Glucose-Treated Manganese Hexacyanoferrate for Sodium-Ion Secondary Battery
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation and Characterization
2.2. Structural Characterization
2.3. Electrochemical Properties
3. Results and Discussion
3.1. Crystal Structure


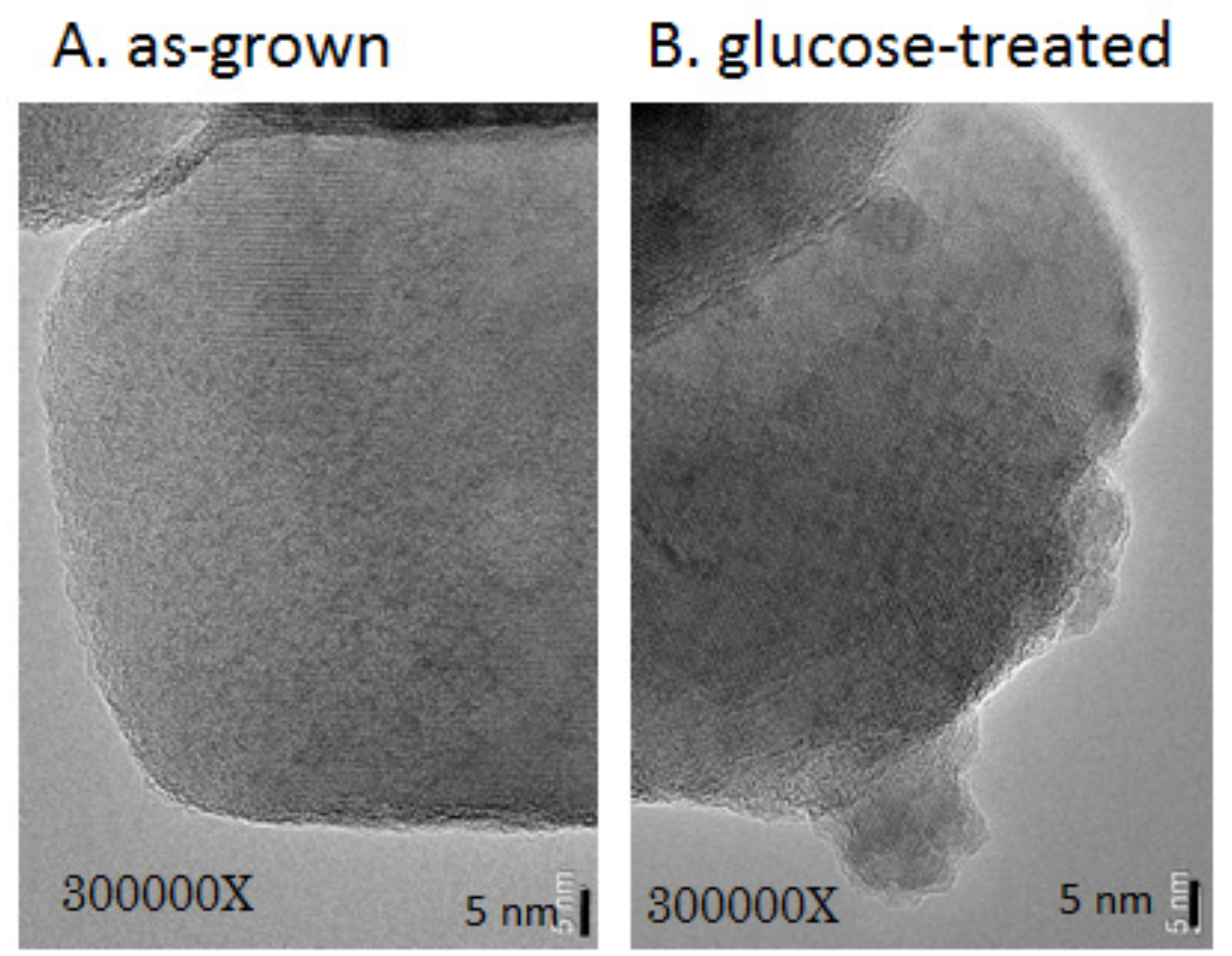
3.2. Surface State

3.3. Electrochemical Properties


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Matsuda, T.; Kim, J.E.; Moritomo, Y. Universal thermal response of the Prussian blue lattice. Phys. Rev. B 2009, 79. [Google Scholar] [CrossRef]
- Imanishi, N.; Morikawa, T.; Kondo, J.; Takeda, Y.; Yamamoto, O.; Kinugasa, N.; Yamagishi, T. Lithium intercalation behavior into iron cyanide complex as positive electrode of lithium secondary battery. J. Power Sources 1999, 79, 215–219. [Google Scholar] [CrossRef]
- Imanishi, N.; Morikawa, T.; Kondo, J.; Yamane, R.; Takeda, Y.; Yamamoto, O.; Sakaebe, H.; Tabuchi, M. Lithium intercalation behavior of iron cyanometallates. J. Power Sources 1999, 81–82, 530–534. [Google Scholar] [CrossRef]
- Okubo, M.; Asakura, D.; Mizuno, Y.; Kim, J.D.; Mizokawa, T.; Kudo, T.; Honnma, I. Switching redox-active sites by valence tautomerism in Prussian blue analogues AxMny[Fe(CN)6]nH2O (A: K, Rb): Robust frameworks for reversible Li storage. J. Phys. Chem. Lett. 2011, 1, 2063–2071. [Google Scholar] [CrossRef]
- Matsuda, T.; Moritomo, Y. Thin film electrode of Prussian blue analogue for Li-ion battery. Appl. Phys. Express 2011, 4, 047101. [Google Scholar] [CrossRef]
- Moritomo, Y.; Takachi, M.; Kurihara, Y.; Matsuda, T. Thin film electrodes of Prussian blue analogues with rapid Li+ intercalation. Appl. Phys. Express 2012, 5, 041801. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Takachi, M.; Moritomo, Y. A sodium manganese ferrocyanide thin film for Na-ion batteries. Chem. Commun. 2013, 49, 2750–2752. [Google Scholar] [CrossRef] [PubMed]
- Takachi, M.; Matsuda, T.; Moritomo, Y. Cobalt hexacyanoferrate as cathode material for Na+ secondary battery. Appl. Phys. Express 2013, 6. [Google Scholar] [CrossRef]
- Yang, D.; Xu, J.; Liao, X.Z.; He, Y.S.; Liu, H.; Ma, Z.F. Structure optimization of Prussian blue analogue cathode materials for advanced sodium ion batteries. Chem. Commum. 2014, 50, 13377–13380. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Wang, R.Y.; Oasta, M.; Lee, S.W.; Liu, N.; Chi, Y. Manganese hexacyanomanganate open framework as a high-capacity positive electrode material for sodium-ion batteries. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, J.; Qiao, R.; Wray, L.A.; Hossain, M.A.; Chung, Y.D.; Yang, W.; Lu, Y.; Evans, D.; Lee, J.J.; et al. Rhombohedral Prussian white as cathode for rechargeable sodium-ion batteries. J. Am. Chem. Soc. 2015, 137, 2548–2554. [Google Scholar]
- Yu, S.; Li, Y.; Lu, Y.; Xu, B.; Wang, Q.; Yan, M.; Jing, Y. A promising cathode material of sodium iron-nickel Hexacyanoferrate for sodium ion batteries. J. Power Sources 2015, 275, 45–49. [Google Scholar] [CrossRef]
- You, Y.; Wu, X.L.; Yin, Y.X.; Guo, Y.G. High-quality Prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries. Energy Environ. Sci. 2014, 7, 1643–1647. [Google Scholar] [CrossRef]
- Ravet, N.; Chouinard, Y.; Magnan, J.F.; Besner, S.; Gauthier, M.; Armand, M. Electroactivity of natural and synthetic triphylite. J. Power Sources 2001, 97–98, 503–507. [Google Scholar] [CrossRef]
- Huang, H.; Yin, S.C.; Nazar, L.F. Approaching theoretical capacity of LiFePO4 at room temperature at high rates. Electrochem. Solid State Lett. 2001, 4, A170–A172. [Google Scholar] [CrossRef]
- Chen, Z.; Dahn, J.R. Reducing carbon in LiFePO4/C composite electrodes to maximize specific energy, volumetric energy, and tap density. J. Electrochem. Soc. 2002, 149, A1184–A1189. [Google Scholar] [CrossRef]
- Chung, S.Y.; Bloking, J.T.; Chiang, Y.M. Electronically conductive phospho-olivines as lithium storage electrodes. Nat. Mat. 2002, 1, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Franger, S.; Bourbon, C.; Gras, F.; Franger, S.; Bourbon, C.; Gras, F. Optimized lithium iron phosphate for high-rate electrochemical applications. J. Electrochem. Soc. 2004, 151, A1024–A1027. [Google Scholar] [CrossRef]
- Moritomo, Y.; Kurihara, Y.; Matsuda, T.; Kim, J.E. Structural Phase Diagram of Mn-Fe Cyanide against Cation Concentration. J. Phys. Soc. Jpn. 2011, 80. [Google Scholar] [CrossRef]
- Moritomo, Y.; Matsuda, T.; Kurihara, Y.; Kim, J.E. Cubic-Rhombohedral Structural Phase Transition in Na1.32Mn[Fe(CN)6]0.833.6H2O. J. Phys. Soc. Jpn. 2011, 80. [Google Scholar] [CrossRef]
- Izumi, F.; Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moritomo, Y.; Goto, K.; Shibata, T. Glucose-Treated Manganese Hexacyanoferrate for Sodium-Ion Secondary Battery. Energies 2015, 8, 9486-9494. https://doi.org/10.3390/en8099486
Moritomo Y, Goto K, Shibata T. Glucose-Treated Manganese Hexacyanoferrate for Sodium-Ion Secondary Battery. Energies. 2015; 8(9):9486-9494. https://doi.org/10.3390/en8099486
Chicago/Turabian StyleMoritomo, Yutaka, Kensuke Goto, and Takayuki Shibata. 2015. "Glucose-Treated Manganese Hexacyanoferrate for Sodium-Ion Secondary Battery" Energies 8, no. 9: 9486-9494. https://doi.org/10.3390/en8099486




