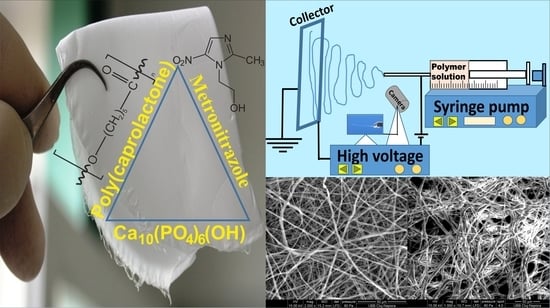
Electrospun Membranes Based on Polycaprolactone, Nano-Hydroxyapatite and Metronidazole
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of nHAP
20NH4NO3 + 20H2O
2.3. Preparation of Experimental EMs
2.4. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (FTIR-ATR)
2.5. X-ray Diffraction
2.6. Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM)
2.7. Mechanical Properties
2.8. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Im, E.; Hong, M.-K. Drug-eluting stents to prevent stent thrombosis and restenosis. Expert Rev. Cardiovasc. Ther. 2015, 14, 87–104. [Google Scholar] [CrossRef]
- Malik, R.; Garg, T.; Goyal, A.K.; Rath, G. Diacerein-loaded novel gastroretentive nanofiber system using PLLA: Development and in vitro characterization. Artif. Cells Nanomed. Biotechnol. 2016, 44, 928–936. [Google Scholar] [PubMed]
- Li, H.; Xu, Y.; Xu, H.; Chang, J. Electrospun membranes: Control of the structure and structure related applications in tissue regen-eration and drug delivery. J. Mater. Chem. B 2014, 2, 5492–5510. [Google Scholar] [CrossRef]
- Patel, K.D.; Kim, T.-H.; Mandakhbayar, N.; Singh, R.K.; Jang, J.-H.; Lee, J.-H.; Kim, H.-W. Coating biopolymer nanofibers with carbon nanotubes accelerates tissue healing and bone regeneration through orchestrated cell- and tissue-regulatory responses. Acta Biomater. 2020, 108, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Zan, J.; Yang, Y.; Peng, S.; Yang, W.; Qi, F.; Shen, L.; Tian, Z. Surface modification enhances interfacial bonding in PLLA/MgO bone scaffold. Mater. Sci. Eng. C 2020, 108, 110486. [Google Scholar] [CrossRef]
- Jo, S.B.; Erdenebileg, U.; Dashnyam, K.; Jin, G.Z.; Cha, J.R.; El-Fiqi, A.; Knowles, J.C.; Patel, K.D.; Lee, H.H.; Lee, J.H.; et al. Nano-graphene oxide/polyurethane nanofibers: Mechanically flexible and myogenic stimulating matrix for skeletal tissue engineering. J. Tissue Eng. 2020, 11, 2041731419900424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Southard, G.; Godowski, K.C. Subgingival controlled release of antimicrobial agents in the treatment of periodontal disease. Int. J. Antimicrob. Agents 1998, 9, 239–253. [Google Scholar] [CrossRef]
- Brogden, K.A.; Guthmiller, J.M. Polymicrobial Diseases; McGraw-Hill Professional: New York, NY, USA, 2015. [Google Scholar]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef] [PubMed]
- Currò, M.; Matarese, G.; Isola, G.; Caccamo, D.; Ventura, V.P.; Cornelius, C.; Lentini, M.; Cordasco, G.; Ientile, R. Differential expression of transglutaminase genes in patients with chronic periodontitis. Oral Dis. 2014, 20, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Leonardi, R. Independent impact of periodontitis and cardiovascular disease on elevated soluble urokinase-type plasminogen activator receptor (suPAR) levels. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Liaw, A.; Miller, C.; Nimmo, A. Comparing the periodontal tissue response to non-surgical scaling and root planing alone, adjunctive azithromycin, or adjunctive amoxicillin plus metronidazole in generalized chronic moderate-to-severe periodontitis: A preliminary randomized controlled trial. Aust. Dent. J. 2019, 64, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, F.; Briguglio, E.; Briguglio, R.; Cafiero, C.; Isola, G. Treatment of infrabony periodontal defects using a resorbable biopolymer of hyaluronic acid: A randomized clinical trial. Quintessence Int. 2013, 44, 231–240. [Google Scholar]
- Kinane, D.F. Local antimicrobial therapies in periodontal disease. Ann. R. Australas. Coll. Dent. Surg. 2000, 15, 57–60. [Google Scholar]
- Joshi, D.; Garg, T.; Goyal, A.K.; Rath, G. Advanced drug delivery approaches against periodontitis. Drug Deliv. 2014, 23, 363–377. [Google Scholar] [CrossRef]
- Murata, M.; Hino, J.; Kabir, M.A.; Yokozeki, K.; Sakamoto, M.; Nakajima, T.; Akazawa, T. Osteoinduction in novel micropores of par-tially dissolved and precipitated hydroxyapatite block in scalp of young rats. Materials 2021, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.L.B.; Gerbi, M.E.M.; Limeira, F.D.A.; Ponzi, E.A.C.; Marques, A.M.C.; Carvalho, C.M.; Santos, R.D.C.; Oliveira, P.C.; Nóia, M.; Ramalho, L.M.P. Bone repair following bone grafting hydroxyapatite guided bone regeneration and infra-red laser photobiomodulation: A histological study in a rodent model. Lasers Med. Sci. 2008, 24, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Furtos, G.; Rivero, G.; Rapuntean, S.; Abraham, G.A. Amoxicillin-loaded electrospun nanocomposite membranes for dental applica-tions. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 966–976. [Google Scholar] [CrossRef]
- Haneke, E. Adverse Effects of Fillers and Their Histopathology. Facial Plast. Surg. 2014, 30, 599–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatto, M.; Groppo, R.; Bloise, N.; Fassina, L.; Visai, L.; Galati, M.; Iuliano, L.; Mengucci, P. Topological, Mechanical and Biological Properties of Ti6Al4V Scaffolds for Bone Tissue Regeneration Fabricated with Reused Powders via Electron Beam Melting. Materials 2021, 14, 224. [Google Scholar] [CrossRef]
- Klug, H.; Alexander, L. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials; John Wiley & Sons, Inc.: London, UK, 1962. [Google Scholar]
- Luo, C.J.; Stride, E.; Edirisinghe, M. Mapping the influence of solubility and dielectricconstant on electrospinning polycaprolactone solutions. Macromolecules 2012, 45, 4669–4680. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Janowski, G.M. A novel spatially designed and functionally graded electrospun membrane for periodontal regeneration. Acta Biomater. 2011, 7, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.H.; Ali, R.; Batakoushy, H.A.; Derayea, S.M. Solid-state FTIR spectroscopic study of two binary mixtures: Cefepime-metronidazole and cefoperazone-sulbactam. J. Spectrosc. 2017, 2017, 5673214. [Google Scholar] [CrossRef]
- Megalai, S.M.; Manjula, P.; Manonmani, K.N.; Kavitha, N.; Baby, N. Metronidazole: A Corrosion Inhibitor for Mild Steel in Aqueous Environment. Port. Electrochim. Acta 2012, 30, 395–403. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Chen, Y.; Majidi, C.; Iida, F.; Askounis, E.; Pei, Q. Controllable and reversible tuning of material rigidity for robot applications. Mater. Today 2018, 21, 563–576. [Google Scholar] [CrossRef]
- Hilal, N.; Ismail, A.F.; Matsuura, T.; Oatley-Radcliffe, D. Mechanical characterization of membranes. In Membrane Characterization; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 13. [Google Scholar]
- Caballé-Serrano, J.; Munar-Frau, A.; Delgado, L.; Pérez, R.; Hernández-Alfaro, F. Physicochemical characterization of barrier mem-branes for bone regeneration. J. Mech. Behav. Biomed. Mater. 2019, 97, 13–20. [Google Scholar] [CrossRef] [PubMed]







| No. | Code | Composition of Experimental EM | ||
|---|---|---|---|---|
| PCL (wt.%) | nHAP (wt.%) | MET (wt.%) | ||
| 1 | PCL | 100 | 0 | 0 |
| 2 | PCL-5% nHAP | 95 | 5 | 0 |
| 3 | PCL-20% MET | 80 | 0 | 20 |
| 4 | PCL-5% nHAP-20% MET | 75 | 5 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirică, I.-C.; Furtos, G.; Lucaciu, O.; Pascuta, P.; Vlassa, M.; Moldovan, M.; Campian, R.-S. Electrospun Membranes Based on Polycaprolactone, Nano-Hydroxyapatite and Metronidazole. Materials 2021, 14, 931. https://doi.org/10.3390/ma14040931
Mirică I-C, Furtos G, Lucaciu O, Pascuta P, Vlassa M, Moldovan M, Campian R-S. Electrospun Membranes Based on Polycaprolactone, Nano-Hydroxyapatite and Metronidazole. Materials. 2021; 14(4):931. https://doi.org/10.3390/ma14040931
Chicago/Turabian StyleMirică, Ioana-Codruţa, Gabriel Furtos, Ondine Lucaciu, Petru Pascuta, Mihaela Vlassa, Mărioara Moldovan, and Radu-Septimiu Campian. 2021. "Electrospun Membranes Based on Polycaprolactone, Nano-Hydroxyapatite and Metronidazole" Materials 14, no. 4: 931. https://doi.org/10.3390/ma14040931
APA StyleMirică, I. -C., Furtos, G., Lucaciu, O., Pascuta, P., Vlassa, M., Moldovan, M., & Campian, R. -S. (2021). Electrospun Membranes Based on Polycaprolactone, Nano-Hydroxyapatite and Metronidazole. Materials, 14(4), 931. https://doi.org/10.3390/ma14040931