Thermodynamic and Kinetic Binding Behaviors of Human Serum Albumin to Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
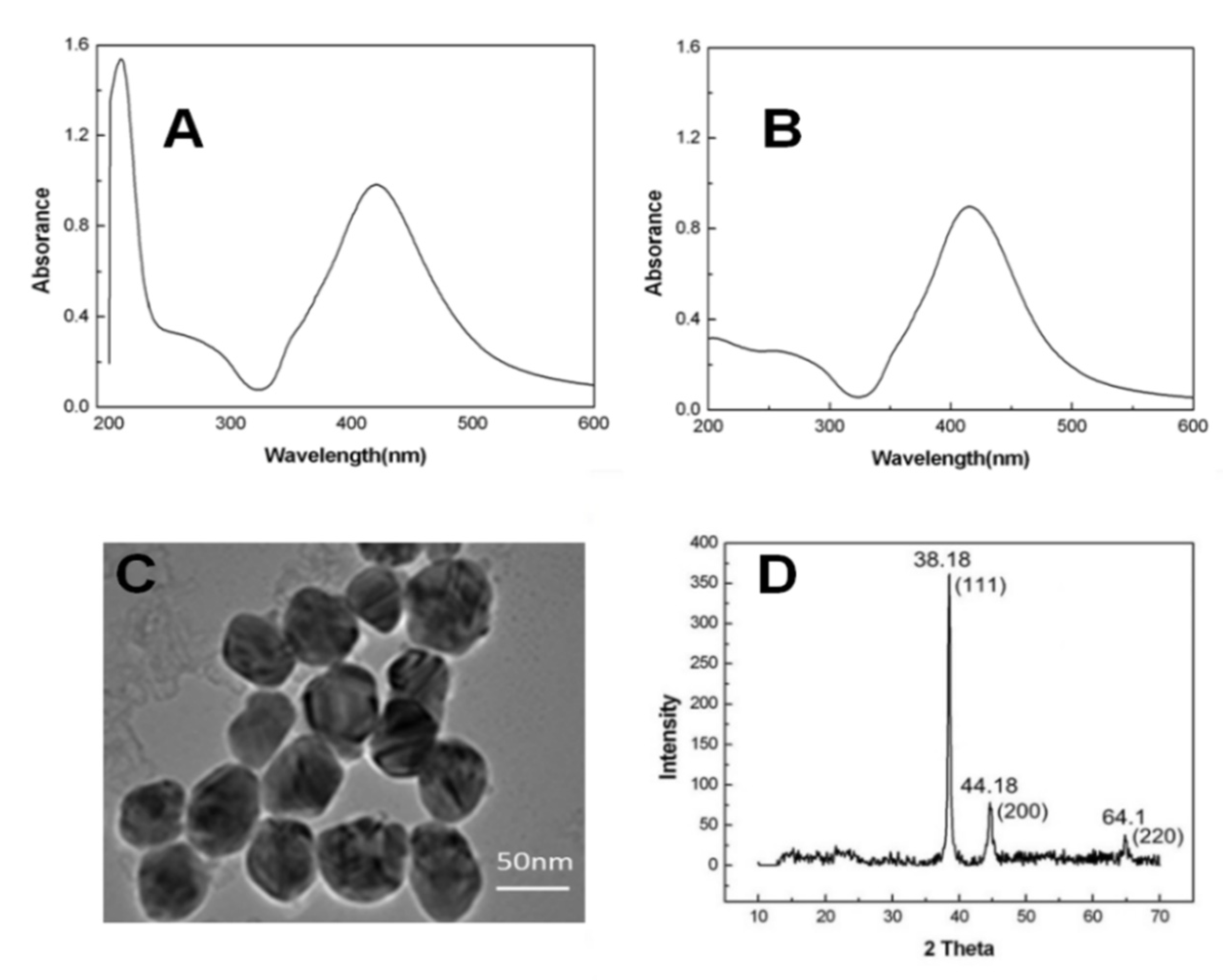
2.2. AgNPs Synthesis and Feature Analysis
2.3. Fluorescence and Synchronous Fluorescence Detection
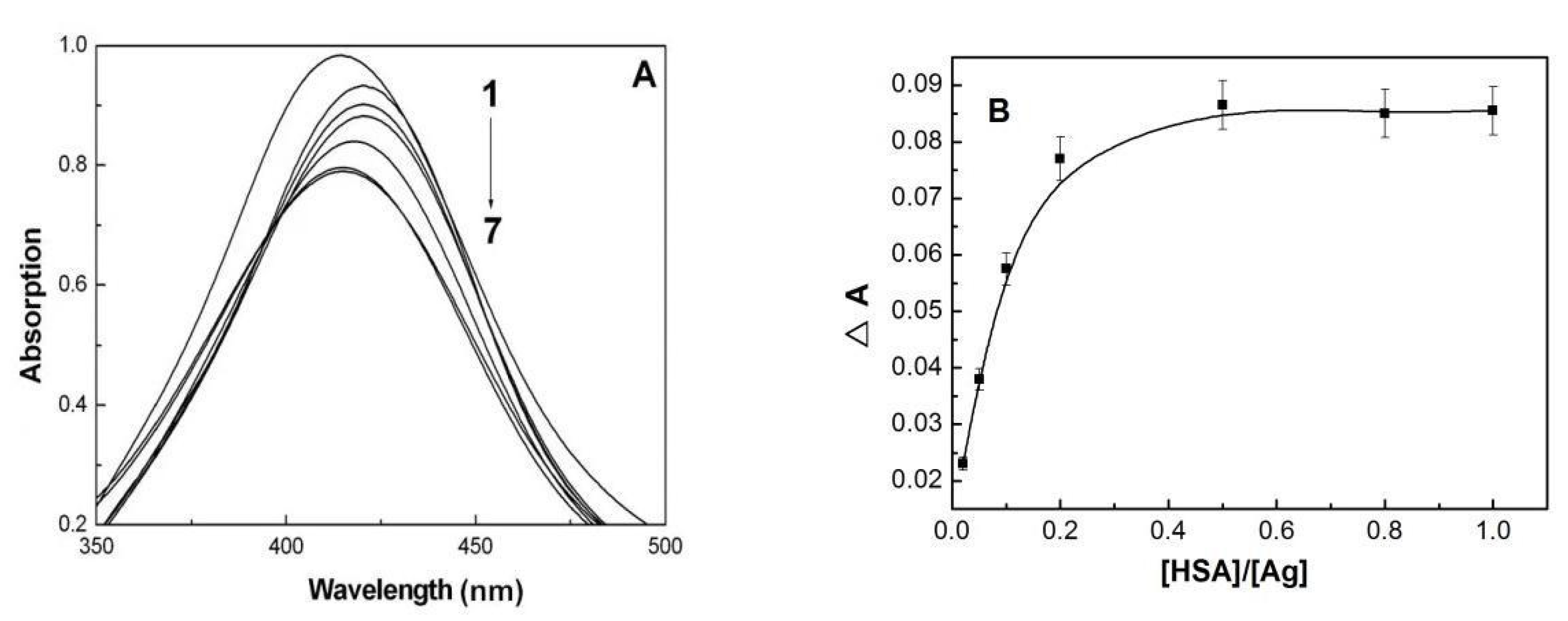
2.4. UV-Vis Absorption Spectroscopy Measurements
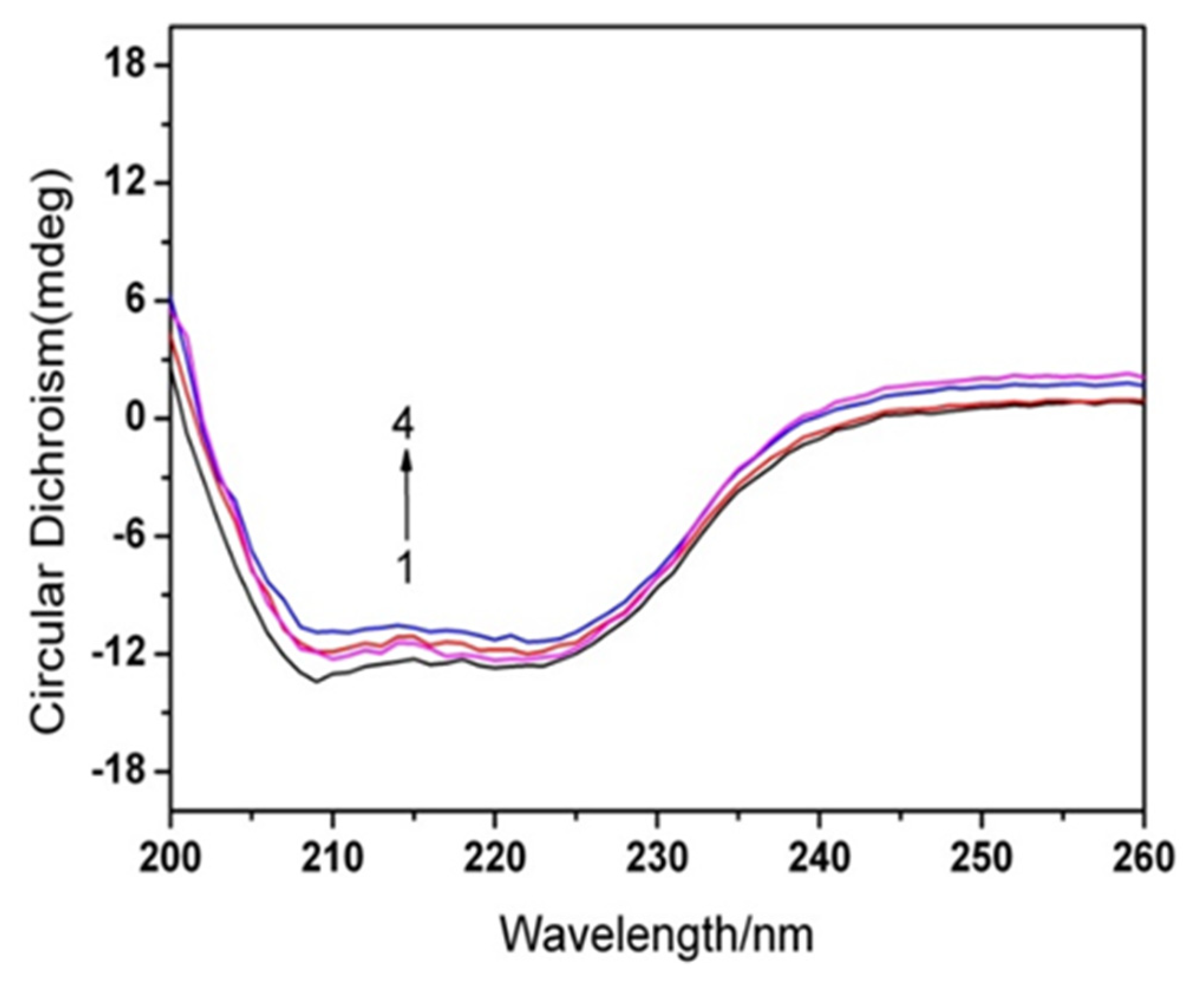
2.5. Circular Dichroism (CD) Detection
3. Results and Discussion
3.1. Characterization of AgNPs
3.2. Fluorescence Quenching Mechanism of HSA by AgNPs
3.3. Binding Mode of HSA with AgNPs
3.4. Conformational Changes of HSA
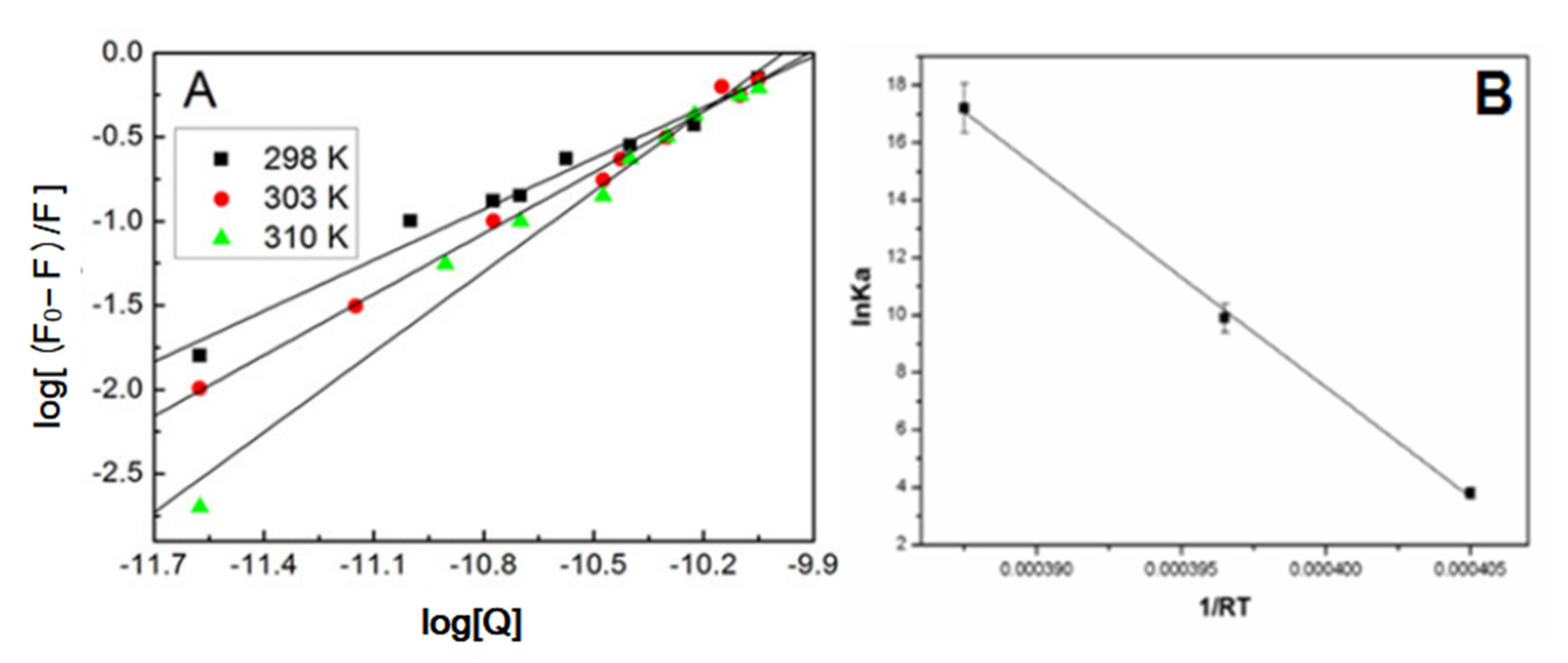
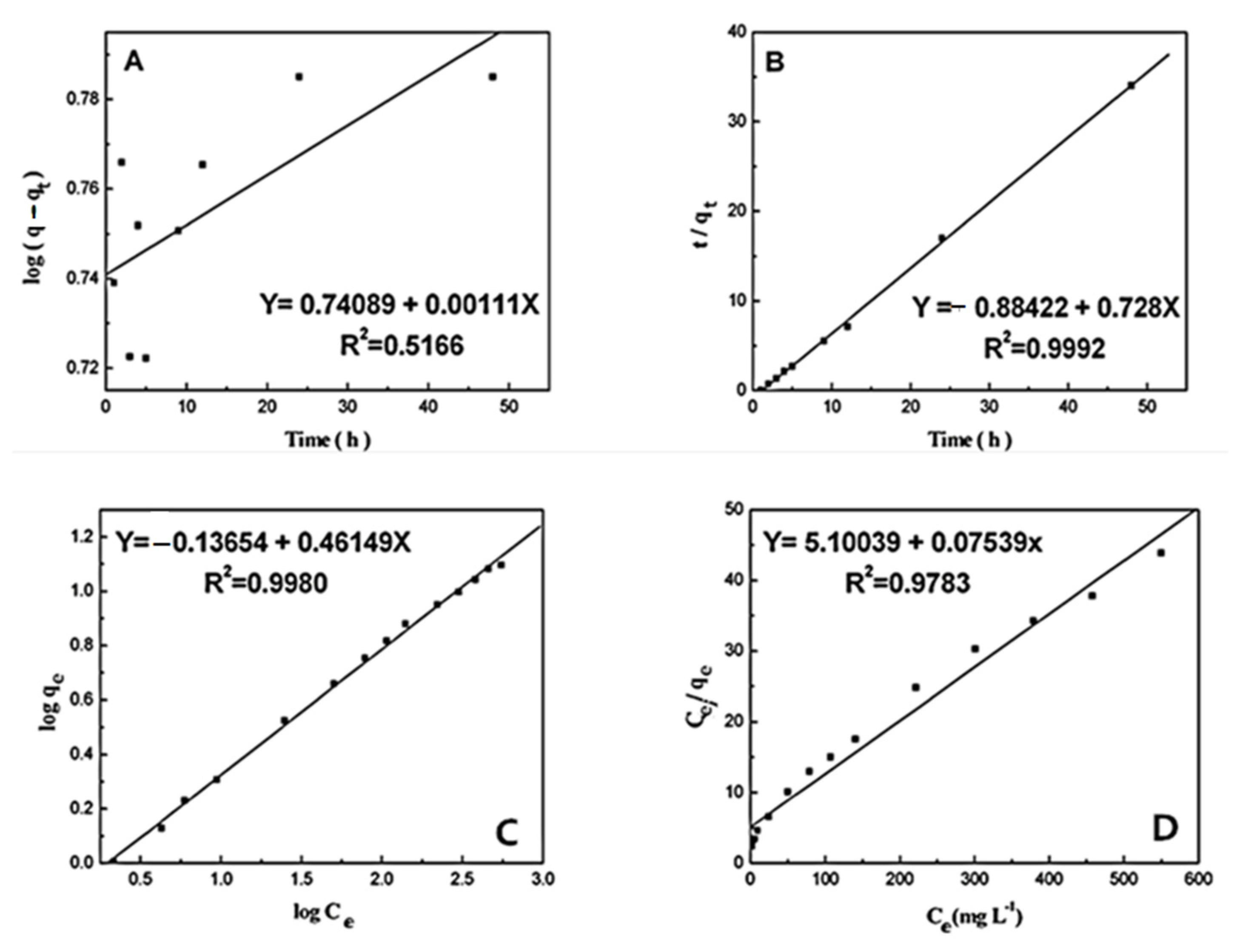
3.5. Kineticstudieson the Adsorption Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doering, W.E.; Nie, S. Single-molecule and single-nanoparticle SERS: Examining the roles of surface active sites and chemical enhancement. J. Phys. Chem. B 2002, 106, 311–317. [Google Scholar] [CrossRef]
- Salas, P.; Odzak, N.; Echegoyen, Y.; Kägi, R.; Sancho, M.C.; Navarro, E. The role of size and protein shells in the toxicity to algal photosynthesis induced by ionic silver delivered from silver nanoparticles. Sci. Total Environ. 2019, 692, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Banjare, M.K.; Behera, K.; Banjare, R.K.; Sahu, R.; Sharma, S.; Pandey, S.; Ghosh, K.K. Interaction of ionic liquid with silver nanoparticles: Potential application in induced structural changes of globular proteins. ACS Sus. Chem. Eng. 2019, 7, 11088–11100. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Cheung, Y.F.; Tam, T.S.C.; Lok, C.N.; Sun, H.; Ng, K.M. Plasmonic metal nanoparticles as efficient mass tags for ion signal amplification and ultrasensitive detection of protein markers. Anal. Chim. Acta 2019, 1055, 1–6. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotech. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Bonilla, J.O.; Alborés, S.V.; Villegas, L.B.; Páez, P.L. Biogenic nanoparticles: Synthesis, stability and biocompatibility mediated by proteins of pseudomonas aeruginosa. Colloids Surf. B 2019, 184, 110517. [Google Scholar] [CrossRef]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef]
- Zolghadri, S.; Saboury, A.A.; Golestani, A.; Divsalar, A.; Rezaei-Zarchi, S.; Moosavi-Movahedi, A.A. Interaction between silver nanoparticle and bovine hemoglobin at different temperatures. J. Nanopart. Res. 2009, 11, 1751–1758. [Google Scholar] [CrossRef]
- Lai, W.J.; Wang, Q.S.; Li, L.; Hu, Z.Y.; Chen, J.K.; Fang, Q.J. Interaction of gold and silver nanoparticles with human plasma: Analysis of protein corona reveals specific binding patterns. Colloids Surf. B 2017, 152, 317–325. [Google Scholar] [CrossRef]
- Ravindran, A.; Singh, A.; Raichur, A.M.; Chandrasekaran, N.; Mukherjee, A. Studies on interaction of colloidal Ag nanoparticles with bovine serum albumin (BSA). Colloids Surf. B 2010, 76, 32–37. [Google Scholar] [CrossRef]
- Kennedy, D.C.; Qian, H.; Gies, V.; Yang, L. Human serum albumin stabilizes aqueous silver nanoparticle suspensions and inhibits particle uptake by cells. Environ. Sci. Nano 2018, 5, 863–867. [Google Scholar] [CrossRef]
- Sen, T.; Mandal, S.; Haldar, S.; Chattopadhyay, K.; Patra, A. Interaction of gold nanoparticle with human serum albumin (HSA) protein using surface energy transfer. J. Phys. Chem. C 2011, 115, 24037–24044. [Google Scholar] [CrossRef]
- Carballal, S.; Radi, R.; Kirk, M.C.; Barnes, S.; Freeman, B.A.; Alvarez, B. Acid formation in human serum albumin by hydrogen peroxide and peroxynitrite. Biochemistry 2003, 42, 9906–9914. [Google Scholar] [CrossRef] [PubMed]
- Petitpas, I.; Bhattacharya, A.A.; Twine, S.; East, M.; Curry, S. Crystal structure analysis of warfarin binding to human serum albumin. J. Biol. Chem. 2001, 271, 22804–22809. [Google Scholar] [CrossRef] [Green Version]
- Azman, N.A.; Nguyen, T.X.; Yong Kah, J.C. Dynamics of human serum albumin corona formation on gold nanorods with different surface ligands in silico. J. Phys. Chem. B 2021, 125, 1181–1195. [Google Scholar] [CrossRef]
- Li, A.P. Screening for human ADME/Tox drug properties in drug discovery. Drug Discov. Today 2001, 6, 357–366. [Google Scholar] [CrossRef]
- Nikolic, D.; Habibi-Goudarzi, S.; Corley, D.G.; Gafner, S.; Pezzuto, J.M.; Breemen, R.B. Evaluation of cyclooxygenase-2 inhibitors using pulsed ultrafiltration mass spectrometry. Anal. Chem. 2000, 7, 23853–23859. [Google Scholar] [CrossRef]
- Watabe, T. Strategic proposals for predicting drug-drug interactions during new drug development: Based on sixteen deaths caused by interactions of the new antiviral sorivudine with 5-fluorouracil prodrugs. J. Toxicol. Sci. 1996, 21, 21299–21300. [Google Scholar] [CrossRef] [Green Version]
- Galloway, T.; Lewis, C.; Dolciotti, I.; Johnston, B.D.; Moger, J.; Regolib, F. Sublethal toxicity of nano-titanium dioxide and carbon nanotubes in a sediment dwelling marine polychaete. Environ. Pollut. 2010, 158, 1748–1755. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-dependent toxicity of metal oxide particles—a comparison between nano- and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef]
- Li, Z.; Greden, K.; Alvarez, P.J.J.; Gregory, K.B.; Lowry, G.V. Adsorbed polymer and nom limits adhesion and toxicity of nano scale zerovalent iron to coli, E. Environ. Sci. Technol. 2010, 44, 3462–3467. [Google Scholar] [CrossRef]
- Klaine, S.J.; Koelmans, A.A.; Horne, N.; Carley, S.; Handy, R.D.; Kapustka, L.; Nowack, B.; Kammer, F. Paradigms to assess the environmental impact of manufactured nanomaterials. Environ. Toxicol. Chem. 2012, 31, 3–14. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Meng, H.; Wang, X.; Lin, S.J.; Ji, Z.X.; Zhang, H.Y. Nanomaterial toxicity testing in the 21st century: Use of a predictive toxicological approach and high-throughput screening. Acc. Chem. Res. 2012, 46, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Siriwardana, K.; Wang, A.; Gadogbe, M.; Collier, W.E.; Fitzkee, N.C.; Zhang, D. Studying the effects of cysteine residues on protein interactions with silver nanoparticles. J. Phys. Chem. C 2015, 119, 2910–2916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munro, C.; Smith, W.; Garner, M.; Clarkson, J.; White, P. Characterization of thesurface of a citrate-reduced colloid optimized for use as a substrate forsurface-enhanced resonance Raman scattering. Langmuir 1995, 11, 3712–3720. [Google Scholar] [CrossRef]
- Thompson, D.G.; Enright, A.; Faulds, K.; Smith, W.E.; Graham, D. Ultrasensitive DNA detection using oligonucleotide-silver nanoparticle conjugates. Anal. Chem. 2008, 80, 2805–2810. [Google Scholar] [CrossRef]
- Henglein, A.; Giersig, M. Formation of colloidal silver nanoparticles: Capping action of citrate. J. Phys. Chem. B 1999, 103, 9533–9539. [Google Scholar] [CrossRef]
- Pillai, Z.S.; Kamat, P.V. What factors control the size and shape of silver nanoparticles in the citrate ion reduction method. J. Phys. Chem. B 2003, 108, 945–951. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of silver nanoparticles using acalyphaindica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B 2010, 76, 50–56. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Plenum Press: New York, NY, USA, 2006; p. 277. [Google Scholar]
- Wang, G.K.; Hou, H.M.; Wang, S.L.; Yan, C.L.; Lu, Y.F. Exploring the interaction of silver nanoparticles with lysozyme: Binding behaviors and kinetics. Colloids Surf. B 2017, 157, 138–145. [Google Scholar] [CrossRef]
- Tian, J.; Liu, J.; He, W.; Hu, Z.; Yao, X.; Chen, X. Probing the binding of scutellarin to human serum albumin by circular dichroism, fluorescence spectroscopy, FTIR, and molecular modeling method. Biomacromolecules 2004, 5, 1956–1961. [Google Scholar] [CrossRef] [PubMed]
- Garabagiu, S. A spectroscopic study on the interaction between gold nanoparticles and hemoglobin. Mater. Res. Bull. 2011, 46, 2474–2477. [Google Scholar] [CrossRef]
- Zhou, Q.J.; Xiang, J.F.; Tang, Y.L.; Liao, J.P.; Yu, C.Y.; Zhang, H.; Li, L.; Yang, Y.Y.; Xu, G.Z. Investigation on the interaction between a heterocyclic aminal derivative, SBDC, and human serum albumin. Colloids Surf. B 2008, 61, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Gerbanowski, A.; Malabat, C.; Rabiller, C.; Gueguen, J.; Agric, J. Grafting of aliphatic and aromatic probes on rapeseed 2S and 12S proteins: Influence on their structural and physicochemical properties. Food Chem. 1999, 47, 5218–5226. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, J.; Han, H. Probing the interaction of magnetic iron oxide nanoparticles with bovine serum albumin by spectroscopic techniques. J. Phys. Chem. B 2009, 113, 10454–10458. [Google Scholar] [CrossRef]
- Tian, J.N.; Liu, J.Q.; Hu, Z.D.; Chen, X.G. Interaction of wogonin with bovine serum albumin. Bioorg. Med. Chem. 2005, 13, 4124–4129. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Némethy, G.; Scheraga, H.A. The structure of water and hydrophobic bonding in proteins. III. The thermodynamic properties of hydrophobic bonds in proteins. J. Phys. Chem. 1962, 66, 1773–1789. [Google Scholar] [CrossRef]
- Bi, S.Y.; Yan, L.L.; Sun, Y.T.; Zhang, H.Q. Investigation of ketoprofen binding to human serum albumin by spectral methods. Spectrochim. Acta A 2011, 78, 410–414. [Google Scholar] [CrossRef]
- Wang, G.K.; Liu, X.B.; Yan, C.L.; Bai, G.Y.; Lu, Y. Probing the binding of trypsin to glutathione-stabilized gold nanoparticles in aqueous solution. Colloids Surf. B 2015, 135, 261–266. [Google Scholar] [CrossRef]
- Erwardt, P.; Roszek, K.; Wiśniewski, M. Determination of Graphene Oxide Adsorption Space by Lysozyme Uptake—Mechanistic Studies. J. Phys. Chem. B 2022, 126, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Kasai, S.; Horie, T.; Mizuma, T.; Mizuma, T.; Awazu, S. Fluorescence energy transfer study of the relationship between the lone tryptophan residue and drug binding sites in human serum albumin. J. Pharm. Sci. 1987, 76, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Y.L.; Gao, S.H.; Wang, G.K.; Yan, C.L.; Chen, D.J. Interaction of quercetin with ovalbumin: Spectroscopic and molecular modeling studies. Lumin, J. 2009, 129, 1048–1054. [Google Scholar] [CrossRef]
- Yuan, T.; Weljie, A.M.; Vogel, H.J. Tryptophan fluorescence quenching by methionine and selenomethionine residues of calmodulin: Orientation of peptide and protein binding. Biochemistry 1998, 37, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Burstein, E.A.; Vedenkina, N.S.; Ivkova, M.N. Fluorescence and the location of tryptophan residues in protein molecules. Photochem. Photobiol. 1973, 18, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.Y.; Zhang, Y.H.; Qin, J.; Chen, X.G. Study of the interaction between esculetin and human serum albumin by multi-spectroscopic method and molecular modeling. J. Mol. Struct. 2008, 888, 25–32. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.M.; Luo, H.J.; Yang, L.Y.; Zhou, Z.Q.; Liu, Y.; Xiao, Q. Conformational structure variation of human serum albumin after binding interaction with black phosphorus quantum dots. Int. J. Biol. Macromol. 2020, 146, 405–414. [Google Scholar] [CrossRef]
- Bhogale, A.; Patel, N.; Mariam, J.; Dongre, P.M.; Miotello, A.; Kothari, D.C. Comprehensive studies on the interaction of copper nanoparticles with bovine serum albumin using various spectroscopies. Colloids Surf. B 2014, 113, 276–284. [Google Scholar] [CrossRef]
- Barbir, R.; Goessler, W.; Ćurlin, M.; Micek, V.; Milić, M.; Vuković, B.; Vinković Vrček, I. Protein corona modulates distribution and toxicological effects of silver nanoparticles in vivo. Part. Part. Syst. Char. 2019, 36, 1900174–1900185. [Google Scholar] [CrossRef]
- Wang, G.K.; Yan, C.L.; Gao, S.Y.; Liu, Y.F. Surface chemistry of gold nanoparticles determines interactions with bovine serum albumin. Mat. Sci. Eng. C 2019, 103, 109856–109866. [Google Scholar] [CrossRef]
- Khan, S.S.; Mukherjee, A.; Chandrasekaran, N. Interaction of colloidal silver nanoparticles (SNPs) with exopolysaccharides (EPS) and its adsorption isotherms and kinetics. Colloids Surf. A 2011, 381, 99–105. [Google Scholar] [CrossRef]
- Hoda, N.; Bayram, E.; Ayranci, E. Kinetic and equilibrium studies on the removal of acid dyes from aqueous solutions by adsorption onto activated carbon cloth. J. Hazard. Mater. 2006, 137, 44–351. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Mukherjee, A.; Chandrasekaran, N. Adsorptive removal of silver nanoparticles (SNPs) from aqueous solution by aeromonas punctata and its adsorption isotherm and kinetics. Colloids Surf. B 2012, 92, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Srivatsan, P.; Vaishnavi, N.; Mukherjee, S.A.; Chandrasekaran, N. Interaction of silver nanoparticles (SNPs) with bacterial extracellular proteins (ECPs) and its adsorption isotherms and kinetics. J. Hazard. Mater. 2011, 192, 299–306. [Google Scholar] [CrossRef]
- Wang, G.K.; Lu, Y.F.; Hou, H.M.; Liu, Y.F. Probing the binding behavior and kinetics of silver nanoparticles with bovine serum albumin. RSC Adv. 2017, 7, 9393–9401. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]

| T (K) | KSV (109 mol·L−1) | Kq (1017 L·mol−1·s−1) | Ra | SDb |
|---|---|---|---|---|
| 298 | 7.639 | 7.639 | 0.9917 | 0.03625 |
| 303 | 7.443 | 7.443 | 0.9927 | 0.03324 |
| 310 | 7.274 | 7.274 | 0.9892 | 0.04161 |
| T (K) | Kb (104 L·mol−1) | Ra | SDb | ∆H0 (kJ·mol−1) | ∆G0 (kJ·mol−1) | ∆S0 (J·mol−1) |
|---|---|---|---|---|---|---|
| 298 | 6.609 | 0.9940 | 0.0531 | 124.68 | −28.71 | 514.73 |
| 303 | 38.616 | 0.9970 | 0.0437 | −31.28 | ||
| 310 | 67.098 | 0.9976 | 0.0407 | −34.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Shi, Z.; Wang, G. Thermodynamic and Kinetic Binding Behaviors of Human Serum Albumin to Silver Nanoparticles. Materials 2022, 15, 4957. https://doi.org/10.3390/ma15144957
Tian J, Shi Z, Wang G. Thermodynamic and Kinetic Binding Behaviors of Human Serum Albumin to Silver Nanoparticles. Materials. 2022; 15(14):4957. https://doi.org/10.3390/ma15144957
Chicago/Turabian StyleTian, Jinjun, Zhenghai Shi, and Gongke Wang. 2022. "Thermodynamic and Kinetic Binding Behaviors of Human Serum Albumin to Silver Nanoparticles" Materials 15, no. 14: 4957. https://doi.org/10.3390/ma15144957





