Effect of Phosphoric Acid on the Preparation of α-Hemihydrate Gypsum Using Hydrothermal Method
Abstract
:1. Introduction
2. Materials and Methods
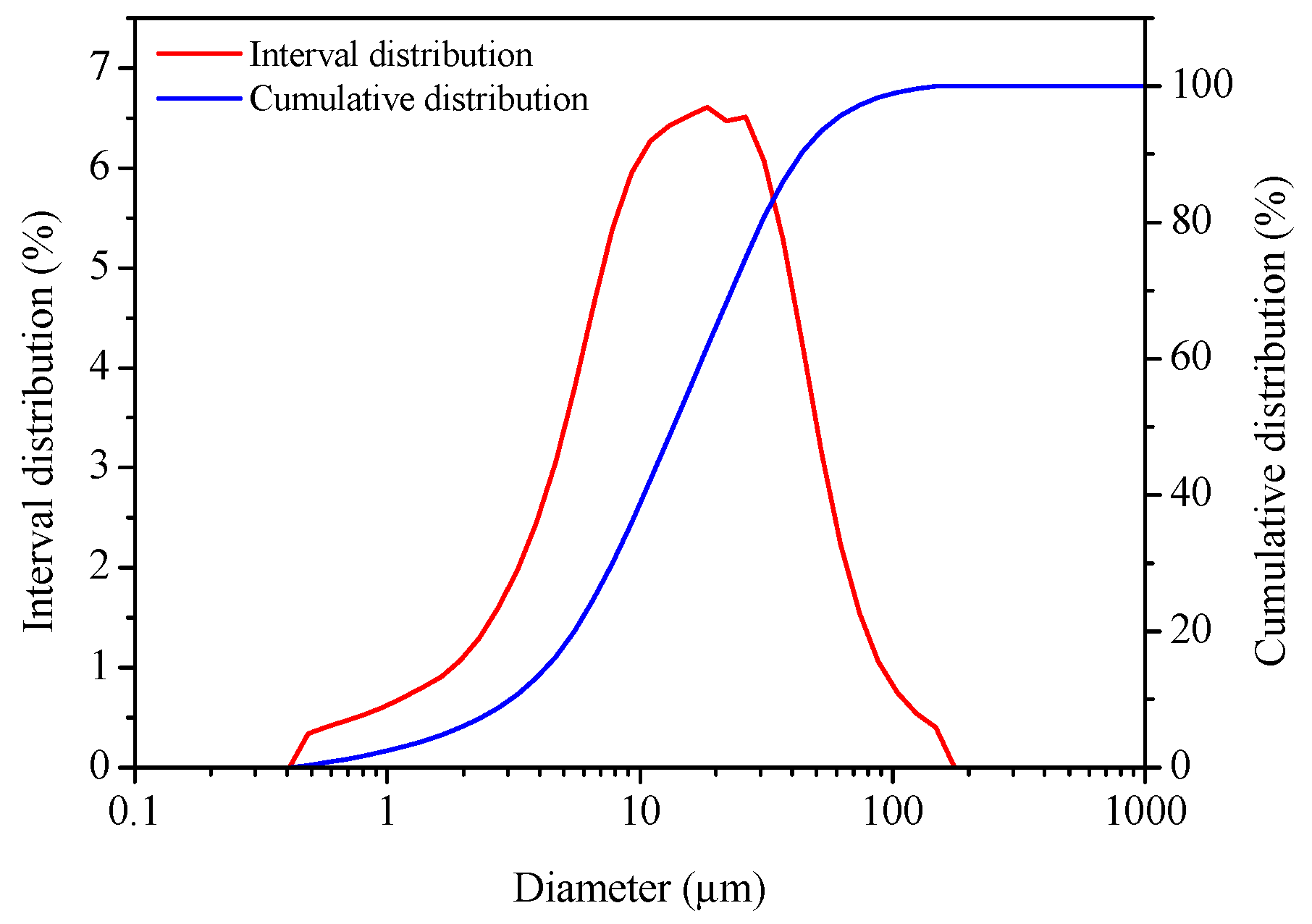
2.1. Materials
2.2. Preparation of α-HH
2.3. Experimental Methods
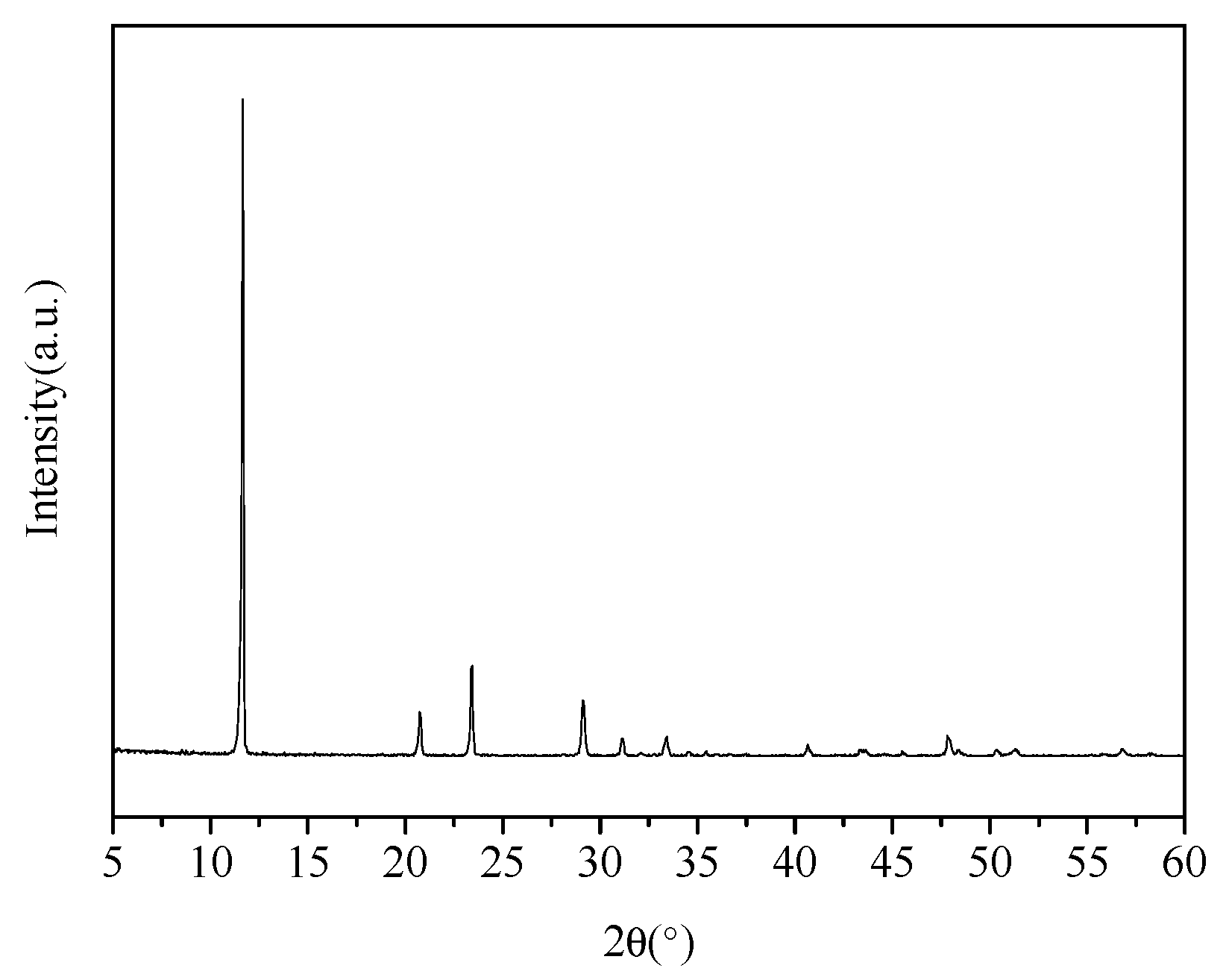
2.3.1. XRD Test
2.3.2. Morphology Test
2.3.3. Infrared Spectrum Test
2.3.4. Determination of Water of Crystal Content
- —Water of crystallization content, %;
- —Mass of sample before calcination, g;
- —Mass of sample after calcination, g.
2.3.5. Physical and Mechanical Properties Test
3. Results and Discussion
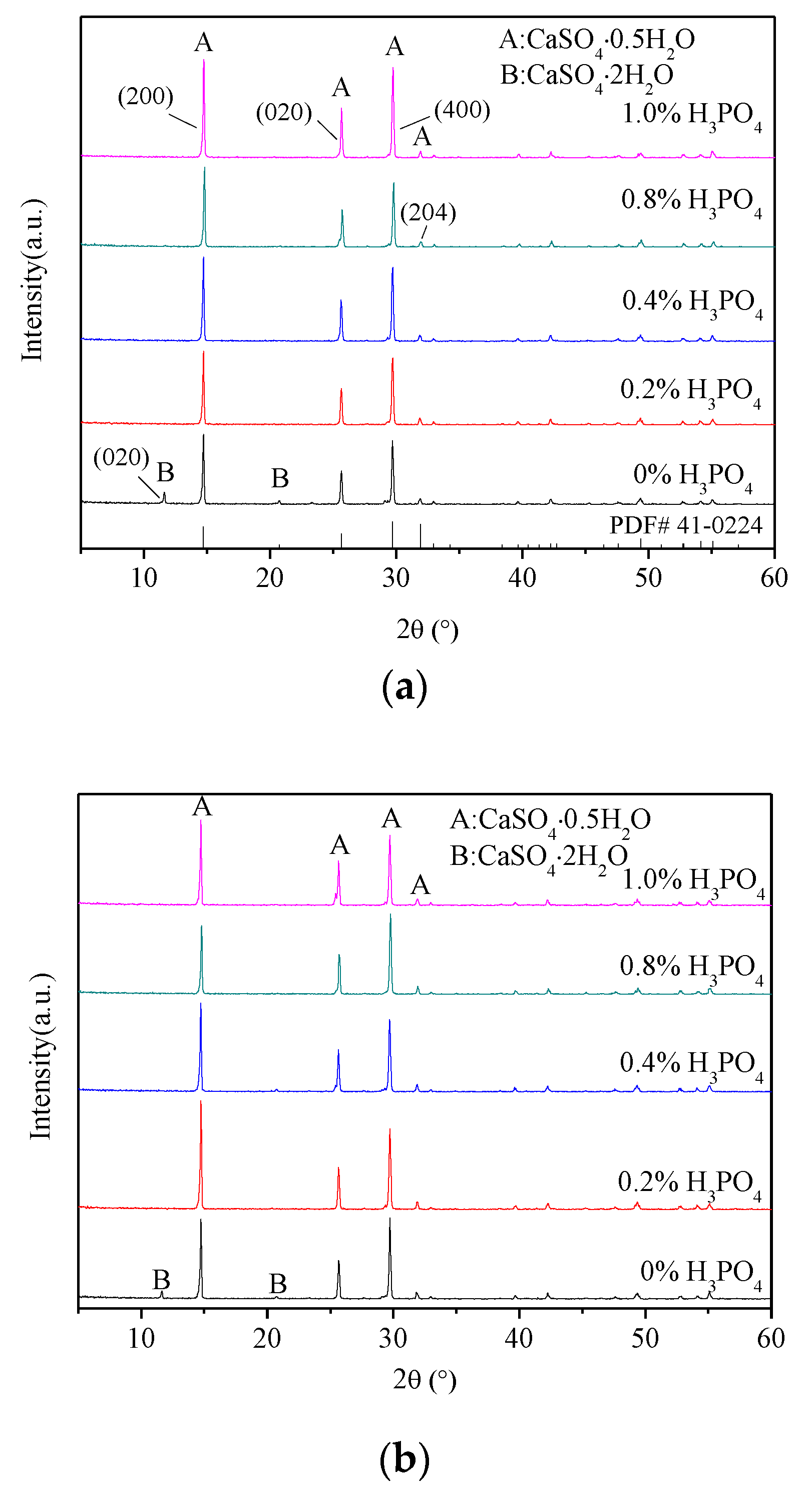
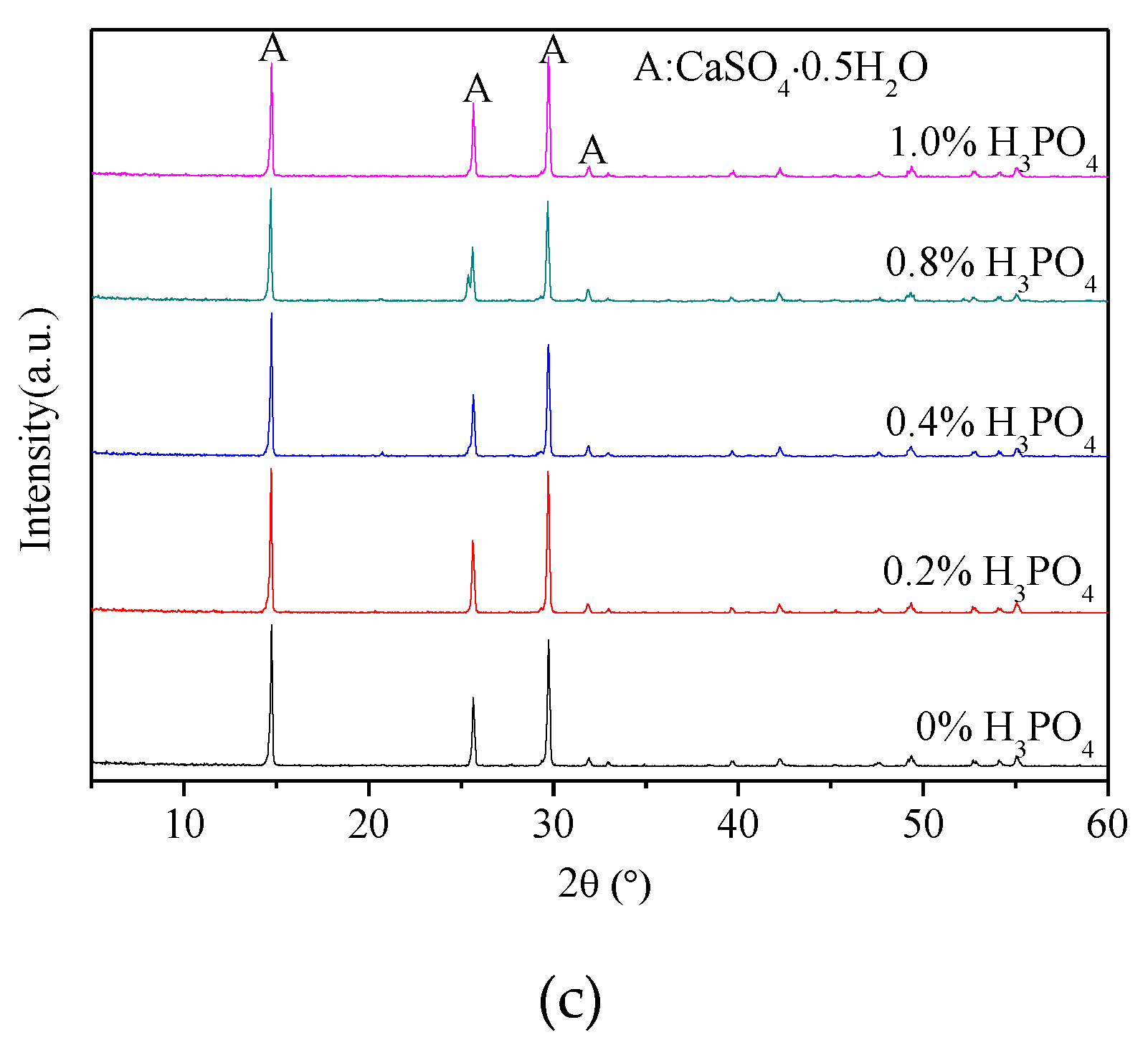
3.1. X-ray Diffraction Analysis
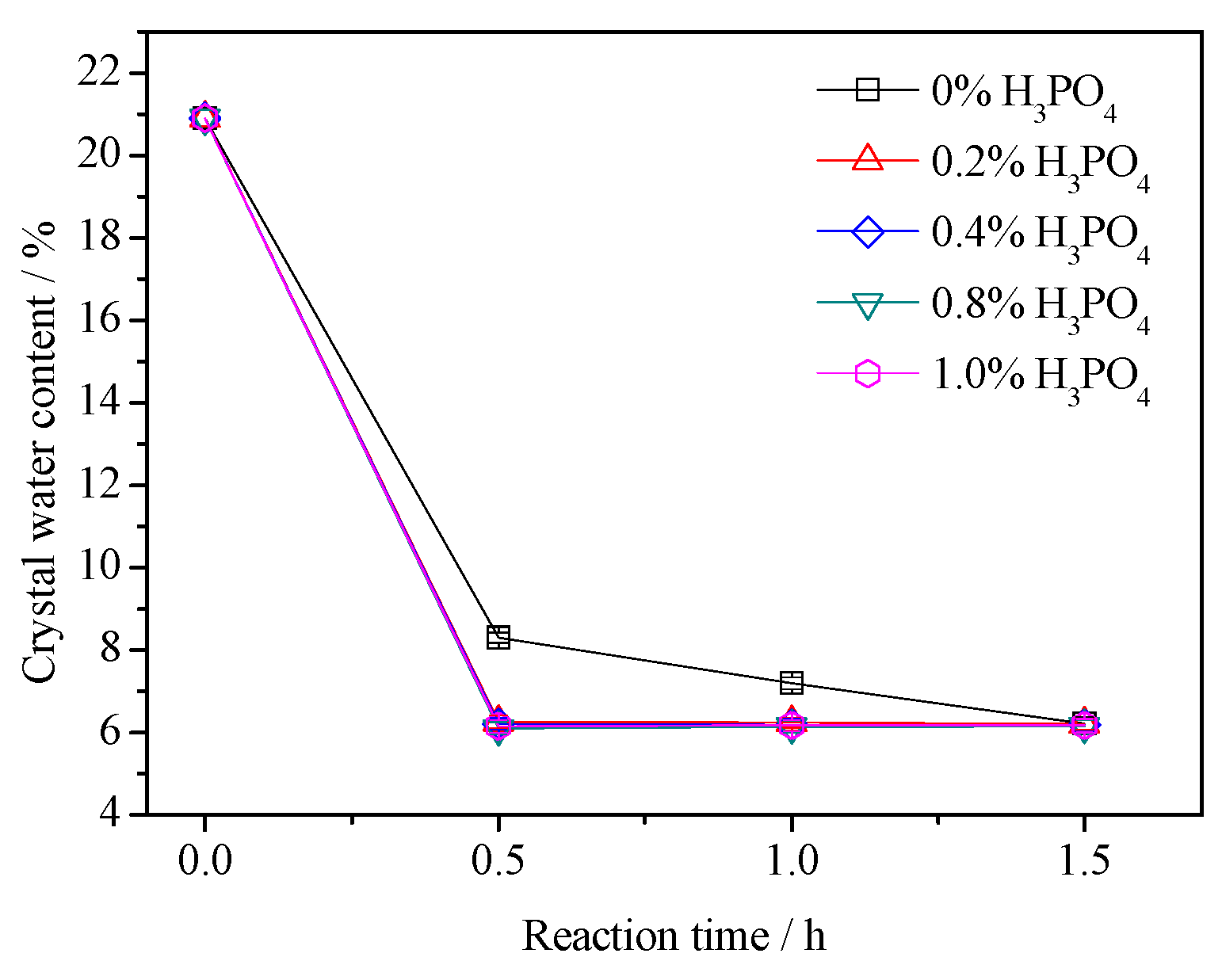
3.2. Crystal Water Content
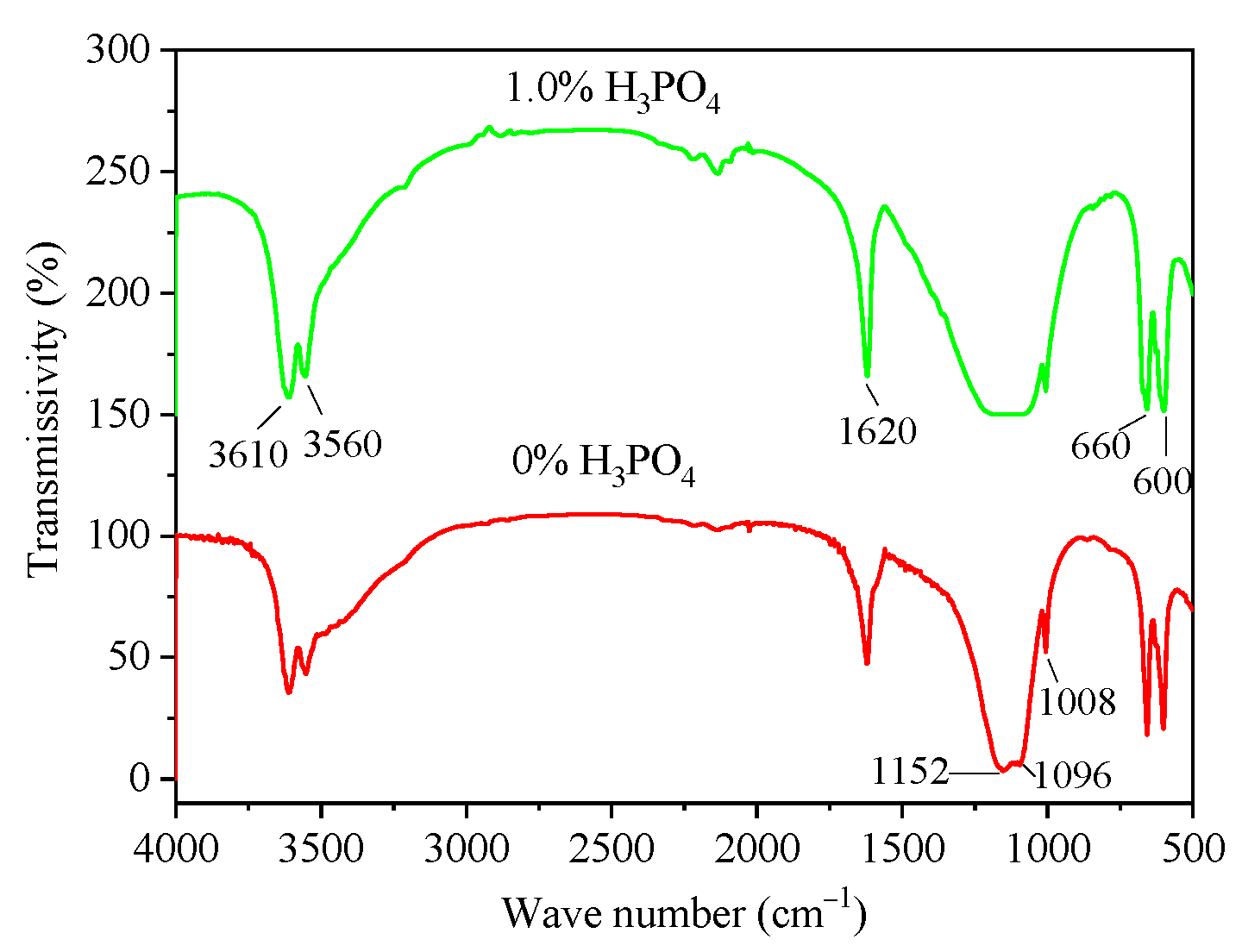
3.3. Infrared Analysis
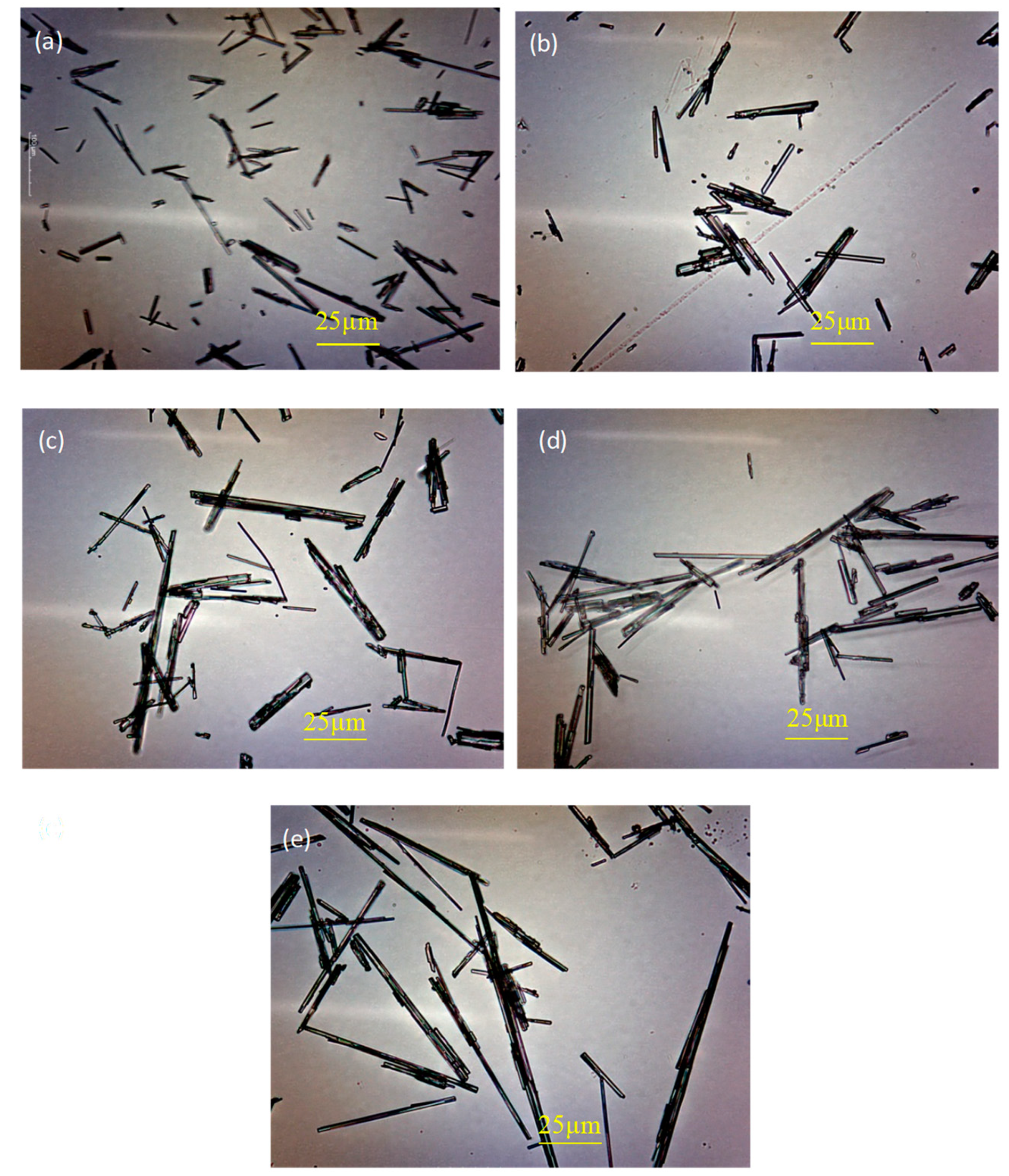
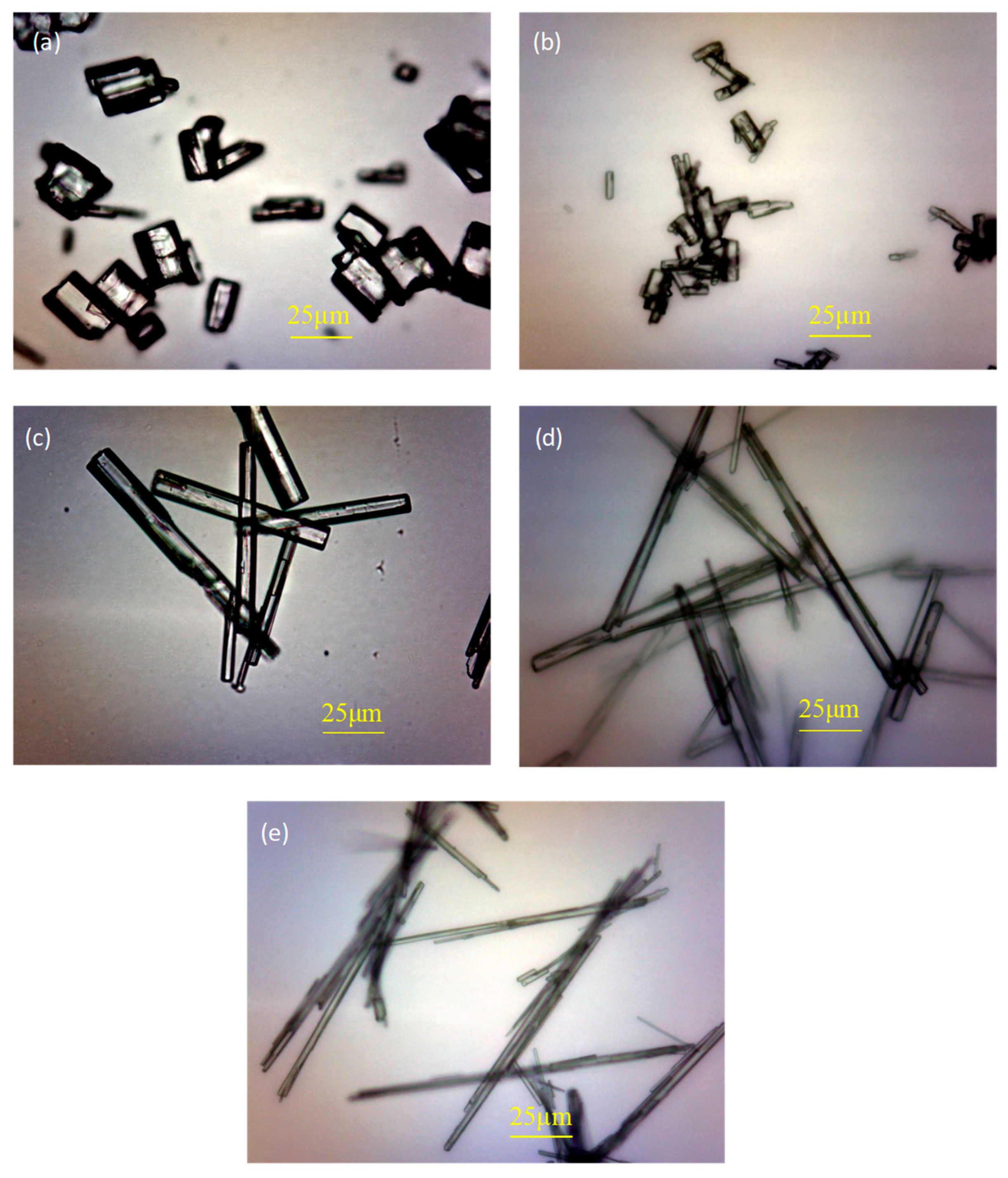
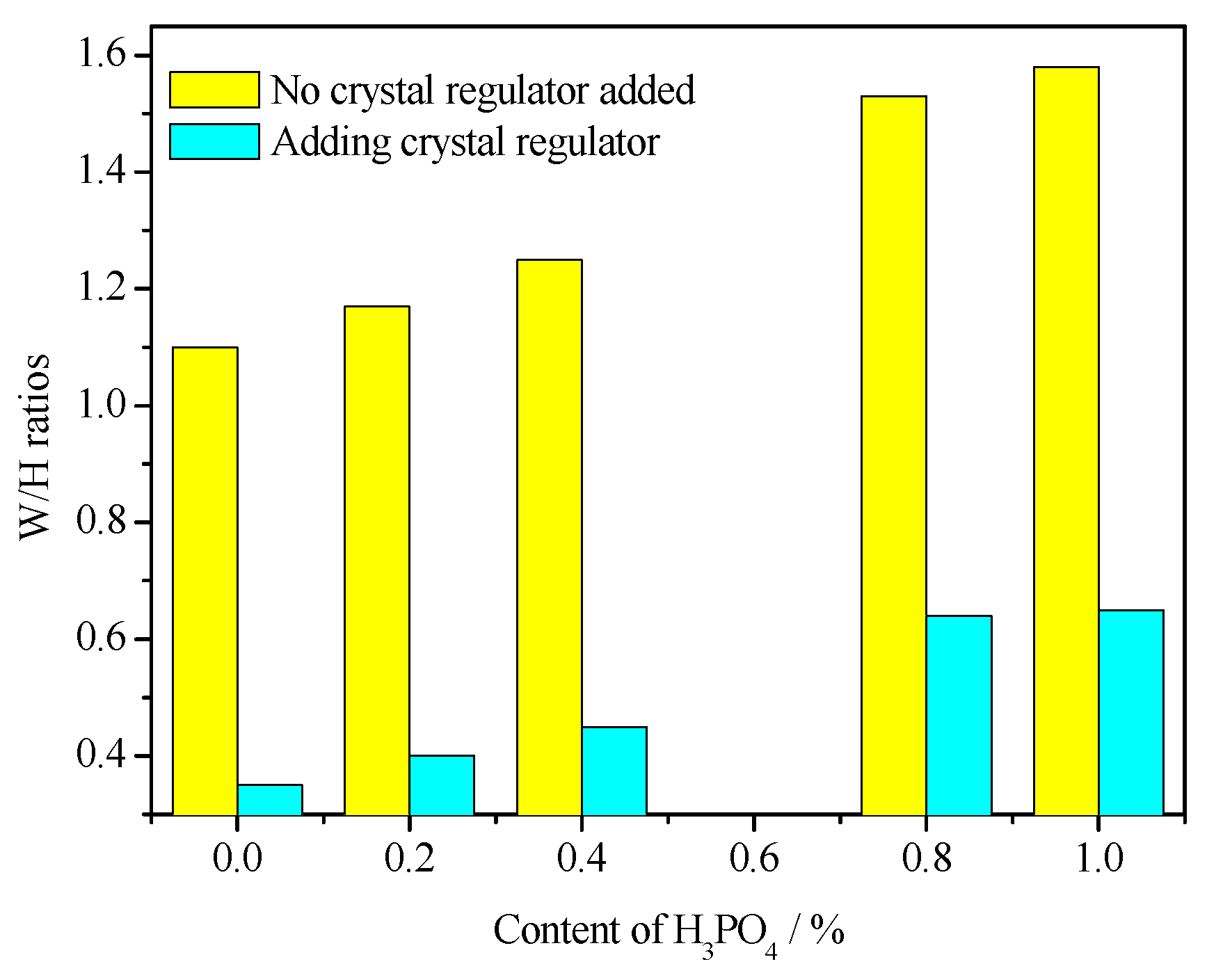
3.4. Morphology Analysis
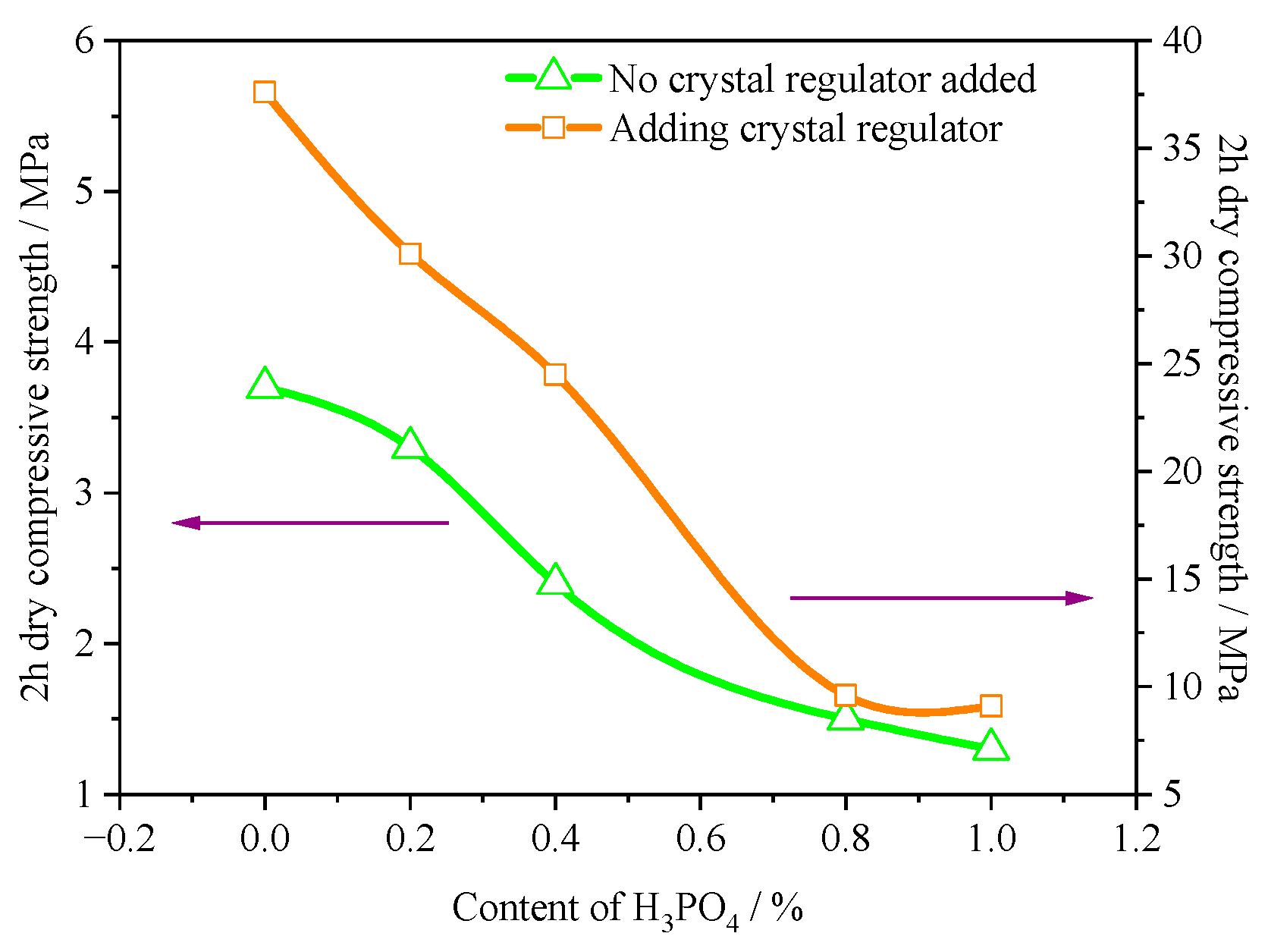
3.5. Water Consumption for Standard Consistency
3.6. Mechanical Properties
4. Conclusions
- (1)
- The presence of H3PO4 can accelerate the formation rate of the α-HH phase. The transition time of all gypsum to the α-HH phase can be shortened from 1.5 h to 0.5 h when only 0.2% H3PO4 was added.
- (2)
- The infrared test results showed that H3PO4 did not enter the α-HH lattice to form eutectic phosphorus solid solution. In addition, the presence of related calcium phosphate was not detected in α-HH, possibly due to the small amount of phosphate content leading to the small amount of calcium phosphate production.
- (3)
- The addition of H3PO4 can significantly affect the crystal shape of α-HH and significantly weaken the regulatory effect of citric acid on the crystal shape of α-HH, resulting in a significant increase in the aspect ratio of α-HH crystals. Moreover, the higher the H3PO4 content, the more significant the negative impact.
- (4)
- Due to the significant increase in the aspect ratio of α-HH caused by H3PO4, the standard consistency water consumption of α-HH gradually increased with the increase in H3PO4 content. Accordingly, the strength of the hardened body of α-HH gradually decreased. However, the prepared α-HH can still meet the requirements of α20 high-strength gypsum when the H3PO4 content was less than 0.4%.
- (5)
- It can be inferred from this study that the soluble P2O5 impurities present in phosphogypsum can effectively promote the rapid formation of the α-HH phase when phosphogypsum is used for hydrothermal preparation of high-strength gypsum, but it can have a significant negative effect on the crystal regulation of α-HH. Therefore, the screening of suitable crystallization agents to avoid the adverse effects of soluble P2O5 will be one of the focuses of future research in this field.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saadaoui, E.; Ghazel, N.; Ben Romdhane, C.; Massoudi, N. Phosphogypsum: Potential uses and problems—A review. Int. J. Environ. Stud. 2017, 74, 558–567. [Google Scholar] [CrossRef]
- Rashad, A.M. Phosphogypsum as a construction material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Sui, Y.; Zhang, F.; Yu, W.; Bo, Y.; Wang, P.; Peng, W.; Jin, J. Preparation of α-calcium sulfate hemihydrate from industrial by-product gypsum: A review. Physicochem. Probl. Miner. Process. 2021, 57, 168–181. [Google Scholar] [CrossRef]
- Islam, G.S.; Chowdhury, F.H.; Raihan, M.T.; Amit, S.K.S.; Islam, M.R. Effect of Phosphogypsum on the Properties of Portland Cement. Procedia Eng. 2017, 171, 744–751. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, A.; Wang, Y.; Lan, W. Low cost and high efficiency utilization of hemihydrate phosphogypsum: Used as binder to prepare filling material. Constr. Build. Mater. 2018, 167, 263–270. [Google Scholar] [CrossRef]
- Wei, Z.; Deng, Z. Research hotspots and trends of comprehensive utilization of phosphogypsum: Bibliometric analysis. J. Environ. Radioact. 2022, 242, 106778. [Google Scholar] [CrossRef] [PubMed]
- Chernysh, Y.; Yakhnenko, O.; Chubur, V.; Roubík, H. Phosphogypsum Recycling: A Review of Environmental Issues, Current Trends, and Prospects. Appl. Sci. 2021, 11, 1575. [Google Scholar] [CrossRef]
- Ding, C.; Sun, T.; Shui, Z.; Xie, Y.; Ye, Z. Physical properties, strength, and impurities stability of phosphogypsum-based cold-bonded aggregates. Constr. Build. Mater. 2022, 331, 127307. [Google Scholar] [CrossRef]
- Du, M.; Wang, J.; Dong, F.; Wang, Z.; Yang, F.; Tan, H.; Fu, K.; Wang, W. The study on the effect of flotation purification on the performance of α-hemihydrate gypsum prepared from phosphogypsum. Sci. Rep. 2022, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Sui, Y.; Yu, W.; Bu, Y.; Zeng, C.; Liu, C.; Zhang, Z.; Gao, Z.; Chi, Z. Deep removal of phosphorus and synchronous preparation of high-strength gypsum from phos-phogypsum by crystal modification in NaCl-HCl solutions. Sep. Purif. Technol. 2022, 298, 121592. [Google Scholar] [CrossRef]
- Li, X.; Gao, W. Conversion of phosphogypsum into α-hemihydrate in the presence of potassium acid phthalate and Ca2+: Ex-perimental and DFT studies. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129906. [Google Scholar] [CrossRef]
- Ma, B.; Lu, W.; Su, Y.; Li, Y.; Gao, C.; He, X. Synthesis of α-hemihydrate gypsum from cleaner phosphogypsum. J. Clean. Prod. 2018, 195, 396–405. [Google Scholar] [CrossRef]
- Fan, H.; Song, X.; Liu, T.; Xu, Y.; Yu, J. Effect of Al3+ on crystal morphology and size of calcium sulfate hemihydrate: Experimental and molecular dynamics simulation study. J. Cryst. Growth 2018, 495, 29–36. [Google Scholar] [CrossRef]
- Garg, M.; Jain, N.; Singh, M. Development of alpha plaster from phosphogypsum for cementitious binders. Constr. Build. Mater. 2009, 23, 3138–3143. [Google Scholar] [CrossRef]
- Feldmann, T.; Demopoulos, G.P. Influence of Impurities on Crystallization Kinetics of Calcium Sulfate Dihydrate and Hemihy-drate in Strong HCl-CaCl2 Solutions. Ind. Eng. Chem. Res. 2013, 52, 6540–6549. [Google Scholar] [CrossRef]
- Lv, X.; Xiang, L. The Generation Process, Impurity Removal and High-Value Utilization of Phosphogypsum Material. Nanomaterials 2022, 12, 3021. [Google Scholar] [CrossRef]
- Cai, Q.; Jiang, J.; Ma, B.; Shao, Z.; Hu, Y.; Qian, B.; Wang, L. Efficient removal of phosphate impurities in waste phosphogypsum for the production of cement. Sci. Total. Environ. 2021, 780, 146600. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, J.; Kang, X.; Yu, J.; Fan, Y.; Dang, Y.; Zhang, W.; Wang, S. Belite-calcium sulfoaluminate cement prepared with phosphogypsum: Influence of P2O5 and F on the clinker formation and cement performances. Constr. Build. Mater. 2019, 203, 432–442. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Chen, Q.; Qi, C.; Su, Z.; Huang, Z. Utilisation of Water-Washing Pre-Treated Phosphogypsum for Cemented Paste Backfill. Minerals 2019, 9, 175. [Google Scholar] [CrossRef]
- Liu, S.; Fang, P.; Ren, J.; Li, S. Application of lime neutralised phosphogypsum in supersulfated cement. J. Clean. Prod. 2020, 272, 122660. [Google Scholar] [CrossRef]
- Li, J.; Peng, X.; Zheng, J.; Mao, M.; Sun, X.; Wang, J.; Li, X.; Chai, X.; Lin, Z.; Liu, W. Simultaneous removal of phosphorus and organic contaminants from phosphogypsum using hydrothermal method for gypsum resource regeneration. J. Environ. Chem. Eng. 2022, 10, 108441. [Google Scholar] [CrossRef]
- Aliedeh, M.A. Factorial Design Study of P2O5 Reduction for Jordanian Phosphogypsum Using Sulfuric and Nitric Acids So-lutions. J. Chem. Technol. Metall. 2018, 53, 437–450. [Google Scholar]
- Al-Thyabat, S.; Zhang, P. REE extraction from phosphoric acid, phosphoric acid sludge, and phosphogypsum. Miner. Process. Extr. Metall. 2015, 124, 143–150. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Xing, D.; Wang, B.; Liu, L.; Tang, J. Effect of maleic acid and pH on the preparation of α-calcium sulfate hemihydrate from phosphogypsum in Mg (NO3)2 solution. J. Mater. Cycles Waste Manag. 2022, 24, 143–154. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Wu, Q.; Xie, X.; Tang, S.; Yang, G.; Luo, L.; Yuan, H. Hydration reaction and microstructural characteristics of hemihydrate phosphogypsum with variable pH. Constr. Build. Mater. 2022, 316, 125891. [Google Scholar] [CrossRef]
- GB/T 17669.4-1999; Gypsum Plasters-Determination of Physical Properties of Pure Paste. State Quality and Technical Supervision: Beijing, China, 1999.
- Ölmez, H.; Yilmaz, V. Infrared study on the refinement of phosphogypsum for cements. Cem. Concr. Res. 1988, 18, 449–454. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Ke, B.; Wang, X.; Li, L.; Li, X.; Mao, S. Insight into the effect of maleic acid on the preparation of α-hemihydrate gypsum from phosphogypsum in Na2SO4 solution. J. Cryst. Growth 2018, 493, 34–40. [Google Scholar] [CrossRef]
- JC/T 2038-2010; α-High Strength Gypsum Plaster. Ministry of Industry and Information Technology: Beijing, China, 2010.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, X.; Hou, P.; Jin, B.; Zhang, X.; Li, Z. Effect of Phosphoric Acid on the Preparation of α-Hemihydrate Gypsum Using Hydrothermal Method. Materials 2023, 16, 5878. https://doi.org/10.3390/ma16175878
Zhang J, Wang X, Hou P, Jin B, Zhang X, Li Z. Effect of Phosphoric Acid on the Preparation of α-Hemihydrate Gypsum Using Hydrothermal Method. Materials. 2023; 16(17):5878. https://doi.org/10.3390/ma16175878
Chicago/Turabian StyleZhang, Jianwu, Xiao Wang, Pengtao Hou, Biao Jin, Xiaoting Zhang, and Zhixin Li. 2023. "Effect of Phosphoric Acid on the Preparation of α-Hemihydrate Gypsum Using Hydrothermal Method" Materials 16, no. 17: 5878. https://doi.org/10.3390/ma16175878




