Detection of Chlorpyrifos Using Bio-Inspired Silver Nanograss
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Fabrication of Bio-Inspired Ag-NG Using Electrochemical Deposition
2.3. SEM and FEM-Based Electromagnetic Simulation of Bio-Inspired Ag-NG
2.4. Optimization of the Sample Preparation
2.5. CPF Detection Using Bio-Inspired Ag-NG
3. Results and Discussion
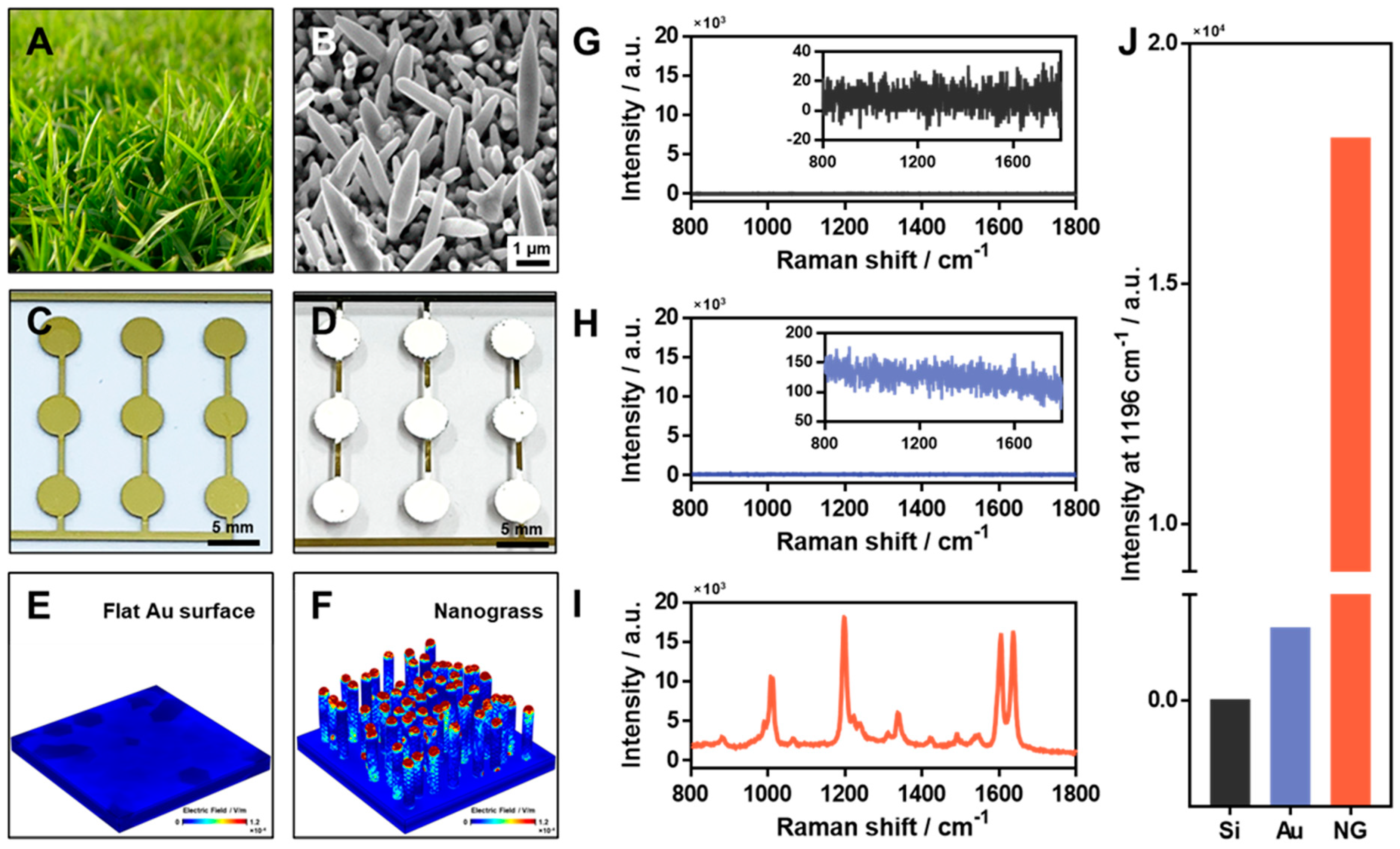
3.1. Fabrication of Bio-Inspired Ag-NG as a SERS Substrate
3.2. Optimization of Sample Preparation Process with Regard to Sample Dispersion Using Sonication
3.3. Optimization of the Sample Preparation Process with Regard to Sample Drying
3.4. Detection of CPF According to the Concentration Using the Ag-NG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agnarsson, I.; Dhinojwala, A.; Sahni, V.; Blackledge, T.A. Spider Silk as a Novel High Performance Biomimetic Muscle Driven by Humidity. J. Exp. Biol. 2009, 212, 1990–1994. [Google Scholar] [CrossRef] [Green Version]
- Heidebrecht, A.; Eisoldt, L.; Diehl, J.; Schmidt, A.; Geffers, M.; Lang, G.; Scheibel, T.; Heidebrecht, A.; Eisoldt, L.; Diehl, J.; et al. Degree of Biomimicry of Artificial Spider Silk Spinning Assessed by NMR Spectroscopy. Angew. Chem. 2017, 129, 12745–12749. [Google Scholar] [CrossRef]
- Keerl, D.; Scheibel, T. Characterization of Natural and Biomimetic Spider Silk Fibers. Bioinspir. Biomim. Nanobiomater. 2012, 1, 83–94. [Google Scholar] [CrossRef]
- Jin, K.; Cremaldi, J.C.; Erickson, J.S.; Tian, Y.; Israelachvili, J.N.; Pesika, N.S. Biomimetic Bidirectional Switchable Adhesive Inspired by the Gecko. Adv. Funct. Mater. 2014, 24, 574–579. [Google Scholar] [CrossRef] [Green Version]
- Zeng, H.; Pesika, N.; Tian, Y.; Zhao, B.; Chen, Y.; Tirrell, M.; Turner, K.L.; Israelachvili, J.N. Frictional Adhesion of Patterned Surfaces and Implications for Gecko and Biomimetic Systems. Langmuir 2009, 25, 7486–7495. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, H.S.; Lee, J.; Kim, S.; Lee, K.H.; Moon, W.; Kwon, T.H. Replication of High-Aspect-Ratio Nanopillar Array for Biomimetic Gecko Foot-Hair Prototype by UV Nano Embossing with Anodic Aluminum Oxide Mold. Microsyst. Technol. 2007, 13, 601–606. [Google Scholar] [CrossRef]
- Jo, Y.K.; Kim, H.J.; Jeong, Y.; Joo, K.I.; Cha, H.J. Biomimetic Surface Engineering of Biomaterials by Using Recombinant Mussel Adhesive Proteins. Adv. Mater. Interfaces 2018, 5, 1800068. [Google Scholar] [CrossRef]
- Goldmann, A.S.; Schödel, C.; Walther, A.; Yuan, J.; Loos, K.; Müller, A.H.E. Enhancing the Adhesion of a Biomimetic Polymer Yields Performance Rivaling Commercial Glues. Adv. Funct. Mater. 2014, 24, 3259–3267. [Google Scholar] [CrossRef]
- Matos-Pérez, C.R.; White, J.D.; Wilker, J.J. Polymer Composition and Substrate Influences on the Adhesive Bonding of a Biomimetic, Cross-Linking Polymer. J. Am. Chem. Soc. 2012, 134, 9498–9505. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Lee, W.; Choi, H.; Lee, G.; Son, J.; Lee, S.W.; Park, J.; Kim, W.; Kim, M.; Yoon, D.S.; et al. Bioinspired Micro Glue Threads Fabricated by Liquid Bridge-to-Solidification as an Effective Sensing Platform. ACS Sens. 2020, 5, 1977–1986. [Google Scholar] [CrossRef]
- Kim, W.; Lee, W.; Park, H.; Park, J.; Kim, W.; Kang, B.; Choi, E.; Kim, C.S.; Park, J.O.; Lee, G.; et al. Biomimetic Nano-Pine-Pollen Structure-Based Surface-Enhanced Raman Spectroscopy Sensing Platform for the Hypersensitive Detection of Toxicants: Cadmium and Amyloid. ACS Sustain. Chem. Eng. 2022, 10, 3180–3190. [Google Scholar] [CrossRef]
- Eddleston, M. Patterns and Problems of Deliberate Self-Poisoning in the Developing World. QJM-Mon. J. Assoc. Physicians 2000, 93, 715–731. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Quezada, M.T.; Lucero, B.A.; Barr, D.B.; Steenland, K.; Levy, K.; Ryan, P.B.; Iglesias, V.; Alvarado, S.; Concha, C.; Rojas, E.; et al. Neurodevelopmental Effects in Children Associated with Exposure to Organophosphate Pesticides: A Systematic Review. Neurotoxicology 2013, 39, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Gunnell, D.; Eddleston, M.; Phillips, M.R.; Konradsen, F. The Global Distribution of Fatal Pesticide Self-Poisoning: Systematic Review. BMC Public Health 2007, 7, 357. [Google Scholar] [CrossRef] [Green Version]
- Buckley, N.A.; Eddleston, M.; Li, Y.; Bevan, M.; Robertson, J. Oximes for Acute Organophosphate Pesticide Poisoning. Cochrane Database Syst. Rev. 2011, CD005085. [Google Scholar] [CrossRef]
- Kuswandi, B.; Fikriyah, C.I.; Gani, A.A. An Optical Fiber Biosensor for Chlorpyrifos Using a Single Sol-Gel Film Containing Acetylcholinesterase and Bromothymol Blue. Talanta 2008, 74, 613–618. [Google Scholar] [CrossRef]
- Zamfir, L.G.; Rotariu, L.; Bala, C. A Novel, Sensitive, Reusable and Low Potential Acetylcholinesterase Biosensor for Chlorpyrifos Based on 1-Butyl-3-Methylimidazolium Tetrafluoroborate/Multiwalled Carbon Nanotubes Gel. Biosens. Bioelectron. 2011, 26, 3692–3695. [Google Scholar] [CrossRef]
- Nagabooshanam, S.; Roy, S.; Mathur, A.; Mukherjee, I.; Krishnamurthy, S.; Bharadwaj, L.M. Electrochemical Micro Analytical Device Interfaced with Portable Potentiostat for Rapid Detection of Chlorpyrifos Using Acetylcholinesterase Conjugated Metal Organic Framework Using Internet of Things. Sci. Rep. 2019, 9, 19862. [Google Scholar] [CrossRef]
- Sandahl, J.F.; Baldwin, D.H.; Jenkins, J.J.; Scholz, N.L. Comparative Thresholds for Acetylcholinesterase Inhibition and Behavioral Impairment in Coho Salmon Exposed to Chlorpyrifos. Environ. Toxicol. Chem. 2005, 24, 136–145. [Google Scholar] [CrossRef]
- Uniyal, S.; Sharma, R.K. Technological Advancement in Electrochemical Biosensor Based Detection of Organophosphate Pesticide Chlorpyrifos in the Environment: A Review of Status and Prospects. Biosens. Bioelectron. 2018, 116, 37–50. [Google Scholar] [CrossRef]
- Ruiyi, L.; Hui, J.; Nana, L.; Dan, X.; Zaijun, L. Electrochemical Detection of Chlorpyrifos in Fruits with Gold-Histidine Functionalized Graphene Quantum Dot-Graphene Hybrid and Target-Induced DNA Cycle Amplification. Sens. Actuators B Chem. 2022, 355, 131314. [Google Scholar] [CrossRef]
- Nana, L.; Ruiyi, L.; Qinsheng, W.; Yongqiang, Y.; Xiulan, S.; Guangli, W.; Zaijun, L. Colorimetric Detection of Chlorpyrifos in Peach Based on Cobalt-Graphene Nanohybrid with Excellent Oxidase-like Activity and Reusability. J. Hazard. Mater. 2021, 415, 125752. [Google Scholar] [CrossRef]
- Kushwaha, A.; Singh, G.; Sharma, M. Colorimetric Sensing of Chlorpyrifos through Negative Feedback Inhibition of the Catalytic Activity of Silver Phosphate Oxygenase Nanozymes. RSC Adv. 2020, 10, 13050–13065. [Google Scholar] [CrossRef] [Green Version]
- Mane, P.C.; Shinde, M.D.; Varma, S.; Chaudhari, B.P.; Fatehmulla, A.; Shahabuddin, M.; Amalnerkar, D.P.; Aldhafiri, A.M.; Chaudhari, R.D. Highly Sensitive Label-Free Bio-Interfacial Colorimetric Sensor Based on Silk Fibroin-Gold Nanocomposite for Facile Detection of Chlorpyrifos Pesticide. Sci. Rep. 2020, 10, 4198. [Google Scholar] [CrossRef]
- Chen, J.; Dong, D.; Ye, S. Detection of Pesticide Residue Distribution on Fruit Surfaces Using Surface-Enhanced Raman Spectroscopy Imaging. RSC Adv. 2018, 8, 4726–4730. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Hu, Y.; Ma, L.; Lu, X. Development of Molecularly Imprinted Polymers-Surface-Enhanced Raman Spectroscopy/Colorimetric Dual Sensor for Determination of Chlorpyrifos in Apple Juice. Sens. Actuators B Chem. 2017, 241, 750–757. [Google Scholar] [CrossRef]
- Zhu, J.; Ahmad, W.; Xu, Y.; Liu, S.; Chen, Q.; Hassan, M.M.; Ouyang, Q. Development of a Novel Wavelength Selection Method for the Trace Determination of Chlorpyrifos on Au@Ag NPs Substrate Coupled Surface-Enhanced Raman Spectroscopy. Analyst 2019, 144, 1167–1177. [Google Scholar] [CrossRef]
- Tang, J.; Chen, W.; Ju, H. Rapid Detection of Pesticide Residues Using a Silver Nanoparticles Coated Glass Bead as Nonplanar Substrate for SERS Sensing. Sens. Actuators B Chem. 2019, 287, 576–583. [Google Scholar] [CrossRef]
- He, Y.; Xiao, S.; Dong, T.; Nie, P. Gold Nanoparticles with Different Particle Sizes for the Quantitative Determination of Chlorpyrifos Residues in Soil by SERS. Int. J. Mol. Sci. 2019, 20, 2817. [Google Scholar] [CrossRef] [Green Version]
- Jia, K.; Xie, J.; He, X.; Zhang, D.; Hou, B.; Li, X.; Zhou, X.; Hong, Y.; Liu, X. Polymeric Micro-Reactors Mediated Synthesis and Assembly of Ag Nanoparticles into Cube-like Superparticles for SERS Application. Chem. Eng. J. 2020, 395, 125123. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, X.; Xu, L.; Ying, Y.; Wu, Y.; Wen, Y.; Yang, H. Template-Free Synthesis of SERS-Active Gold Nanopopcorn for Rapid Detection of Chlorpyrifos Residues. Sens. Actuators B Chem. 2017, 241, 1008–1013. [Google Scholar] [CrossRef]
- Khurana, S.C.; McLaren, J.S. The Influence of Leaf Area, Light Interception and Season on Potato Growth and Yield. Potato Res. 1982, 25, 329–342. [Google Scholar] [CrossRef]
- Wells, R.; Meredith, W.R.; Williford, J.R. Heterosis in Upland Cotton. II. Relationship of Leaf Area to Plant Photosynthesis. Crop Sci. 1988, 28, 522–525. [Google Scholar] [CrossRef]
- Bang, D.; Chang, Y.W.; Park, J.; Lee, T.; Park, J.; Yeo, J.S.; Kim, E.K.; Yoo, K.H.; Huh, Y.M.; Haam, S. One-Step Electrochemical Fabrication of Vertically Self-Organized Silver Nanograss. J. Mater. Chem. A 2013, 1, 4851–4857. [Google Scholar] [CrossRef]
- Lu, H.; Jin, M.; Ma, Q.; Yan, Z.; Liu, Z.; Wang, X.; Akinoglu, E.M.; van den Berg, A.; Zhou, G.; Shui, L. Ag Nano-Assemblies on Si Surface via CTAB-Assisted Galvanic Reaction for Sensitive and Reliable Surface-Enhanced Raman Scattering Detection. Sens. Actuators B Chem. 2020, 304, 127224. [Google Scholar] [CrossRef]
- Mohapatra, S.; Siddhanta, S.; Ravinder Kumar, D.; Narayana, C.; Kumar Maji, T. Facile and Green Synthesis of SERS Active and Ferromagnetic Silver Nanorods. Eur. J. Inorg. Chem. 2010, 2010, 4969–4974. [Google Scholar] [CrossRef]
- Gómez-Graña, S.; Pérez-Juste, J.; Alvarez-Puebla, R.A.; Guerrero-Martínez, A.; Liz-Marzán, L.M.; Gómez-Graña, S.; Pérez-Juste, J.; Liz-Marzán, L.M.; Alvarez-Puebla, R.A.; Domingo, M.; et al. Self-Assembly of Au@ Ag Nanorods Mediated by Gemini Surfactants for Highly Efficient SERS-active Supercrystals. Wiley Online Libr. 2013, 1, 477–481. [Google Scholar] [CrossRef]
- Botta, R.; Eiamchai, P.; Horprathum, M.; Limwichean, S.; Chananonnawathorn, C.; Patthanasettakul, V.; Nuntawong, N. Investigation of Silver Nanorods as Reusable SERS-Active Substrates for Trace Level Detection of 2-MIB Volatile Organic Compound. Sens. Actuators B Chem. 2018, 271, 122–127. [Google Scholar] [CrossRef]
- Keikavousi Behbahan, A.; Mahdavi, V.; Roustaei, Z.; Bagheri, H. Preparation and Evaluation of Various Banana-Based Biochars Together with Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry for Determination of Diverse Pesticides in Fruiting Vegetables. Food Chem. 2021, 360, 130085. [Google Scholar] [CrossRef]
- Skotadis, E.; Kanaris, A.; Aslanidis, E.; Kalatzis, N.; Chatzipapadopoulos, F.; Marianos, N.; Tsoukalas, D. Identification of Two Commercial Pesticides by a Nanoparticle Gas-Sensing Array. Sensors 2021, 21, 5803. [Google Scholar] [CrossRef]
- Chen, M.J.; Yang, H.L.; Si, Y.M.; Tang, Q.; Chow, C.F.; Gong, C. bin A Hollow Visible-Light-Responsive Surface Molecularly Imprinted Polymer for the Detection of Chlorpyrifos in Vegetables and Fruits. Food Chem. 2021, 355, 129656. [Google Scholar] [CrossRef] [PubMed]
- Weerathunge, P.; Behera, B.K.; Zihara, S.; Singh, M.; Prasad, S.N.; Hashmi, S.; Mariathomas, P.R.D.; Bansal, V.; Ramanathan, R. Dynamic Interactions between Peroxidase-Mimic Silver NanoZymes and Chlorpyrifos-Specific Aptamers Enable Highly-Specific Pesticide Sensing in River Water. Anal. Chim. Acta 2019, 1083, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Sahoo, D.; Sarkar, P.; Chakraborty, K.; Das, S. Fluorescence Turn-on and Turn-off Sensing of Pesticides by Carbon Dot-Based Sensor. New J. Chem. 2019, 43, 12137–12151. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.; Park, S.J.; Kwon, C.; Noh, H. Development of Colorimetric Paper Sensor for Pesticide Detection Using Competitive-Inhibiting Reaction. BioChip J. 2018, 12, 326–331. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Park, J.; Lee, G.; Kim, W.; Park, J. Detection of Chlorpyrifos Using Bio-Inspired Silver Nanograss. Materials 2022, 15, 3454. https://doi.org/10.3390/ma15103454
Park H, Park J, Lee G, Kim W, Park J. Detection of Chlorpyrifos Using Bio-Inspired Silver Nanograss. Materials. 2022; 15(10):3454. https://doi.org/10.3390/ma15103454
Chicago/Turabian StylePark, Hyunjun, Joohyung Park, Gyudo Lee, Woong Kim, and Jinsung Park. 2022. "Detection of Chlorpyrifos Using Bio-Inspired Silver Nanograss" Materials 15, no. 10: 3454. https://doi.org/10.3390/ma15103454







