Enhancing Azo Dye Mineralization and Bioelectricity Generation through Biocathode-Microbial Fuel Cell Integration with Aerobic Bioreactor
Abstract
:1. Introduction
2. Results and Discussion
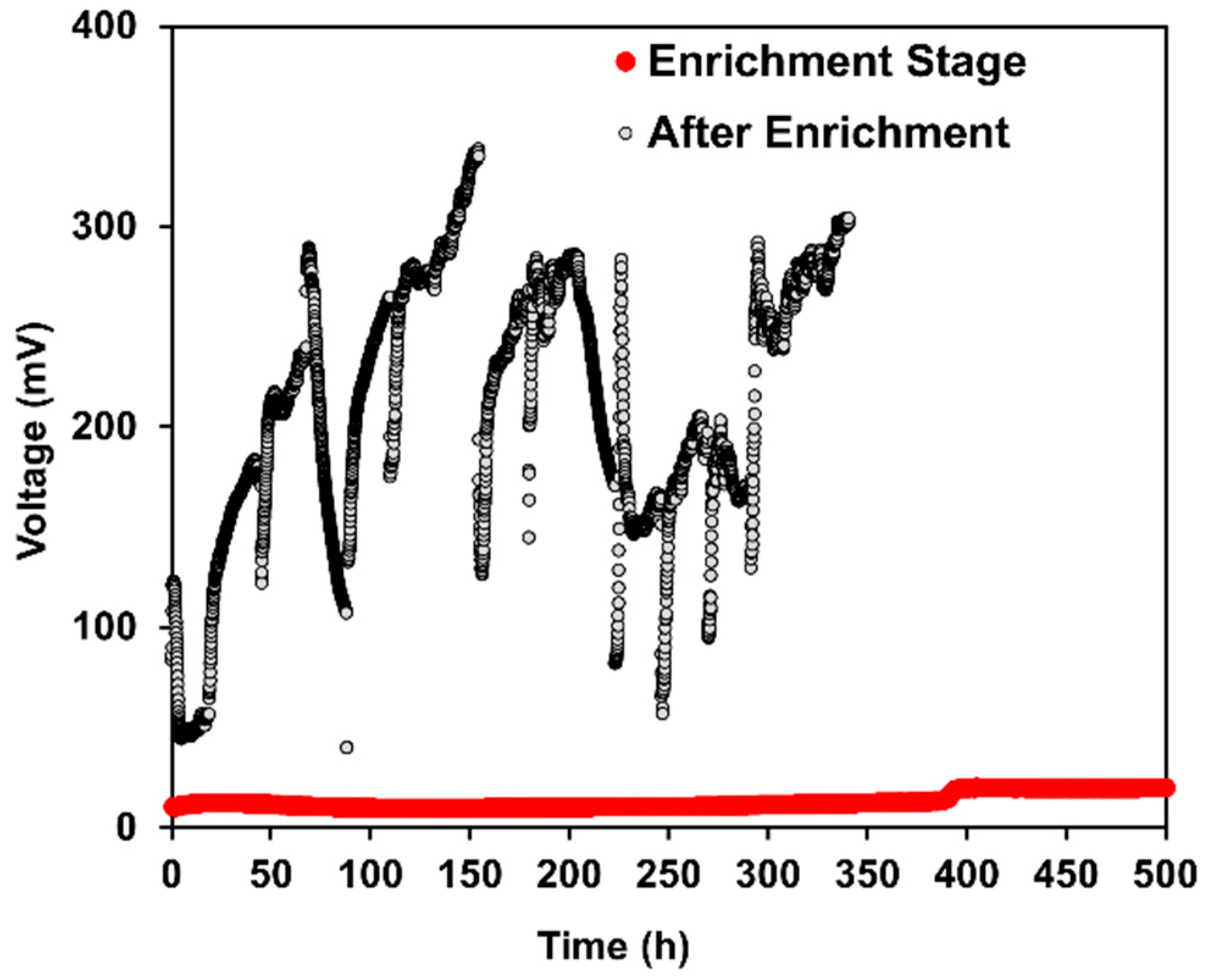
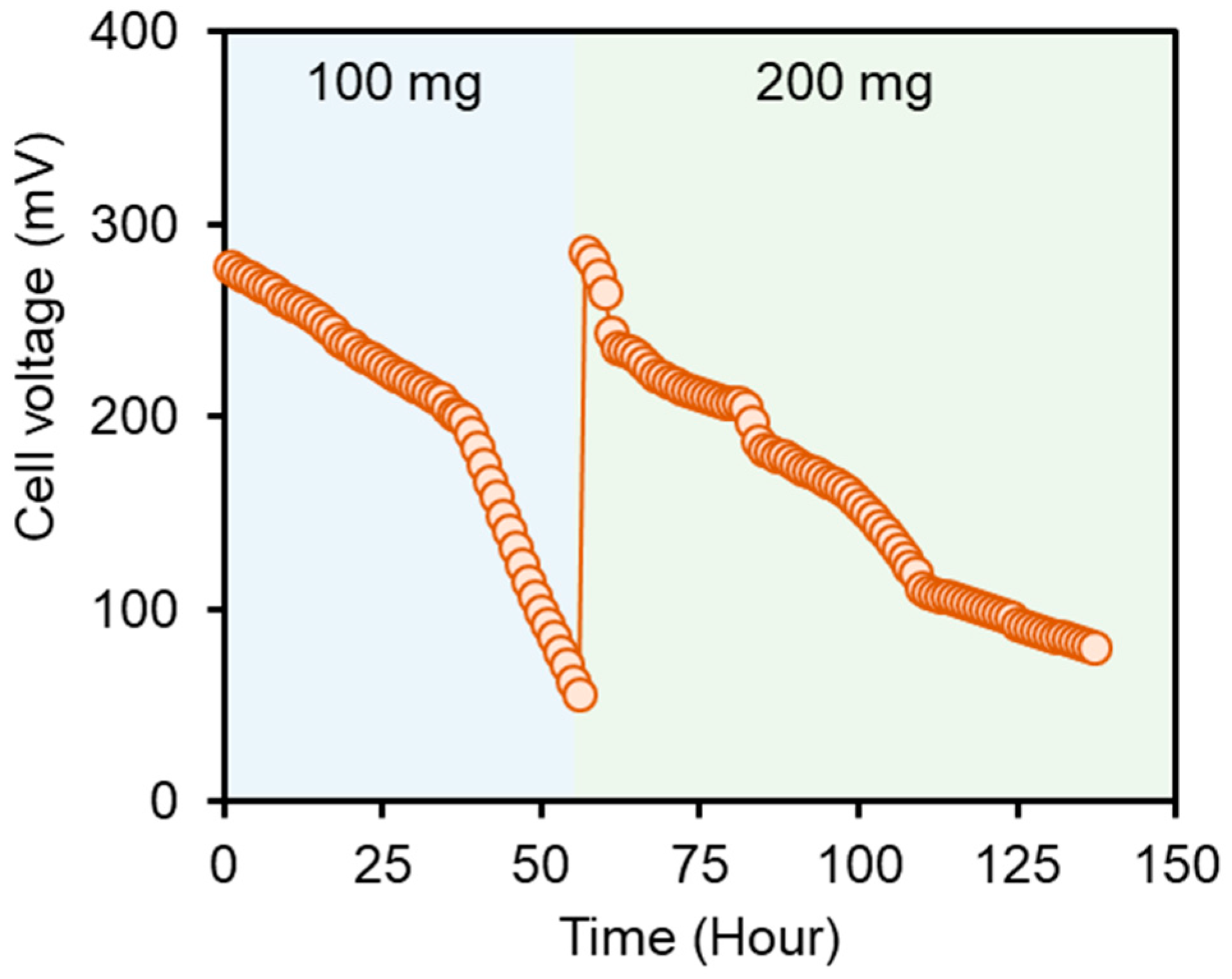
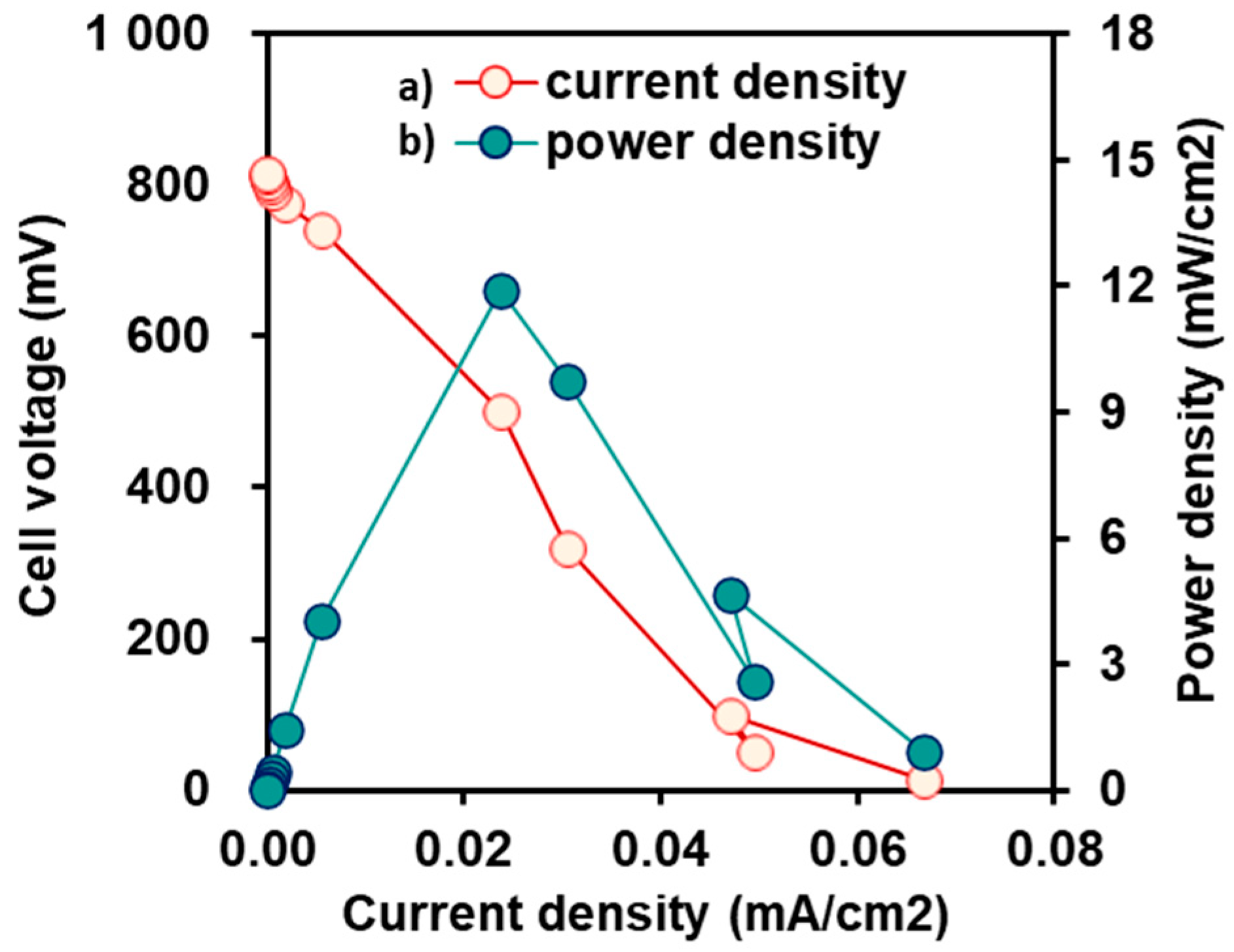
2.1. Bioelectrochemical Performance of DCMFCs
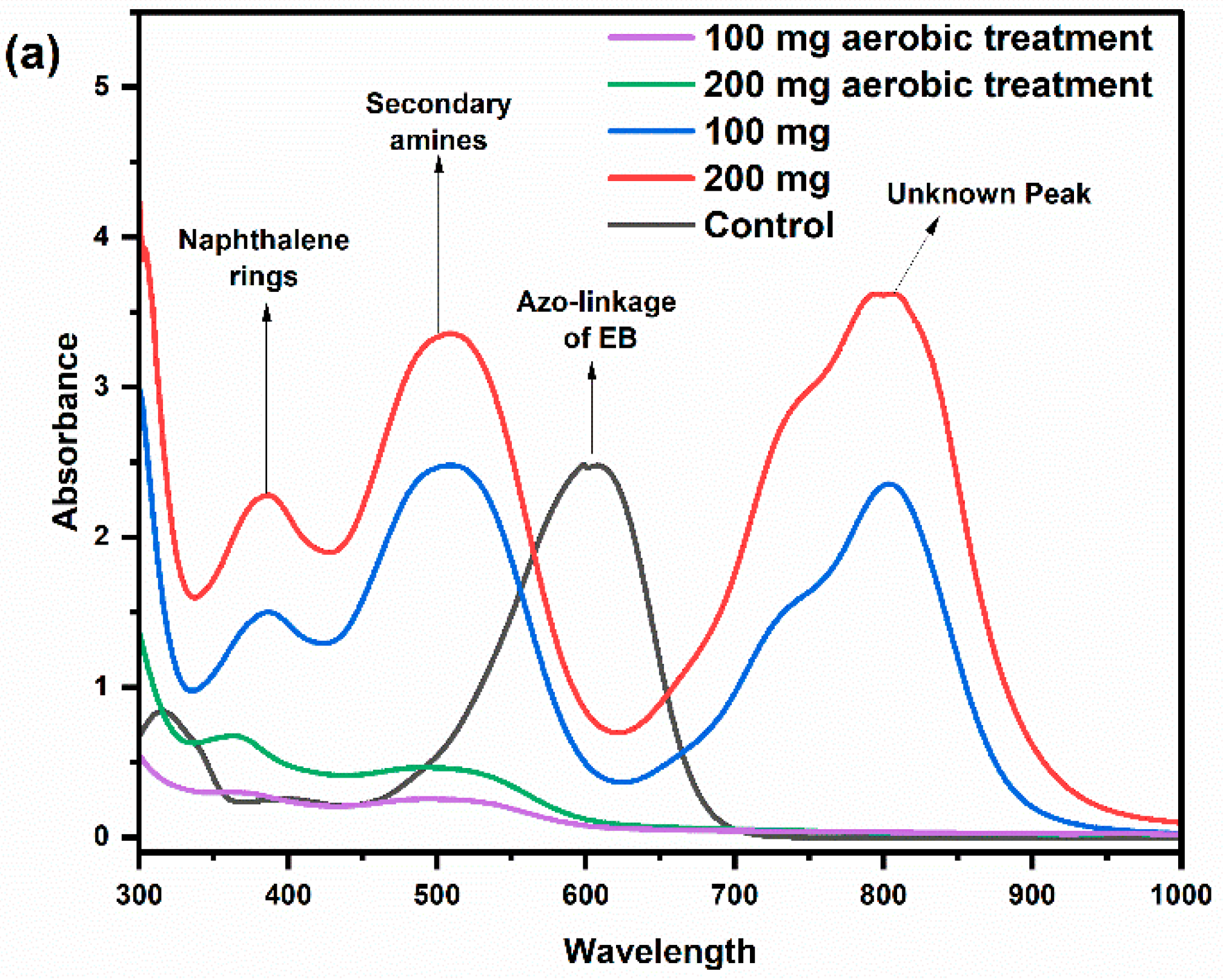
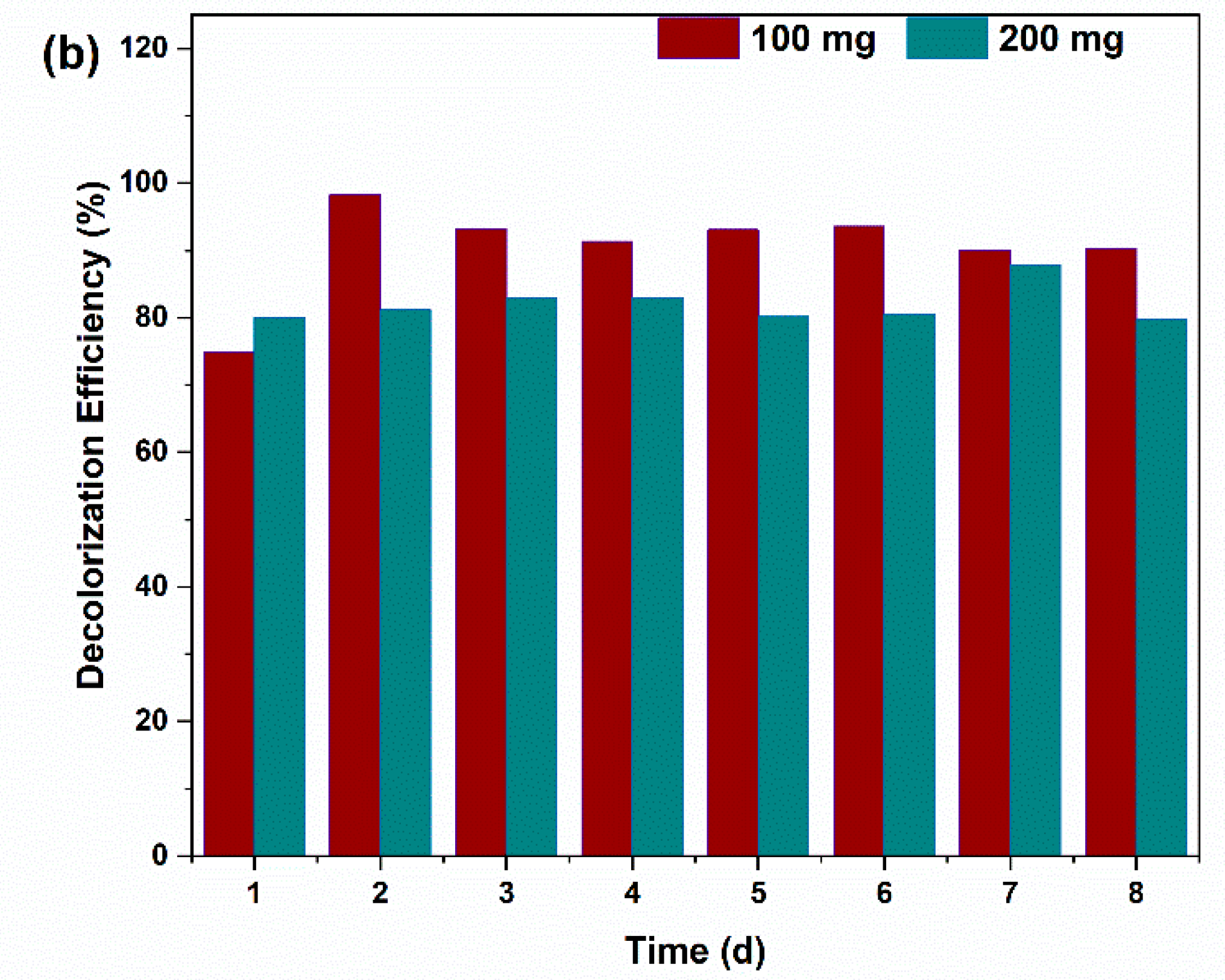
2.2. Decolorization Efficiency of the Dyes
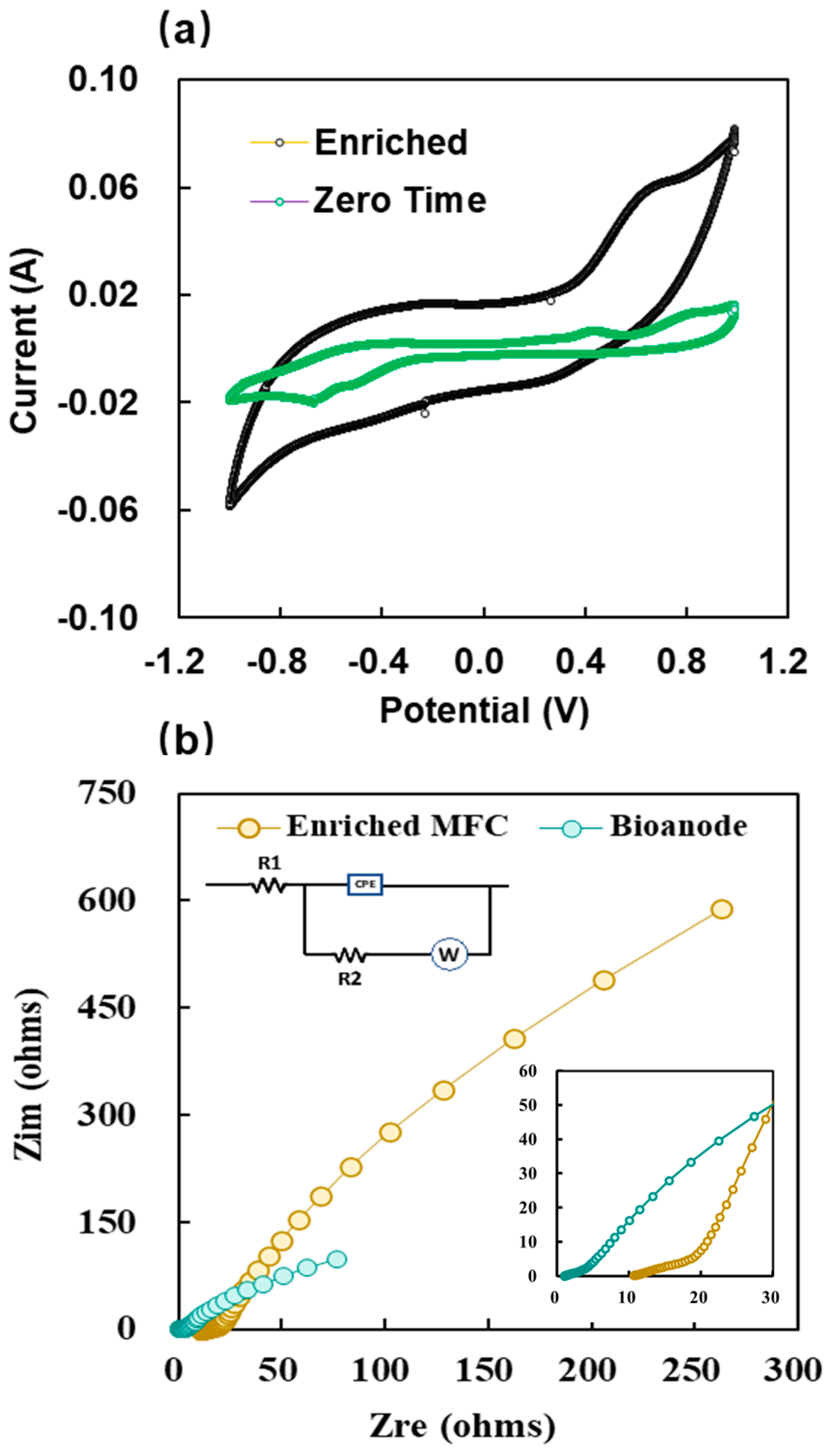
2.3. Electrochemical Characteristics of the Anode Biofilms
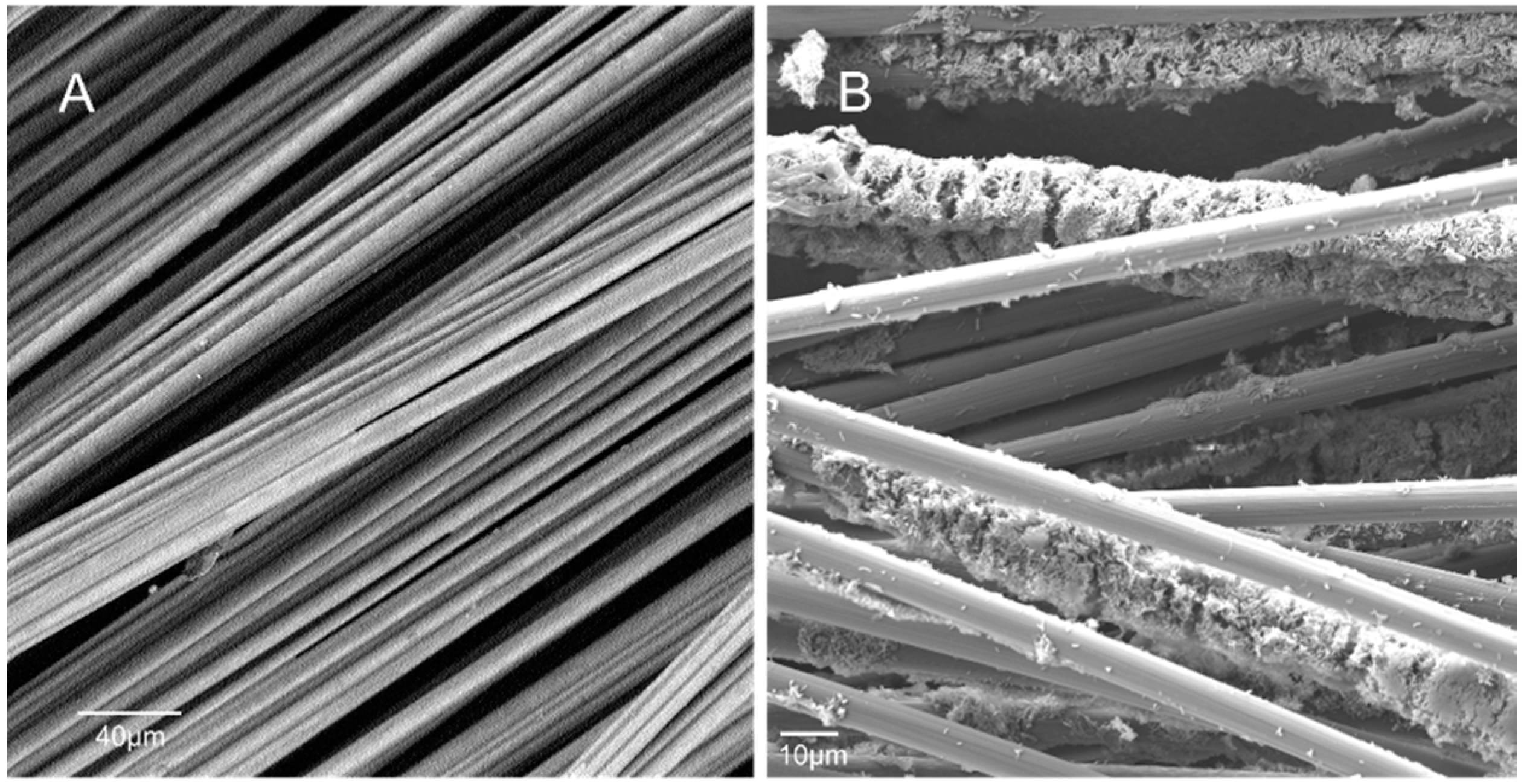
2.4. Surface Characterization and Microbial Biofilm Community Analysis
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Anodic and Cathodic Media
3.3. Inoculum Preparation
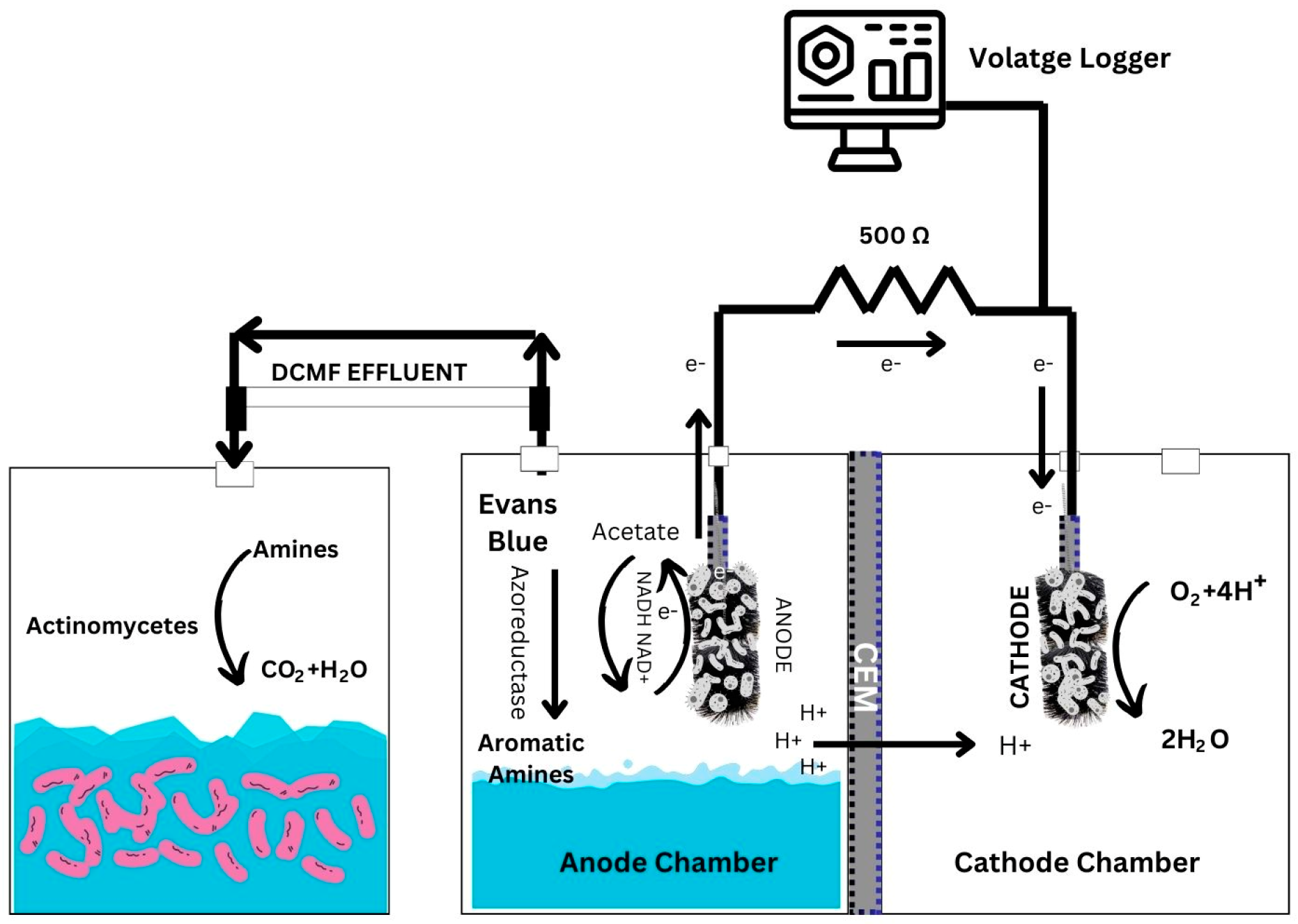
3.4. The Design and Functioning of the Integrated DCMFC–Aerobic Reactor System
3.5. Detection Method for Evans Blue Decolorization
3.6. Monitoring of MFC Performance
3.7. Scanning Electron Microscopy (SEM) of the Anode Surface
3.8. DNA Extraction, PCR Amplification and Illumina Sequencing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rabaey, K.; Verstraete, W. Microbial Fuel Cells: Novel Biotechnology for Energy Generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Zafar, Z.; Ayaz, K.; Nasir, M.H.; Yousaf, S.; Sharafat, I.; Ali, N. Electrochemical Performance of Biocathode Microbial Fuel Cells Using Petroleum-Contaminated Soil and Hot Water Spring. Int. J. Environ. Sci. Technol. 2019, 16, 1487–1500. [Google Scholar] [CrossRef]
- Kyazze, G. Taking the Waste out of Dye Wastewater Using Microbial Fuel Cells. In Proceedings of the 5th EU-ISMET Conference, Online, 13–15 September 2021. [Google Scholar]
- Rawat, D.; Mishra, V.; Sharma, R.S. Detoxification of Azo Dyes in the Context of Environmental Processes. Chemosphere 2016, 155, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ahmad, R. Biosorption of Hazardous Crystal Violet Dye from Aqueous Solution onto Treated Ginger Waste (TGW). Desalination 2011, 265, 112–118. [Google Scholar] [CrossRef]
- Popli, S.; Patel, U.D. Destruction of Azo Dyes by Anaerobic–Aerobic Sequential Biological Treatment: A Review. Int. J. Environ. Sci. Technol. 2015, 12, 405–420. [Google Scholar] [CrossRef]
- Ilamathi, R.; Jayapriya, J. Microbial Fuel Cells for Dye Decolorization. Environ. Chem. Lett. 2018, 16, 239–250. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Adhami, A.; Darvari, S.; Zirepour, A.; Oh, S.E. Microbial Fuel Cell as New Technol Ogy for Bioelectricity Generation: A Review. Alex. Eng. J. 2015, 54, 745–756. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial Fuel Cells: An Overview of Current Technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Mani, P.; Fidal, V.T.; Bowman, K.; Breheny, M.; Chandra, T.S.; Keshavarz, T.; Kyazze, G. Degradation of Azo Dye (Acid Orange 7) in a Microbial Fuel Cell: Comparison Between Anodic Microbial-Mediated Reduction and Cathodic Laccase-Mediated Oxidation. Front. Energy Res. 2019, 7, 101. [Google Scholar] [CrossRef]
- Yao, L.; Xue, X.; Yu, P.; Ni, Y.; Chen, F. Evans Blue Dye: A Revisit of Its Applications in Biomedicine. Contrast Media Mol. Imaging 2018, 2018, 7628037. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Hu, Y.; Hou, B.; Zhang, Y.; Li, S. Understanding the Degradation of Congo Red and Bacterial Diversity in an Air-Cathode Microbial Fuel Cell Being Evaluated for Simultaneous Azo Dye Removal from Wastewater and Bioelectricity Generation. Appl. Microbiol. Biotechnol. 2013, 97, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kumar, P.; Rawat, D.; Garg, S.; Mukherjee, P.; Farooqi, F.; Roy, A.; Sundaram, S.; Sharma, R.S.; Mishra, V. Microbial Fuel Cells for Mineralization and Decolorization of Azo Dyes: Recent Advances in Design and Materials. Sci. Total Environ. 2022, 826, 154038. [Google Scholar] [PubMed]
- Meenakshi, S.; Hiremath, J.; Meenakshi, M.H.; Shivaveerakumar, S. Actinomycetes: Isolation, Cultivation and Its Active Biomolecules. J. Pure Appl. Microbiol. 2024, 18, 118–143. [Google Scholar] [CrossRef]
- Kong, F.; Ren, H.Y.; Pavlostathis, S.G.; Wang, A.; Nan, J.; Ren, N.Q. Enhanced Azo Dye Decolorization and Microbial Community Analysis in a Stacked Bioelectrochemical System. Chem. Eng. J. 2018, 354, 351–362. [Google Scholar] [CrossRef]
- Sun, J.; Cai, B.; Zhang, Y.; Peng, Y.; Chang, K.; Ning, X.; Liu, G.; Yao, K.; Wang, Y.; Yang, Z.; et al. Regulation of Biocathode Microbial Fuel Cell Performance with Respect to Azo Dye Degradation and Electricity Generation via the Selection of Anodic Inoculum. Int. J. Hydrogen Energy 2016, 41, 5141–5150. [Google Scholar] [CrossRef]
- Sultana, S.; Khan, M.D.; Sabir, S.; Gani, K.M.; Oves, M.; Khan, M.Z. Bio-Electro Degradation of Azo-Dye in a Combined Anaerobic–Aerobic Process along with Energy Recovery. New J. Chem. 2015, 39, 9461–9470. [Google Scholar] [CrossRef]
- Khan, M.D.; Li, D.; Tabraiz, S.; Shamurad, B.; Scott, K.; Khan, M.Z.; Yu, E.H. Integrated Air Cathode Microbial Fuel Cell-Aerobic Bioreactor Set-up for Enhanced Bioelectrodegradation of Azo Dye Acid Blue 29. Sci. Total Environ. 2021, 756, 143752. [Google Scholar] [CrossRef]
- Thung, W.E.; Ong, S.A.; Ho, L.N.; Wong, Y.S.; Ridwan, F.; Oon, Y.L.; Oon, Y.S.; Lehl, H.K. A Highly Efficient Single Chambered Up-Flow Membrane-Less Microbial Fuel Cell for Treatment of Azo Dye Acid Orange 7-Containing Wastewater. Bioresour. Technol. 2015, 197, 284–288. [Google Scholar] [CrossRef]
- Katuri, K.P.; Scott, K.; Head, I.M.; Picioreanu, C.; Curtis, T.P. Microbial Fuel Cells Meet with External Resistance. Bioresour. Technol. 2011, 102, 2758–2766. [Google Scholar] [CrossRef]
- Potrykus, S.; León-Fernández, L.F.; Nieznański, J.; Karkosiński, D.; Fernandez-Morales, F.J. The Influence of External Load on the Performance of Microbial Fuel Cells. Energies 2021, 14, 612. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, Y.; Chen, L.; He, Q.; Zhu, D.; Cao, H.; Cheng, J. Highly Efficient Single Fluorescent Probe for Multiple Amine Vapours via Reaction between Amine and Aldehyde/Dioxaborolane. RSC Adv. 2014, 4, 46631–46634. [Google Scholar] [CrossRef]
- Quan, X.; Xu, H.; Sun, B.; Xiao, Z. Anode Modification with Palladium Nanoparticles Enhanced Evans Blue Removal and Power Generation in Microbial Fuel Cells. Int. Biodeterior. Biodegrad. 2018, 132, 94–101. [Google Scholar] [CrossRef]
- Long, X.; Cao, X.; Liu, S.; Nishimura, O.; Li, X. The Azo Dye Degradation and Differences between the Two Anodes on the Microbial Community in a Double-Anode Microbial Fuel Cell. Water. Air. Soil Pollut. 2019, 230, 265. [Google Scholar] [CrossRef]
- Fang, Z.; Song, H.L.; Cang, N.; Li, X.N. Electricity Production from Azo Dye Wastewater Using a Microbial Fuel Cell Coupled Constructed Wetland Operating under Different Operating Conditions. Biosens. Bioelectron. 2015, 68, 135–141. [Google Scholar] [CrossRef]
- Sanchez-Herrera, D.; Pacheco-Catalan, D.; Valdez-Ojeda, R.; Canto-Canche, B.; Dominguez-Benetton, X.; Domínguez-Maldonado, J.; Alzate-Gaviria, L. Characterization of Anode and Anolyte Community Growth and the Impact of Impedance in a Microbial Fuel Cell. BMC Biotechnol. 2014, 14, 102. [Google Scholar] [CrossRef]
- Wang, H.; Long, X.; Sun, Y.; Wang, D.; Wang, Z.; Meng, H.; Jiang, C.; Dong, W.; Lu, N. Electrochemical Impedance Spectroscopy Applied to Microbial Fuel Cells: A Review. Front. Microbiol. 2022, 13, 973501. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Khan, M.D.; Nizami, A.S.; Rehan, M.; Shaida, A.; Ahmad, A.; Khan, M.Z. Energy Generation through Bioelectrochemical Degradation of Pentachlorophenol in Microbial Fuel Cell. RSC Adv. 2018, 8, 20726–20736. [Google Scholar] [CrossRef] [PubMed]
- Offei, F.; Thygesen, A.; Mensah, M.; Tabbicca, K.; Fernando, D.; Petrushina, I.; Daniel, G. A Viable Electrode Material for Use in Microbial Fuel Cells for Tropical Regions. Energies 2016, 9, 35. [Google Scholar] [CrossRef]
- Yang, S.; Du, F.; Liu, H. Characterization of Mixed-Culture Biofilms Established in Microbial Fuel Cells. Biomass Bioenergy 2012, 46, 531–537. [Google Scholar] [CrossRef]
- Pham, T.H.; Boon, N.; Aelterman, P.; Clauwaert, P.; De Schamphelaire, L.; Vanhaecke, L.; De Maeyer, K.; Höfte, M.; Verstraete, W.; Rabaey, K. Metabolites Produced by Pseudomonas Sp. Enable a Gram-Positive Bacterium to Achieve Extracellular Electron Transfer. Appl. Microbiol. Biotechnol. 2008, 77, 1119–1129. [Google Scholar] [CrossRef]
- Hemdan, B.A.; El-Taweel, G.E.; Naha, S.; Goswami, P. Bacterial Community Structure of Electrogenic Biofilm Developed on Modified Graphite Anode in Microbial Fuel Cell. Sci. Rep. 2023, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Arbour, T.J.; Gilbert, B.; Banfield, J.F. Diverse Microorganisms in Sediment and Groundwater Are Implicated in Extracellular Redox Processes Based on Genomic Analysis of Bioanode Communities. Front. Microbiol. 2020, 11, 508335. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, L.; Kulko, M.; Kim, R.; Jashari, T.; Choe, T. 16S RRNA Analysis of Electrogenic Bacterial Communities in Microbial Fuel Cells Developed from Temperate Soils. Bios 2020, 91, 9–20. [Google Scholar] [CrossRef]
- De La Cruz-Noriega, M.; Benites, S.M.; Rojas-Flores, S.; Otiniano, N.M.; Sabogal Vargas, A.M.; Alfaro, R.; Cabanillas-Chirinos, L.; Rojas-Villacorta, W.; Nazario-Naveda, R.; Delfín-Narciso, D. Use of Wastewater and Electrogenic Bacteria to Generate Eco-Friendly Electricity through Microbial Fuel Cells. Sustainability 2023, 15, 10640. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, S.; Liu, H.; Huang, J.; Li, L. Sulfide-Mediated Azo Dye Degradation and Microbial Community Analysis in a Single-Chamber Air Cathode Microbial Fuel Cell. Bioelectrochemistry 2020, 131, 107349. [Google Scholar] [CrossRef]
- Rathour, R.; Patel, D.; Shaikh, S.; Desai, C. Eco-Electrogenic Treatment of Dyestuff Wastewater Using Constructed Wetland-Microbial Fuel Cell System with an Evaluation of Electrode-Enriched Microbial Community Structures. Bioresour. Technol. 2019, 285, 121349. [Google Scholar] [CrossRef]
- He, Y.Q.; Chen, R.W.; Li, C.; Shi, S.B.; Cui, L.Q.; Long, L.J.; Tian, X.P. Actinomarinicola tropica Gen. Nov. Sp. Nov., a New Marine Actinobacterium of the Family Iamiaceae, Isolated from South China Sea Sediment Environments. Int. J. Syst. Evol. Microbiol. 2020, 70, 3852–3858. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Mo, Y.; Zhang, L. A Mini Review on Bio-Electrochemical Systems for the Treatment of Azo Dye Wastewater: State-of-the-Art and Future Prospects. Chemosphere 2022, 294, 133801. [Google Scholar] [CrossRef]
- Antonin, V.S.; Garcia-Segura, S.; Santos, M.C.; Brillas, E. Degradation of Evans Blue Diazo Dye by Electrochemical Processes Based on Fenton’s Reaction Chemistry. J. Electroanal. Chem. 2015, 747, 1–11. [Google Scholar] [CrossRef]
- Dai, M.; Zhang, Y.; Wu, Y.; Sun, R.; Zong, W.; Kong, Q. Mechanism Involved in the Treatment of Sulfamethoxazole in Wastewater Using a Constructed Wetland Microbial Fuel Cell System. J. Environ. Chem. Eng. 2021, 9, 106193. [Google Scholar] [CrossRef]
- Choi, Y.; Jung, E.; Park, H.; Paik, S.R.; Jung, S.; Kim, S. Construction of Microbial Fuel Cells Using Thermophilic Microorganisms, Bacillus Licheniformis and Bacillus Thermoglucosidasius. Bull. Korean Chem. Soc. 2004, 25, 813–818. [Google Scholar] [CrossRef]
- Fu, Q.; Kobayashi, H.; Kawaguchi, H.; Vilcaez, J.; Wakayama, T.; Maeda, H.; Sato, K. Electrochemical and Phylogenetic Analyses of Current-Generating Microorganisms in a Thermophilic Microbial Fuel Cell. J. Biosci. Bioeng. 2013, 115, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Jong, B.C.; Kim, B.H.; Chang, I.S.; Liew, P.W.Y.; Choo, Y.F.; Kang, G.S. Enrichment, Performance, and Microbial Diversity of a Thermophilic Mediatorless Microbial Fuel Cell. Environ. Sci. Technol. 2006, 40, 6449–6454. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Li, Y.; Cao, J. Study on Dyeing Wastewater Treatment at High Temperature by MBBR and the Thermotolerant Mechanism Based on Its Microbial Analysis. Process Biochem. 2015, 50, 1934–1941. [Google Scholar] [CrossRef]
- Zhang, G.; Feng, S.; Jiao, Y.; Lee, D.J.; Xin, Y.; Sun, H. Cathodic Reducing Bacteria of Dual-Chambered Microbial Fuel Cell. Int. J. Hydrogen Energy 2017, 42, 27607–27617. [Google Scholar] [CrossRef]
- Nazina, T.N.; Bidzhieva, S.K.; Grouzdev, D.S.; Sokolova, D.S.; Tourova, T.P.; Parshina, S.N.; Avtukh, A.N.; Poltaraus, A.B.; Talybly, A.K. Soehngenia longivitae sp. nov., a Fermenting Bacterium Isolated from a Petroleum Reservoir in Azerbaijan, and Emended Description of the Genus Soehngenia. Microorganisms 2020, 8, 1967. [Google Scholar] [CrossRef]
- Naz, I.; Hodgson, D.; Smith, A.; Marchesi, J.; Sehar, S.; Ahmed, S.; Lynch, J.; Avignone-Rossa, C.; Saroj, D.P. Investigation of the Active Biofilm Communities on Polypropylene Filter Media in a Fixed Biofilm Reactor for Wastewater Treatment. J. Chem. Technol. Biotechnol. 2018, 93, 3264–3275. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Y.; Cai, J.; Wen, H.; Mao, Z.; Zhang, H.; Wang, X.; Ma, L.; Zhu, M. Electricity Production and the Analysis of the Anode Microbial Community in a Constructed Wetland-Microbial Fuel Cell. RSC Adv. 2019, 9, 21460. [Google Scholar] [CrossRef]
- Fischer, F.; Merino, N.; Sugnaux, M.; Huguenin, G.; Nealson, K.H. Microbial Community Diversity Changes during Voltage Reversal Repair in a 12-Unit Microbial Fuel Cell. Chem. Eng. J. 2022, 446, 137334. [Google Scholar] [CrossRef]
- Brune, A.; Ludwig, W.; Schink, B. Propionivibrio limicola Sp. Nov., a Fermentative Bacterium Specialized in the Degradation of Hydroaromatic Compounds, Reclassification of Propionibacter Pelophilus as Propionivibrio Pelophilus Comb. Nov. and Amended Description of the Genus Propionivibrio. Int. J. Syst. Evol. Microbiol. 2002, 52 Pt 2, 441–444. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Y.; Zhang, X.; Zhang, K.; Chen, S.; Wu, H.; He, J. Proteiniclasticum sediminis Sp. Nov., an Obligate Anaerobic Bacterium Isolated from Anaerobic Sludge. Antonie Van Leeuwenhoek 2021, 114, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Díaz, M.I.; Zárate-Segura, P.; Bermeo-Fernández, L.A.; Nirmalkar, K.; Bastida-González, F.; García-Mena, J.; Jan-Roblero, J.; Guerrero-Barajas, C. Bacterial Consortium from Hydrothermal Vent Sediments Presents Electrogenic Activity Achieved under Sulfate Reducing Conditions in a Microbial Fuel Cell. J. Environ. Health Sci. Eng. 2020, 18, 1189. [Google Scholar] [CrossRef]
- Ravadelli, M.; Da Costa, R.E.; Lobo-Recio, M.A.; Akaboci, T.R.V.; Bassin, J.P.; Lapolli, F.R.; Belli, T.J. Anoxic/Oxic Membrane Bioreactor Assisted by Electrocoagulation for the Treatment of Azo-Dye Containing Wastewater. J. Environ. Chem. Eng. 2021, 9, 105286. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Sun, Y.; Zhao, X.; Li, Y. Cathodic Microbial Community Adaptation to the Removal of Chlorinated Herbicide in Soil Microbial Fuel Cells. Environ. Sci. Pollut. Res. 2018, 25, 16900–16912. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xue, H.; Feng, X.; Pyo, S.H. Electrostimulation for Promoted Microbial Community and Enhanced Biodegradation of Refractory Azo Dyes. J. Environ. Chem. Eng. 2022, 10, 108626. [Google Scholar] [CrossRef]
- Zhong, D.; Liu, Y.; Liao, X.; Zhong, N.; Xu, Y. Facile Preparation of Binder-Free NiO/MnO2-Carbon Felt Anode to Enhance Electricity Generation and Dye Wastewater Degradation Performances of Microbial Fuel Cell. Int. J. Hydrogen Energy 2018, 43, 23014–23026. [Google Scholar] [CrossRef]
- Kameche, K.; Amrani, S.; Mouzaoui, S.; Aït-Amar, H. Biodegradation of Diazo Dye Evans Blue by Four Strains of Streptomyces Isolated from Soils of Algeria. Biocatal. Agric. Biotechnol. 2022, 46, 102529. [Google Scholar] [CrossRef]
- Ayaz, K.; Zabłocka-Godlewska, E. Screening of Actinomycetes Decolorizing the Synthetic Dyes from Rotten Poplar Wood and Garden Compost. In Contemporary Problems of Power Engineering and Environmental Protection; Pikoń, K., Bogacka, M., Eds.; Politechnika Śląska: Gliwice, Poland, 2022; pp. 119–131. [Google Scholar]
- Adenan, N.H.; Lim, Y.Y.; Ting, A.S.Y. Identification and Optimization of Triphenylmethane Dyes Removal by Streptomyces Sp. from Forest Soil. Sustain. Environ. Res. 2021, 31, 8. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Yadav, R.S.; He, W.; Liang, D.; Li, C.; Yu, Y.; Feng, Y. Enrichment of Geobacter on Anode Biofilms from Domestic Wastewater without Posing Anode Potential in Microbial Electrochemical Cells. Microbiol. Res. 2024, 15, 1859–1869. [Google Scholar] [CrossRef]
- Yadav, R.S.; He, W.; Liang, D.; Li, C.; Yu, Y.; Ayaz, K.; Feng, Y. High-Efficiency Hydrogen Recovery from Corn Straw Hydrolysate Using Functional Bacteria and Negative Pressure with Microbial Electrolysis Cells. Water 2024, 16, 2423. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayaz, K.; Zabłocka-Godlewska, E.; Li, C. Enhancing Azo Dye Mineralization and Bioelectricity Generation through Biocathode-Microbial Fuel Cell Integration with Aerobic Bioreactor. Energies 2024, 17, 4896. https://doi.org/10.3390/en17194896
Ayaz K, Zabłocka-Godlewska E, Li C. Enhancing Azo Dye Mineralization and Bioelectricity Generation through Biocathode-Microbial Fuel Cell Integration with Aerobic Bioreactor. Energies. 2024; 17(19):4896. https://doi.org/10.3390/en17194896
Chicago/Turabian StyleAyaz, Kamran, Ewa Zabłocka-Godlewska, and Chao Li. 2024. "Enhancing Azo Dye Mineralization and Bioelectricity Generation through Biocathode-Microbial Fuel Cell Integration with Aerobic Bioreactor" Energies 17, no. 19: 4896. https://doi.org/10.3390/en17194896




