Preparation of W-C-Co Composite Micropowder with Spherical Shaped Particles Using Plasma Technologies
Abstract
:1. Introduction
- W-C-Co system nanopowders synthesis by interaction of tungsten oxide WO3 and cobalt powder mixture with methane in a flow of hydrogen-containing thermal plasma of electric arc plasm torch.
- Nanopowders granulation in a spray dryer to produce nanopowder microgranules, and classification of microgranules into a given fraction.
- Densification and spheroidization in a thermal plasma of the separated fractions of microgranules.
2. Materials and Methods
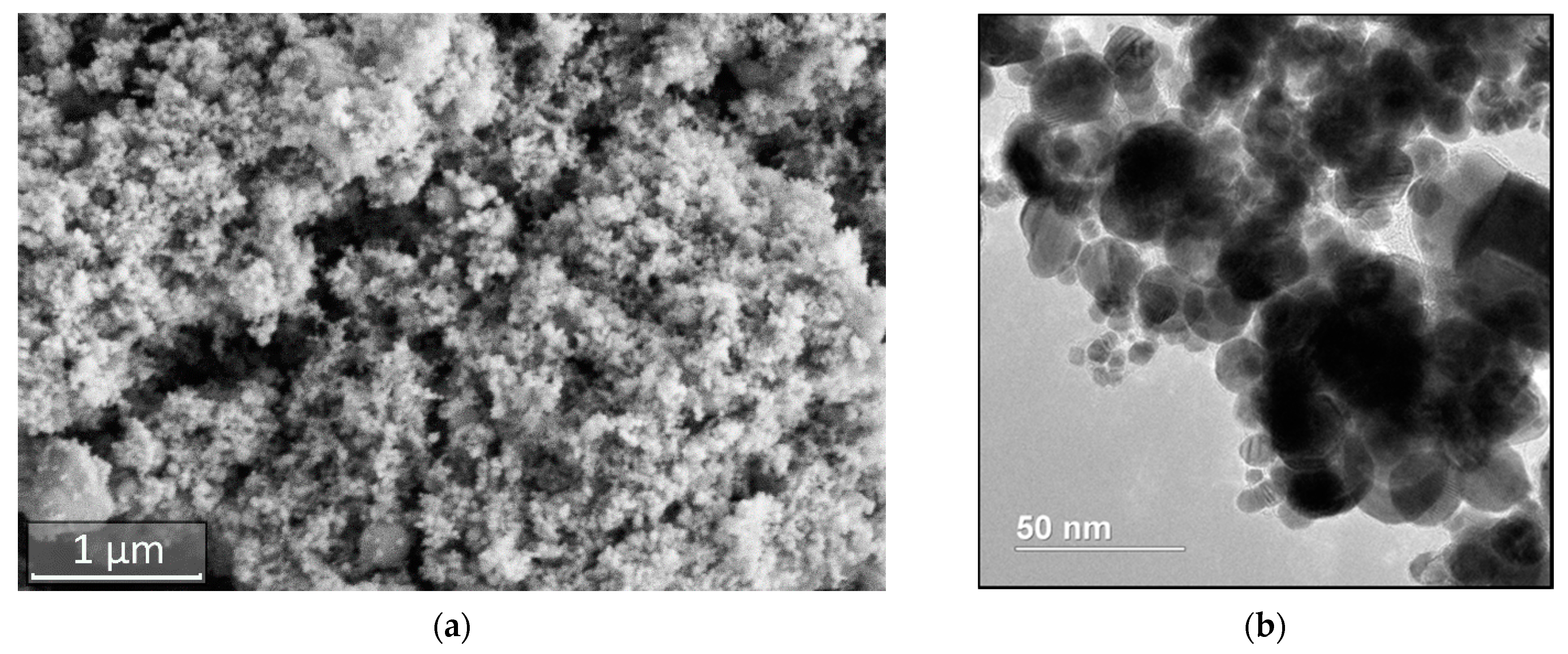
2.1. Obtaining Nanopowder of W-C-Co System
2.2. W-C-Co System Nanopowder Microgranules Production
- Preparation of an alcohol suspension consisting of composite nanoparticles of the W-C-Co system and polyvinyl butyral (C8H14O2)n (PVB) used as an organic binder to ensure the strength of the obtained microgranules.
- Spray drying of the obtained suspension with an ultrasonic nozzle. Nitrogen was used as a working gas in the process of granulating the nanopowder using a Buchi B-295 circulation gas circuit.
- Separation of the 25.63 μm fraction of microgranules on a Retsch AS 200 sieve machine (Haan, Germany).
2.3. Plasma Processing of W-C-Co Nanopowders
- Scanning, transmission electron and optical microscopy—Scios SEM microscope (FEI, Hillsboro, OR, USA) with elemental energy dispersive X-ray microanalysis (EDS), Osiris TEM microscope (FEI, Hillsboro, OR, USA) and Olympus CX31 OM microscope (Tokyo, Japan), respectively. ImageScope M software (Aperio Technologies, Vista, CA, USA) was used for statistical image processing.
- Measurement of the specific surface area of nanopowders by the BET method on a Micromeritics TriStar 3000 specific surface analyzer (Norcross, GA USA).
- Amount of total oxygen measurements with a TC-600 (LECO, St. Joseph, MO, USA) analyzer during reduction smelting in a graphite crucible and detection of the resulting gases with an infrared radiation sensor.
- Amount of total carbon in a CS-600 (LECO, St. Joseph, MO, USA) analyzer by burning the sample in a stream of oxygen and detecting the resulting gases using an infrared radiation sensor.
- Determination of metallic elements (Co, W) by X-ray fluorescence spectroscopy (XRFMS) in the powder layer on an Orbis analyzer (EDAX, Mahwah, NJ, USA).
- Particle size distribution of micropowders measured with a Mastersizer 2000M laser diffraction particle size analyzer (Malvern, Worcestershire, UK) with Hydro S liquid sample feeder.
- Phase analysis on an Ultima-4 X-ray diffractometer (RIGAKU, Tokyo, Japan) with a monochromator in filtered Cu-Kα radiation.
- Separation of nanoparticles in spheroidal micropowders by fractional separation in liquid by sedimentation of aqueous suspension after treatment on ultrasonic dispersant Bandelin Sonopuls HD3100 (Berlin, Germany).
- Determination of flowability of powders was carried out using a calibrated funnel (Hall device) and a stopwatch for samples weighing 50 g.
3. Results and Discussion
3.1. Preparation of W-C-Co System Nanopowders
3.2. Preparation of W-C-Co microgranules
3.3. Plasma Treatment of W-C-Co System Microgranules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Falkovskiy, V.A.; Klyachko, L.I. Tverdye Splavi; Ruda i Metally: Moscow, Russia, 2005. (In Russian) [Google Scholar]
- Kurlov, A.S.; Gusev, A.I. Tungsten Carbides: Structure, Properties and Application in Hardmetals; Springer Series in Materials Science 184; Springer: Berlin/Heidelberg, Germany, 2016; p. 256. [Google Scholar]
- Konyashin, I. Cemented carbides for mining, construction and wear parts. In Comprehensive Hard Materials; Mari, D., Miguel, L., Nebel, C.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 425–451. [Google Scholar]
- Yang, Y.; Zhang, C.; Wang, D.; Nie, L.; Wellmann, D.; Tian, Y. Additive manufacturing of WC-Co hardmetals: A review. Int. J. Adv. Manuf. Technol. 2020, 108, 1653–1673. [Google Scholar] [CrossRef]
- Qian, M. Metal powder for additive manufacturing. JOM 2015, 67, 536–537. [Google Scholar] [CrossRef] [Green Version]
- Saheb, S.H.; Durgam, V.K.; Chandrashekhar, A. A review on metal powders in additive manufacturing. AIP Conf. Proc. 2020, 2281, 020018. [Google Scholar]
- Petrunichev, V.A.; Mihalev, V.I. K razrabotke protsessa plazmennogo polucheniya sfericheskih poroshkov iz tugoplavkih materialov. Izvestiya AN SSSR. Metalli 1966, 6, 154–158. (In Russian) [Google Scholar]
- Rykalin, N.N.; Petrunichev, V.A.; Sorokin, L.M. Poluchenie sfericheskih tonkodispersnih poroshkov v nizkotemperaturnoi plazme. In Plazmennie Protsessi v Metallurgii i Tehnologii Neorganicheskih Materialov; Nauka: Moscow, Russia, 1973; pp. 220–230. (In Russian) [Google Scholar]
- Boulos, M. Plasma power can make better powders. Met. Powder Rep. 2004, 59, 16–21. [Google Scholar] [CrossRef]
- Vert, R.; Pontone, R.; Dolbec, R.; Dionne, L.; Boulos, M.I. Induction plasma technology applied to powder manufacturing: Example of titanium-based materials. In Proceedings of the 22nd International Symposium on Plasma Chemistry, Antwerp, Belgium, 5–10 July 2015. [Google Scholar]
- Ji, L.; Wang, C.; Wu, W.; Tan, C.; Wang, G.; Duan, X.M. Spheroidization by plasma processing and characterization of stainless steel powder for 3d printing. Metall. Mat. Trans. A 2017, 48, 4831–4841. [Google Scholar] [CrossRef]
- Kotlyarov, V.I.; Beshkarev, V.T.; Kartsev, V.E.; Ivanov, V.V.; Gasanov, A.A.; Yuzhakova, E.A.; Samokhin, A.V.; Fadeev, A.A.; Alekseev, N.V.; Sinayskiy, M.A.; et al. Production of spherical powders on the basis of group IV metals for additive manufacturing. Inorg. Mater. Appl. Res. 2017, 8, 452–458. [Google Scholar] [CrossRef]
- Samokhin, A.; Alekseev, N.; Sinayskiy, M.; Astashov, A.; Kirpichev, D.; Fadeev, A.; Tsvetkov, Y.; Kolesnikov, A. Nanopowders Production and Micron-Sized Powders Spheroidization in DC Plasma Reactors. In Powder Technology; Cavalheiro, A.A., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Samokhin, A.V.; Fadeev, A.A.; Alekseev, N.V.; Sinaysky, M.A.; Sufiyarov, V.S.; Borisov, E.V.; Korznikov, O.V.; Fedina, T.V.; Vodovozova, G.S.; Baryshkov, S.V. Spheroidization of Fe-based powders in plasma jet of DC arc plasma torch and application of these powders in selective laser melting. Inorg. Mater. Appl. Res. 2020, 11, 579–585. [Google Scholar] [CrossRef]
- Itagaki, H.; Yachi, T.; Ogiso, H.; Sato, H.; Yamashita, Y.; Yasuoka, J.; Funada, Y. DC arc plasma treatment for defect reduction in WC-Co granulated powder. Metals 2020, 10, 975. [Google Scholar] [CrossRef]
- Yan, Z.; Xiao, M.; Mao, X.; Khanlari, K.; Shi, Q.; Liu, X. Fabrication of spherical WC-Co powders by radio frequency inductively coupled plasma and a consequent heat treatment. Powder Technol. 2021, 385, 160–169. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Wang, X.; Ryu, T.; Hwang, K.S.; Sohn, H.Y. Synthesis, sintering, and mechanical properties of nanocrystalline cemented tungsten carbide—A review. Int. J. Refract. Met. Hard Mater. 2009, 27, 288–299. [Google Scholar] [CrossRef]
- Lisovskii, A.F. On the development of nanostructured WC-Co hard alloys. J. Superhard Mater. 2010, 32, 389–395. [Google Scholar] [CrossRef]
- GOST 19440-94. Metallic Powders. Determination of Apparent Density; IPC Publishing House of Standards: Moscow, Russia, 1996. [Google Scholar]
- ISO 3923-1:2018. Metallic Powders—Determination of Apparent Density—Part 1: Funnel Method; Technical Committee ISO: Geneva, Switzerland, 2018. [Google Scholar]
- ISO 3923-2. Metallic Powders—Determination of Apparent Density—Part 2: Scott Volumeter Method; Technical Committee ISO: Geneva, Switzerland, 1981. [Google Scholar]
- Krasovskii, P.V.; Malinovskaya, O.S.; Samokhin, A.V.; Blagoveshchenskiy, Y.V.; Kazakov, V.A.; Ashmarin, A.A. XPS study of surface chemistry of tungsten carbides nanopowders produced through DC thermal plasma/hydrogen annealing process. Appl. Surf. Sci. 2015, 339, 46–54. [Google Scholar] [CrossRef]










| Microgranules | Spheroidized Powder Produced at Different Enthalpy | ||
|---|---|---|---|
| 2.8 kW∙h/m3 | 4.8 kW∙h/m3 | ||
| Dav, μm | 39.1 | 16.7 | 19.8 |
| Apparent density, g/cm3 | 2.6 | 8.8 | 9.5 |
| Flowability, s/50 g | 29 | 11 | 10 |
| Nanopowder | Microgranules | Spheroidized Powder Produced at Different Enthalpy | ||
|---|---|---|---|---|
| 2.8 kW∙h/m3 | 4.9 kW∙h/m3 | |||
| Carbon, wt.% | 4.7 | 6.4 | 4.7 | 3.9 |
| Cobalt, wt.% | 7.7 | 7.0 | 4.6 | 3.7 |
| Oxygen, wt.% | 0.5 | 1.0 | 0.05 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samokhin, A.; Alekseev, N.; Astashov, A.; Dorofeev, A.; Fadeev, A.; Sinayskiy, M.; Kalashnikov, Y. Preparation of W-C-Co Composite Micropowder with Spherical Shaped Particles Using Plasma Technologies. Materials 2021, 14, 4258. https://doi.org/10.3390/ma14154258
Samokhin A, Alekseev N, Astashov A, Dorofeev A, Fadeev A, Sinayskiy M, Kalashnikov Y. Preparation of W-C-Co Composite Micropowder with Spherical Shaped Particles Using Plasma Technologies. Materials. 2021; 14(15):4258. https://doi.org/10.3390/ma14154258
Chicago/Turabian StyleSamokhin, Andrey, Nikolay Alekseev, Aleksey Astashov, Aleksey Dorofeev, Andrey Fadeev, Mikhail Sinayskiy, and Yulian Kalashnikov. 2021. "Preparation of W-C-Co Composite Micropowder with Spherical Shaped Particles Using Plasma Technologies" Materials 14, no. 15: 4258. https://doi.org/10.3390/ma14154258







