Carbon dioxide capture and storage (CCS), which involves capture, transport and storage, is proposed as an important strategy to reduce greenhouse gas emissions significantly [
1]. Of these three steps, CO
2 capture is recognized as a promising and relatively quick solution to reduce global CO
2 emissions. Many different types of CO
2 capture methods have been studied by researchers all over the world. The main CO
2 capture methods contain chemical absorption, physical adsorption, membrane separation, and cryogenic distillation. Among these technologies, cryogenic CO
2 capture is attracting increased attention due to its energy saving and environmental friendliness [
2]. Kelley
et al. [
3] proposed the Controlled Freeze Zone™ technology which is capable of removing CO
2 and H
2S from natural gas. In the process, CO
2 is frozen out and remelted in a distillation tower. Song
et al. [
2] developed a novel CO
2 capture process based on Stirling coolers (SC); the CO
2 in the gas stream can be captured in solid form under the cryogenic condition, and frosted on the cold head of an SC. Theunissen
et al. [
4] reported condensed rotational separations for CO
2 removal from contaminated natural gas. Zanganeh
et al. [
5] designed a CO
2 cryogenic separate system where flue gas is compressed, cooled and dried, then the CO
2 is condensed into a liquid. The above strategies capture CO
2 by liquefaction or desublimation separately, however, at a nominal 14% CO
2 in typical flue gas, no liquid forms at any temperature or pressure occur, and desublimation separation is too energy-intensive to apply alone. For these reasons, a new liquefaction combined with desublimation system is proposed. The phase equilibrium of CO
2-mixtures is of great importance to the design and operation of a cryogenic system. Depending on the sources of CO
2-mixtures, the purity of CO
2 stream is varied. Generally, the common impurities in flue gas include N
2, O
2, Ar, CH
4, H
2S, SO
2 and H
2O [
6]. After removal of water and toxic gas, the main impurity in typical boiler gases is N
2 [
7], the permanent gas N
2 is the main obstacle in the separation of CO
2. Therefore, N
2/CO
2 phase equilibrium analysis at a low temperature is crucial for the investigation and design of a cryogenic CO
2 capture system. The experimental data of N
2/CO
2 covers pressures from 0.6 MPa to 13.95 MPa and temperatures from 218.15 K to 403.15 K, with CO
2 liquid mole fractions ranging from 0.43 to 1, and CO
2 gas mole fractions ranging from 0.153 to 1 [
8]. However, the experimental data are not continuous, there are some gaps between the available experimental data and the requirements of engineering applications. Therefore, semi-empirical equations of state (EOS) are usually used to satisfy the requirements with respect to the design and operation of cryogenic CO
2 capture system. Cubic EOS offer a compromise between the generality and simplicity that are often used for many technical applications [
9].
For N
2/CO
2 phase equilibrium calculations, different cubic EOSs and their reliability have been studied by some investigators. Dorau
et al. [
10] conducted N
2/CO
2 equilibrium experiments and the results showed the Peng-Robinson (PR) EOS correlates the experimental data well. However, the concrete accuracy was not given and experiments were conducted only at 223.15 K and 273.15 K. Thiery
et al. [
11] investigated Soave-Redlich-Kwong (SRK) EOS for the vapor liquid equilibrium (VLE) calculations of N
2/CO
2. The results indicated that the average deviation for the saturated pressures was around 4% in the temperature range of 218.15–273.15 K. Duan and Hu [
12] developed a new cubic EOS, modeling the VLE properties of natural fluids based on SRK EOS. The calculations of the VLE were in good agreement with experiments for the N
2/CO
2 mixture. Li and Yan [
13] evaluated the reliabilities of seven cubic EOS for predicting volumes of binary CO
2 mixtures. Comparatively, the calculated results of PR and Patel-Teja (PT) are in good agreement with the experimental data especially in the liquid phase for the N
2/CO
2 mixture, the PR is superior in the calculation of liquid volumes, where the absolute average deviations is 1.74%, but the analysis was only carried out at 270 K. Ahmad
et al. [
14] stated that SRK provides satisfactory predictions that match well with the experimental data, especially with high concentration of CO
2 (97.5%) in the case of N
2/CO
2 mixtures. For O
2/CO
2 mixtures, the SRK can correctly predict the dew-point line and the bubble point line with high concentrations of CO
2.
Due to the semi-empirical EOS that were developed by using pure component data, the application of EOSs for multi-component is based on different mixing rules. Some EOS are not of sufficient accuracy, mainly because of inadequate use of the mixing rules. In general, the same equation of state, combining different mixing rules, may lead to different results. Raabe and Köhler [
15] investigated the performances of SRK and PR cubic EOS with different types of mixing rules (including van der Waals (vdW) mixing rule and some Gibbs energy (
GE) mixing rules that incorporate an activity coefficient model into the EOS model by equating the excess
GE) in the N
2/C
2H
6 system. The results showed that the vdW2 mixing rule, which introduced two variable parameters, was able to achieve high accuracy. Faúndez and Valderrama [
16] used PR EOS combined with the Wong-Sandler (WS) mixing rule to study the hydrocarbon + alcohol mixtures at low and moderate pressure. It was concluded that the model could correlate equilibrium data in complex mixtures with accuracy similar to that of other models. However, it is difficult to evaluate precisely and systematically the relative merits of different mixing rules from existing papers for N
2/CO
2 mixture, since there is no widely used basis of comparison. In general, all of the studied EOS and mixing rules have various performances for various mixtures.
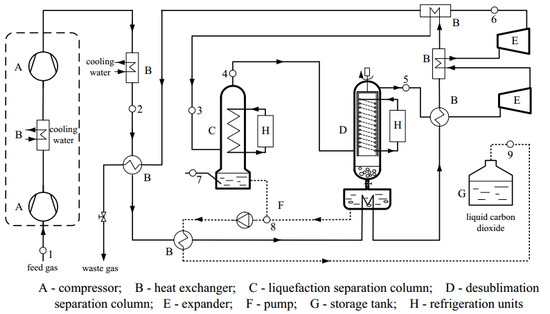
In this paper, the reliability of different cubic EOS combined with various mixing rules for the VLE properties of N2/CO2 mixture are evaluated. Based on the comparison with the collected experimental data, the most accurate model to conduct N2/CO2 phase equilibrium analysis at low temperature is identified. Additionally, the thermodynamic properties of N2/CO2 at low temperature are analyzed based on the selected calculation model. According to the results, a new liquefaction combined with desublimation system is proposed. Finally, the recovery and purity of CO2 and the energy consumption of the new system are analyzed.