drop tube furnace. Fuel 2016, 170, 16–26. [Google Scholar] [CrossRef]
- Abelha, P.; Vilela, C.M.; Nanou, P.; Carbo, M.; Janssen, A.; Leiser, S. Combustion improvements of upgraded biomass by washing and torrefaction. Fuel 2019, 253, 1018–1033. [Google Scholar] [CrossRef]
- Saddawi, A.; Jones, J.M.; Williams, A.; Le Coeur, C. Commodity fuels from biomass through pretreatment and torrefaction: Effects of mineral content on torrefied fuel characteristics and quality. Energy Fuels 2012, 26, 6466–6474. [Google Scholar] [CrossRef]
- Chen, D.; Mei, J.; Li, H.; Li, Y.; Lu, M.; Ma, T.; Ma, Z. Combined pretreatment with torrefaction and washing using torrefaction liquid products to yield upgraded biomass and pyrolysis products. Bioresour. Technol. 2017, 228, 62–68. [Google Scholar] [CrossRef]
- Nebyvaev, A.; Klimov, D.; Ryzhenkov, A.; Brulé, M. Preliminary Results of Innovative Two-Stage Torrefaction Technology Applied for Thermochemical Treatment of Sunflower Husk. Processes 2023, 11, 2486. [Google Scholar] [CrossRef]
- Kongto, P.; Palamanit, A.; Chaiprapat, S.; Tippayawong, N. Enhancing the fuel properties of rubberwood biomass by moving bed torrefaction process for further applications. Renew. Energy 2021, 170, 703–713. [Google Scholar] [CrossRef]
- Zhang, D.; Han, P.; Zheng, H.; Yan, Z. Torrefaction of walnut oil processing wastes by superheated steam: Effects on products characteristics. Sci. Total Environ. 2021, 830, 154649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Han, P.; Yang, R.; Wang, H.; Lin, W.; Zhou, W.; Yan, Z.; Qi, Z. Fuel properties and combustion behaviors of fast torrefied pinewood in a heavily loaded fixed-bed reactor by superheated steam. Bioresour. Technol. 2021, 342, 125929. [Google Scholar] [CrossRef]
- Singh, R.k.; Sarkar, A.; Chakraborty, J.P. Effect of torrefaction on the physicochemical properties of pigeon pea stalk (Cajanus cajan) and estimation of kinetic parameters. Renew. Energy 2019, 138, 805–819. [Google Scholar] [CrossRef]
- Vamvuka, D.; Zografos, D. Predicting the behaviour of ash from agricultural wastes during combustion. Fuel 2004, 83, 2051–2057. [Google Scholar] [CrossRef]


| Characteristics | Raw SFH | Torrefied SFH | ||
|---|---|---|---|---|
| 220 °C | 265 °C | 295 °C | ||
| Moisture, % | 7.06 | 3.56 | 3.45 | 3.89 |
| Ash, % | 2.86 | 3.86 | 4.11 | 7.01 |
| S, % | 0.02 | <0.01 | <0.01 | <0.01 |
| C, % | 47.8 | 53.7 | 56.0 | 66.7 |
| H2, % | 5.49 | 5.16 | 5.08 | 3.98 |
| N2, % | 0.63 | 0.54 | 0.57 | 0.71 |
| O2, % | 36.14 | 33.17 | 30.78 | 17.7 |
| Volatile substances (in analytical sample) VM, % | 70.85 | 64.92 | 60.62 | 36.29 |
| Volatile substances, % (dry state) | 76.23 | 67.31 | 62.78 | 37.76 |
| Fixed carbon content, %, FC | 22.09 | 31.52 | 35.93 | 59.82 |
| Fuel ratio (FR = FC/VM) | 0.31 | 0.51 | 0.59 | 1.65 |
| Combustion index CI (MJ/kg) = HHV × (115 − Ash) × VM/105 × FC | 65.08 | 45.55 | 38.49 | 15.7 |
| LCV, MJ/kg | 17.7 | 19.6 | 20.5 | 24.1 |
| HHV, MJ/kg | 19.0 | 20.9 | 21.6 | 25.1 |
| Mass loss of sample, % | - | 8 | 15 | 40 |
| MY | - | 92 | 85 | 60 |
| EE | - | 1.11 | 1.16 | 1.36 |
| EY | - | 102.12 | 98.6 | 81.6 |
| DT | - | 8.4 | 17.6 | 50.5 |
| Elemental Composition of Ash Per Calcined Material (550 °C), wt. % | Raw SFH | Torrefied SFH | ||
|---|---|---|---|---|
| 220 °C | 265 °C | 295 °C | ||
| CO2 carbonates | 17.8 | 27.6 | 24.7 | 21.2 |
| SiO2 | 2.78 | 5.85 | 3.98 | 4.82 |
| TiO2 | 0.04 | 0.04 | 0.04 | 0.04 |
| Al2O3 | 0.58 | 0.79 | 0.69 | 0.7 |
| Fe2O3 | 0.35 | 1.36 | 0.69 | 0.82 |
| CaO | 7.14 | 6.16 | 6.29 | 6.47 |
| MgO | 12.04 | 10.74 | 9.78 | 10.78 |
| K2O | 38.19 | 30.80 | 36.40 | 35.87 |
| Na2O | 0.58 | 0.50 | 0.29 | 0.31 |
| P2O5 | 6.78 | 4.06 | 4.69 | 3.2 |
| SO3 | 5.65 | 5.04 | 5.61 | 6.57 |
| Cl | 1.06 | 0.68 | 0.44 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milovanov, O.; Klimov, D.; Kuzmin, S.; Grigoriev, S.; Mikhalev, A.; Isemin, R.; Brulé, M. Application of Torrefaction for Improved Fuel Properties of Sunflower Husks. Energies 2024, 17, 4643. https://doi.org/10.3390/en17184643
Milovanov O, Klimov D, Kuzmin S, Grigoriev S, Mikhalev A, Isemin R, Brulé M. Application of Torrefaction for Improved Fuel Properties of Sunflower Husks. Energies. 2024; 17(18):4643. https://doi.org/10.3390/en17184643
Chicago/Turabian StyleMilovanov, Oleg, Dmitry Klimov, Sergey Kuzmin, Sergey Grigoriev, Alexander Mikhalev, Rafail Isemin, and Mathieu Brulé. 2024. "Application of Torrefaction for Improved Fuel Properties of Sunflower Husks" Energies 17, no. 18: 4643. https://doi.org/10.3390/en17184643





