Rheological Properties of MWCNT-Doped Titanium-Oxo-Alkoxide Gel Materials for Fiber Drawing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Precursors
2.2. Rheological Measurements
2.3. Fibers Preparation and Characterization
3. Results and Discussions
3.1. Preparation of Precursors
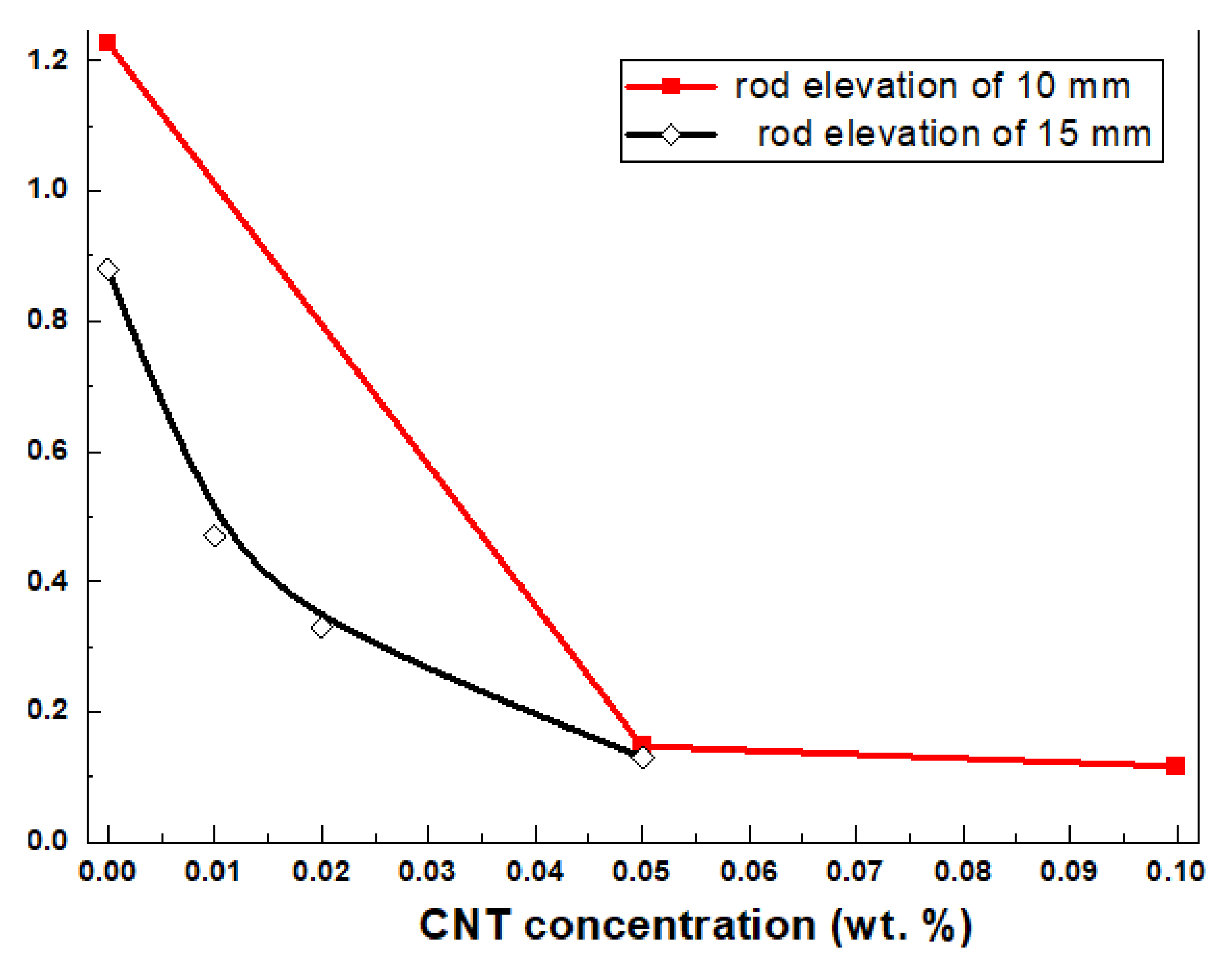
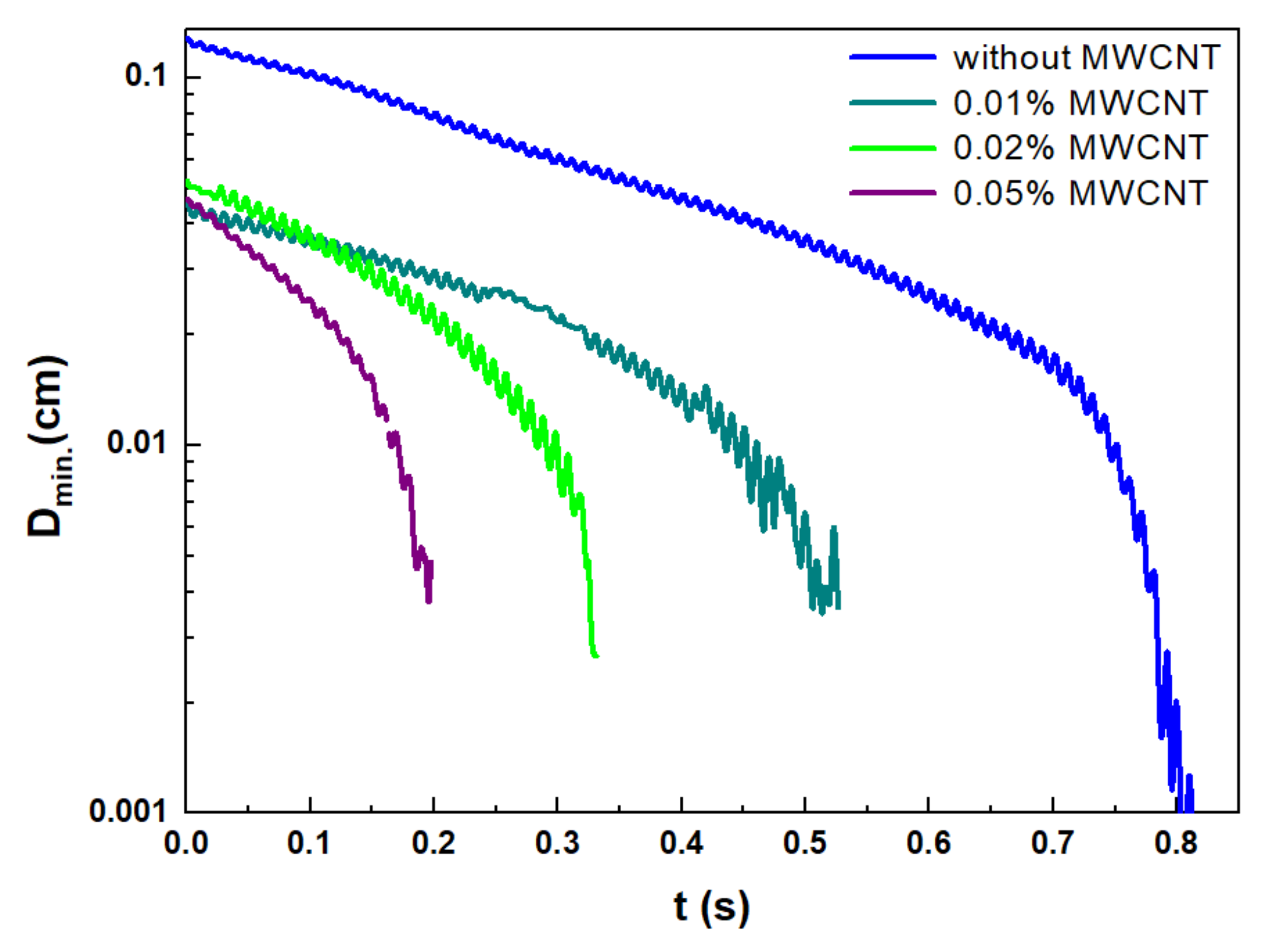
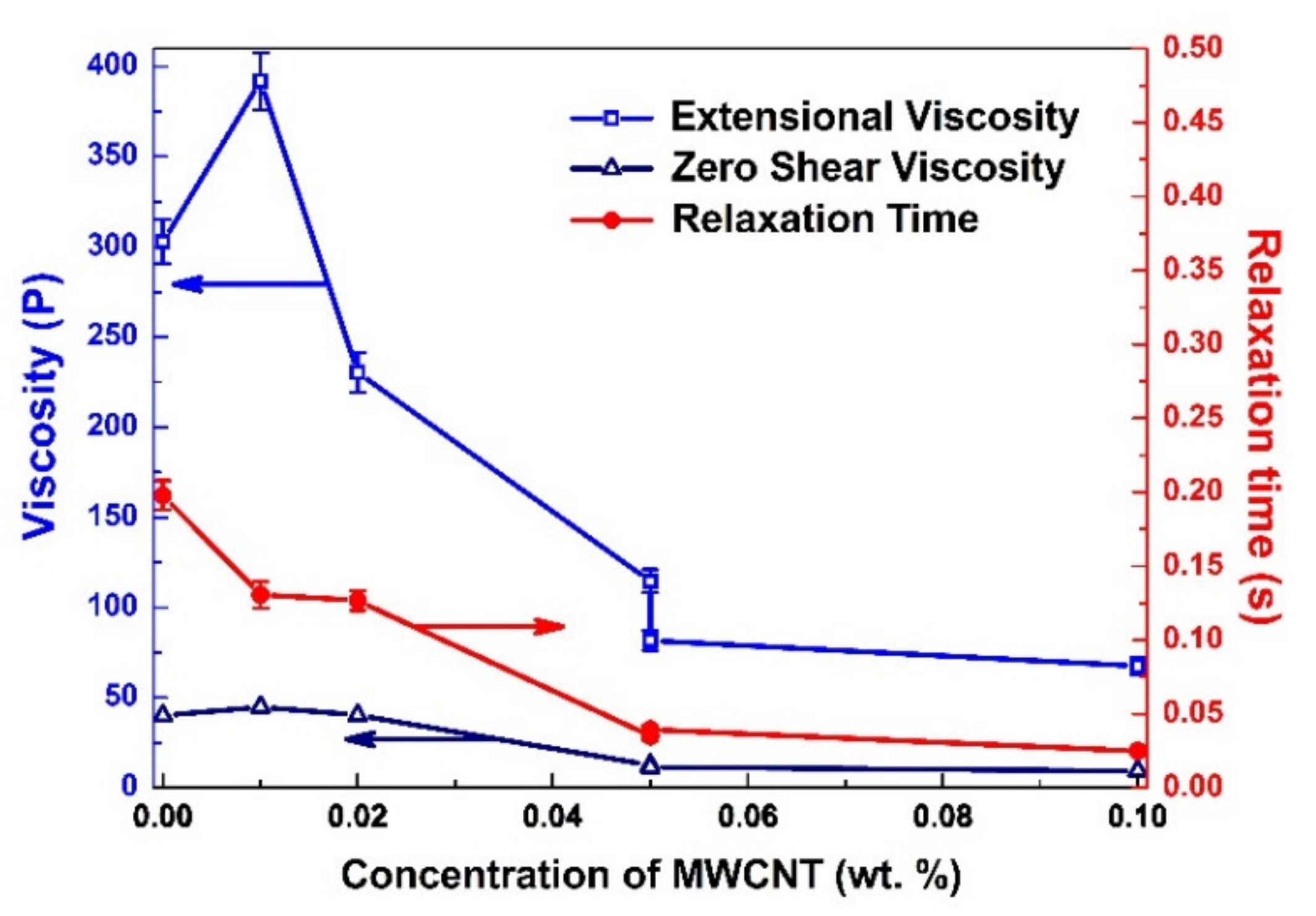
3.2. The Rheological Properties of the CNT-Doped Metal Alkoxide Precursors
- (i)
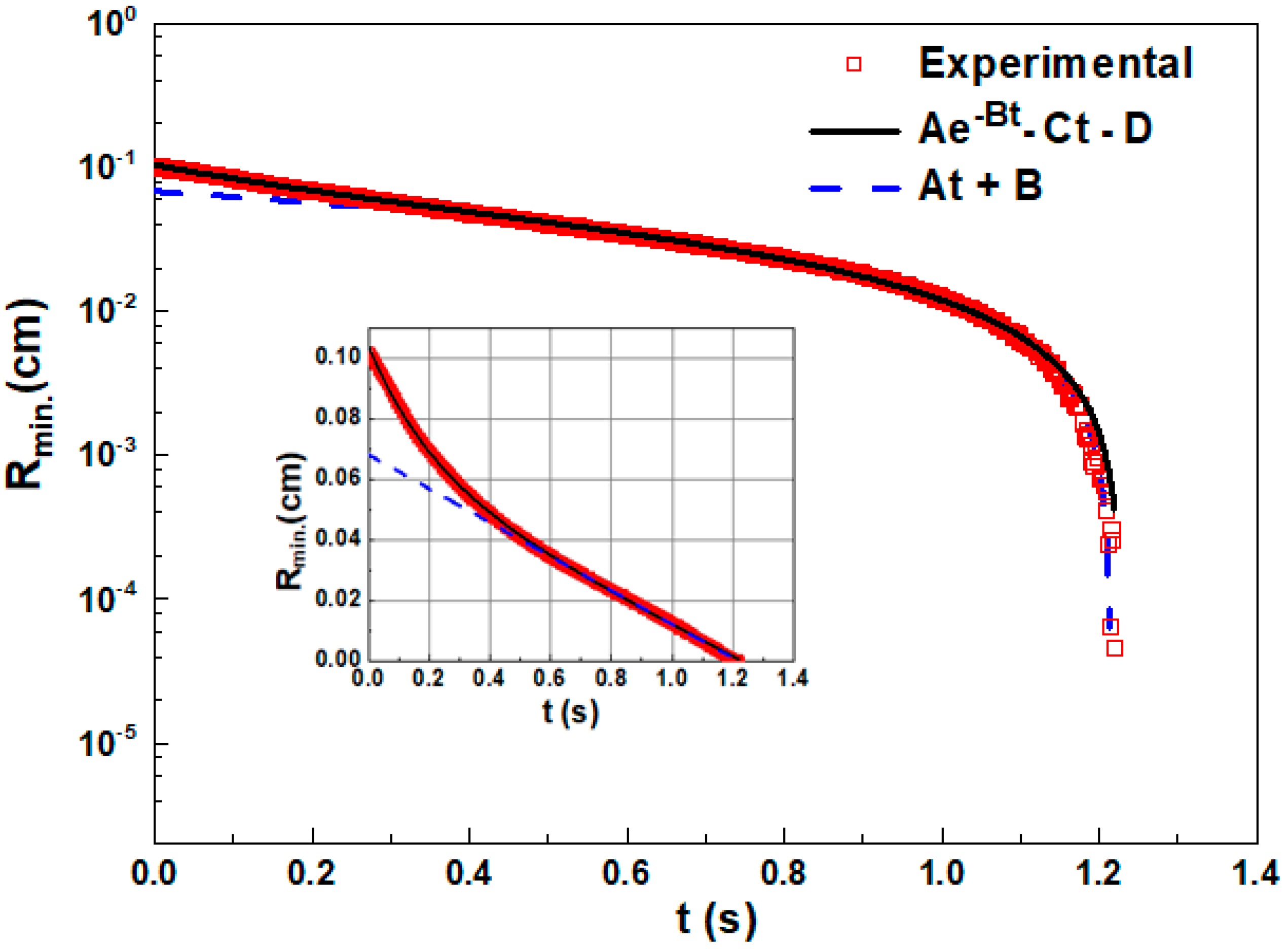
- approach for initial and intermediate stages of evolution described by the equation [50]:Rmin = A × exp(−B × t) − C × t + D
- (ii)
- approach at the final stage of the filament evolution described by linear law:where t is time and A, B, C, D are fitting parameters.Rmin = A × t + B
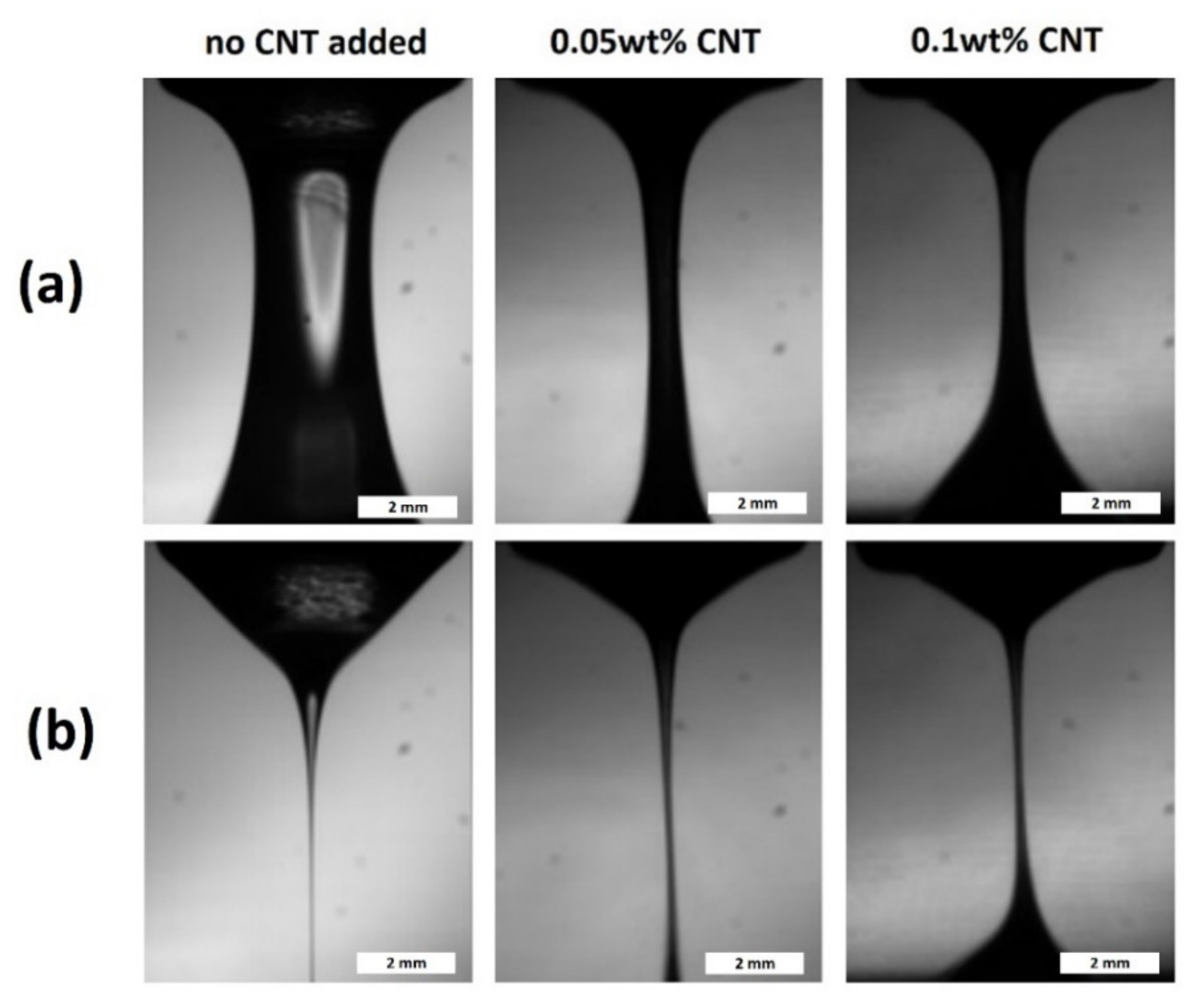
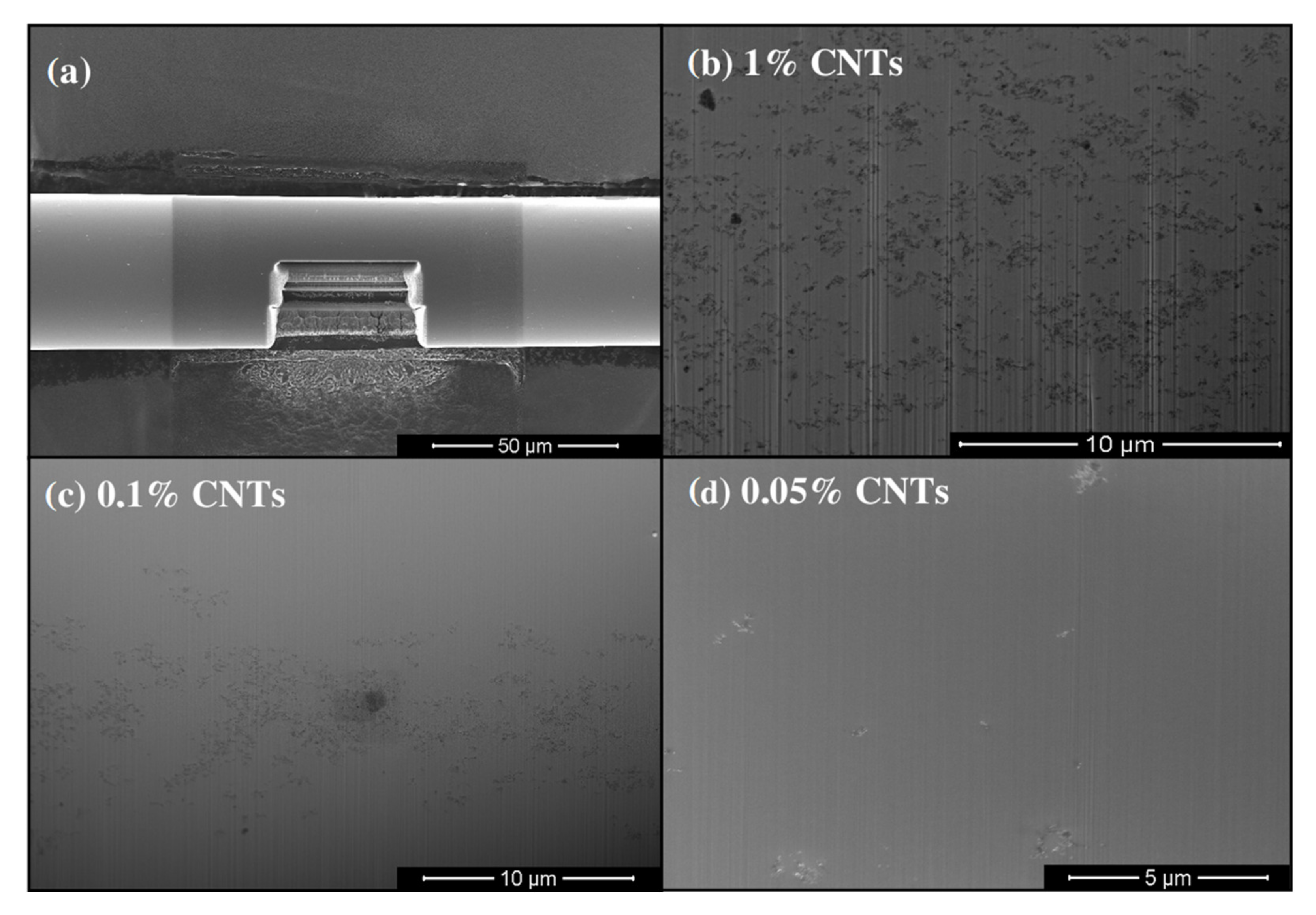
3.3. CNT-Metal Oxide Fiber Preparation and Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Cheng, H.-M. Carbon nanotubes: Controlled growth and application. Mater. Today 2013, 16, 19–28. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, Y.; Ren, Z.F. Physics and applications of aligned carbon nanotubes. Adv. Phys. 2011, 60, 553–678. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, K.; Pan, W. Microstructure-toughening relation in alumina based multiwall carbon nanotube ceramic composites. J. Eur. Ceram. Soc. 2015, 35, 663–671. [Google Scholar] [CrossRef]
- Xin, X.; Zhou, X.; Wu, J.; Yao, X.; Liu, Z. Scalable Synthesis of TiO2/Graphene Nanostructured Composite with High-Rate Performance for Lithium Ion Batteries. ACS Nano 2012, 6, 11035–11043. [Google Scholar] [CrossRef]
- Kazazi, M.; Zafar, Z.A.; Delshad, M.; Cervenka, J.; Chen, C. TiO2/CNT nanocomposite as an improved anode material for aqueous rechargeable aluminum batteries. Solid State Ionics 2018, 320, 64–69. [Google Scholar] [CrossRef]
- Cho, J.; Boccaccini, A.R.; Shaffer, M.S.P. Ceramic matrix composites containing carbon nanotubes. J. Mater. Sci. 2009, 44, 1934–1951. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Zhao, N.; Shi, C.; Song, S. Mechanical properties and microstructures of carbon nanotube-reinforced Al matrix composite fabricated by in situ chemical vapor deposition. J. Alloys Compd. 2009, 487, 258–262. [Google Scholar] [CrossRef]
- Leonov, A.A.; Khasanov, A.O.; Danchenko, V.A.; Khasanov, O.L. Spark plasma sintering of ceramic matrix composite based on alumina, reinforced by carbon nanotubes. IOP Conf. Ser. Mater. Sci. Eng. 2017, 286, 012034. [Google Scholar] [CrossRef]
- Candelario, V.M.; Moreno, R.; Shen, Z.; Ortiz, A.L. Aqueous colloidal processing of nano-SiC and its nano-Y3Al5O12 liquid-phase sintering additives with carbon nanotubes. J. Eur. Ceram. Soc. 2015, 35, 3363–3368. [Google Scholar] [CrossRef]
- Rehman, M.A.U.; Chen, Q.; Braem, A.; Shaffer, M.S.P.; Boccaccini, A.R. Electrophoretic deposition of carbon nanotubes: Recent progress and remaining challenges. Int. Mater. Rev. 2020, 66, 1–30. [Google Scholar] [CrossRef]
- Esquivias, L.; Rivero-Antúnez, P.; Zamora-Ledezma, C.; Domínguez-Rodríguez, A.; Morales-Flórez, V. Intragranular carbon nanotubes in alumina-based composites for reinforced ceramics. J. Sol Gel Sci. Technol. 2018, 90, 162–171. [Google Scholar] [CrossRef]
- Satapathy, S.; Prabakaran, P.; Prasad, E.; Satapathy, S. Augmenting Photoinduced Charge Transport in a Single-Component Gel System: Controlled In Situ Gel-Crystal Transformation at Room Temperature. Chem. A Eur. J. 2018, 24, 6217–6230. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.; Prasad, E. Charge Transfer Modulated Self-Assembly in Poly(aryl ether) Dendron Derivatives with Improved Stability and Transport Characteristics. ACS Appl. Mater. Interfaces 2016, 8, 26176–26189. [Google Scholar] [CrossRef] [PubMed]
- Joly, C.; Goizet, S.; Schrotter, J.; Sanchez-Marcano, J.; Escoubes, M. Sol-gel polyimide-silica composite membrane: Gas transport properties. J. Membr. Sci. 1997, 130, 63–74. [Google Scholar] [CrossRef]
- Zapata-Solvas, E.; García, D.G.; Dominguez-Rodriguez, A. Towards physical properties tailoring of carbon nanotubes-reinforced ceramic matrix composites. J. Eur. Ceram. Soc. 2012, 32, 3001–3020. [Google Scholar] [CrossRef]
- Sakka, S.; Yoko, T. Fibers from gels. J. Non Cryst. Solids 1992, 147–148, 394–403. [Google Scholar] [CrossRef]
- Part, M.; Hanschmidt, K.; Jõgi, J.; Rauwel, E.; Seisenbaeva, G.A.; Kessler, V.G.; Tätte, T. Study of the curing mechanism of metal alkoxide liquid threads for the synthesis of metal oxide fibers or microtubes. RSC Adv. 2014, 4, 12545–12554. [Google Scholar] [CrossRef]
- Tätte, T.; Part, M.; Talviste, R.; Hanschmidt, K.; Utt, K.; Mäeorg, U.; Jõgi, I.; Kiisk, V.; Mändar, H.; Nurk, G.; et al. Yttria stabilized zirconia microtubes for microfluidics under extreme conditions. RSC Adv. 2014, 4, 17413–17419. [Google Scholar] [CrossRef]
- Nadiv, R.; Vasilyev, G.; Shtein, M.; Peled, A.; Zussman, E.; Regev, O. The multiple roles of a dispersant in nanocomposite systems. Compos. Sci. Technol. 2016, 133, 192–199. [Google Scholar] [CrossRef]
- Nadiv, R.; Shachar, G.; Peretz-Damari, S.; Varenik, M.; Levy, I.; Buzaglo, M.; Ruse, E.; Regev, O. Performance of nano-carbon loaded polymer composites: Dimensionality matters. Carbon 2018, 126, 410–418. [Google Scholar] [CrossRef]
- Katepalli, H.; John, V.T.; Tripathi, A.; Bose, A. Microstructure and rheology of particle stabilized emulsions: Effects of particle shape and inter-particle interactions. J. Colloid Interface Sci. 2017, 485, 11–17. [Google Scholar] [CrossRef]
- Gossard, A.; Frances, F.; Aloin, C. Rheological properties of TiO2 suspensions varied by shifting the electrostatic inter-particle interactions with an organic co-solvent. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 425–432. [Google Scholar] [CrossRef]
- Morris, J.F. Toward a fluid mechanics of suspensions. Phys. Rev. Fluids 2020, 5, 110519. [Google Scholar] [CrossRef]
- Kawaguchi, M. Dispersion stability and rheological properties of silica suspensions in aqueous solutions. Adv. Colloid Interface Sci. 2020, 284, 102248. [Google Scholar] [CrossRef]
- Esfe, M.H.; Saedodin, S.; Asadi, A.; Karimipour, A. Thermal conductivity and viscosity of Mg(OH)2-ethylene glycol nanofluids. J. Therm. Anal. 2015, 120, 1145–1149. [Google Scholar] [CrossRef]
- Tuteja, A.; Duxbury, P.M.; Mackay, M.E. Multifunctional Nanocomposites with Reduced Viscosity. Macromolecules 2007, 40, 9427–9434. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, H.-Y.; Shi, R.; Lin, W.-F.; Jia, X.-M.; Qian, H.-J.; Lu, Z.-Y.; Zhang, X.-X.; Li, Y.-K.; Sun, Z.-Y. An unexpected N-dependence in the viscosity reduction in all-polymer nanocomposite. Nat. Commun. 2019, 10, 5552. [Google Scholar] [CrossRef] [Green Version]
- Motahar, S.; Nikkam, N.; Alemrajabi, A.A.; Khodabandeh, R.; Toprak, M.S.; Muhammed, M. Experimental investigation on thermal and rheological properties of n-octadecane with dispersed TiO2 nanoparticles. Int. Commun. Heat Mass Transf. 2014, 59, 68–74. [Google Scholar] [CrossRef]
- Advani, S.G.; Fan, Z. Effect of Dispersion State on the Rheology of Multi-walled Carbon nanotube Suspensions in Shear Flow. AIP Conf. Proc. 2004, 712, 1619–1623. [Google Scholar] [CrossRef]
- Kharchenko, S.B.; Douglas, J.F.; Obrzut, J.; Grulke, E.A.; Migler, K. Flow-induced properties of nanotube-filled polymer materials. Nat. Mater. 2004, 3, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Esfe, M.H.; Arani, A.A.A.; Madadi, M.R.; Alirezaie, A. A study on rheological characteristics of hybrid nano-lubricants containing MWCNT-TiO2 nanoparticles. J. Mol. Liq. 2018, 260, 229–236. [Google Scholar] [CrossRef]
- Tian, Z.; Rostami, S.; Taherialekouhi, R.; Karimipour, A.; Moradikazerouni, A.; Yarmand, H.; Zulkifli, N.W.B.M. Prediction of rheological behavior of a new hybrid nanofluid consists of copper oxide and multi wall carbon nanotubes suspended in a mixture of water and ethylene glycol using curve-fitting on experimental data. Phys. A Stat. Mech. Appl. 2020, 549, 124101. [Google Scholar] [CrossRef]
- Yan, S.-R.; Toghraie, D.; Abdulkareem, L.A.; Alizadeh, A.; Barnoon, P.; Afrand, M. The rheological behavior of MWCNTs–ZnO/Water–Ethylene glycol hybrid non-Newtonian nanofluid by using of an experimental investigation. J. Mater. Res. Technol. 2020, 9, 8401–8406. [Google Scholar] [CrossRef]
- Chanklin, W.; Laowongkotr, J.; Chibante, L.F. Electrical property validation of percolation modeling in different polymer structures of carbon-based nanocomposites. Mater. Today Commun. 2018, 17, 153–160. [Google Scholar] [CrossRef]
- Warren, H.; Gately, R.D.; O’Brien, P.; Gorkin, R., III; Panhuis, M.I.H. Electrical conductivity, impedance, and percolation behavior of carbon nanofiber and carbon nanotube containing gellan gum hydrogels. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 864–871. [Google Scholar] [CrossRef] [Green Version]
- Azizi, S.; Azizi, M.; Sabetzadeh, M. The Role of Multiwalled Carbon Nanotubes in the Mechanical, Thermal, Rheological, and Electrical Properties of PP/PLA/MWCNTs Nanocomposites. J. Compos. Sci. 2019, 3, 64. [Google Scholar] [CrossRef] [Green Version]
- Nadiv, R.; Fernandes, R.M.; Ochbaum, G.; Dai, J.; Buzaglo, M.; Varenik, M.; Biton, R.; Furó, I.; Regev, O. Polymer nanocomposites: Insights on rheology, percolation and molecular mobility. Polymer 2018, 153, 52–60. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, S. Polymer/Carbon Nanotube Nano Composite Fibers—A Review. ACS Appl. Mater. Interfaces 2014, 6, 6069–6087. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, I.; Fambri, L.; Pegoretti, A.; Slouf, M.; Vackova, T.; Kolarik, J. Spinning, drawing and physical properties of polypropylene nanocomposite fibers with fumed nanosilica. Express Polym. Lett. 2015, 9, 277–290. [Google Scholar] [CrossRef]
- Lu, M.; Gulgunje, P.V.; Arias-Monje, P.J.; Luo, J.; Ramachandran, J.; Sahoo, Y.; Agarwal, S.; Kumar, S. Structure, properties, and applications of polyacrylonitrile/carbon nanotube (CNT) fibers at low CNT loading. Polym. Eng. Sci. 2020, 60, 2143–2151. [Google Scholar] [CrossRef]
- Basu, B.; Balani, K. Advanced Structural Ceramics; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Hussainov, M.; Tätte, T.; Hussainova, I. Technique for extensional rheology characterization of highly reactive viscoelastic liquids. Rheol. Acta 2012, 51, 729–742. [Google Scholar] [CrossRef]
- Tatte, T.; Hussainov, M.; Paalo, M.; Part, M.; Talviste, R.; Kiisk, V.; Mändar, H.; Põhako, K.; Pehk, T.; Reivelt, K.; et al. Alkoxide-based precursors for direct drawing of metal oxide micro- and nanofibres. Sci. Technol. Adv. Mater. 2011, 12, 34412. [Google Scholar] [CrossRef]
- Kisand, V.; Shulga, J.; Tätte, T.; Visk, U.; Natali, M.; Mistura, G.; Paalo, M.; Lobjakas, M.; Kink, I. Preparation of structured sol–gel films using tape casting method. Mater. Sci. Eng. B 2007, 137, 162–165. [Google Scholar] [CrossRef]
- Kessler, V.G. The chemistry behind the sol-gel synthesis of complex oxide nanoparticles for bio-imaging applications. J. Sol-Gel Sci. Technol. 2009, 51, 264–271. [Google Scholar] [CrossRef]
- Spijksma, G.I.; Seisenbaeva, G.A.; Fischer, A.; Bouwmeester, H.J.M.; Blank, D.H.A.; Kessler, V.G. The molecular composition of non-modified and acac-modified propoxide and butoxide precursors of zirconium and hafnium dioxides. J. Sol-Gel Sci. Technol. 2009, 51, 10–22. [Google Scholar] [CrossRef]
- Senouci, A.; Yaakoub, M.; Huguenard, C.; Henry, M. Molecular templating using titanium(iv)(oxo)alkoxides and titanium(iv)(oxo)aryloxides. J. Mater. Chem. 2004, 14, 3215–3230. [Google Scholar] [CrossRef]
- Anna, S.; McKinley, G. Elasto-capillary thinning and breakup of model elastic liquids. J. Rheol. 2001, 45, 115–138. [Google Scholar] [CrossRef] [Green Version]
- Pötschke, P.; Fornes, T.; Paul, D. Rheological behavior of multiwalled carbon nanotube/polycarbonate composites. Polymer 2002, 43, 3247–3255. [Google Scholar] [CrossRef]
- Miyazono, K.; Kagarise, C.D.; Koelling, K.W.; Mahboob, M.; Bechtel, S.E. Shear and extensional rheology and flow-induced orientation of carbon nanofiber/polystyrene melt composites. J. Appl. Polym. Sci. 2011, 119, 1940–1951. [Google Scholar] [CrossRef]
- Brukh, R.; Mitra, S. Kinetics of carbon nanotubeoxidation. J. Mater. Chem. 2007, 17, 619–623. [Google Scholar] [CrossRef]
- Du, J.-H.; Bai, J.; Cheng, H.-M. The present status and key problems of carbon nanotube based polymer composites. Express Polym. Lett. 2007, 1, 253–273. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tätte, T.; Hussainov, M.; Amiri, M.; Vanetsev, A.; Paalo, M.; Hussainova, I. Rheological Properties of MWCNT-Doped Titanium-Oxo-Alkoxide Gel Materials for Fiber Drawing. Materials 2022, 15, 1186. https://doi.org/10.3390/ma15031186
Tätte T, Hussainov M, Amiri M, Vanetsev A, Paalo M, Hussainova I. Rheological Properties of MWCNT-Doped Titanium-Oxo-Alkoxide Gel Materials for Fiber Drawing. Materials. 2022; 15(3):1186. https://doi.org/10.3390/ma15031186
Chicago/Turabian StyleTätte, Tanel, Medhat Hussainov, Mahsa Amiri, Alexander Vanetsev, Madis Paalo, and Irina Hussainova. 2022. "Rheological Properties of MWCNT-Doped Titanium-Oxo-Alkoxide Gel Materials for Fiber Drawing" Materials 15, no. 3: 1186. https://doi.org/10.3390/ma15031186







