Homogenization of Extrusion Billets of a Novel Al-Mg-Si-Cu Alloy with Increased Copper Content
Abstract
:1. Introduction
2. Materials and Methods
3. Results
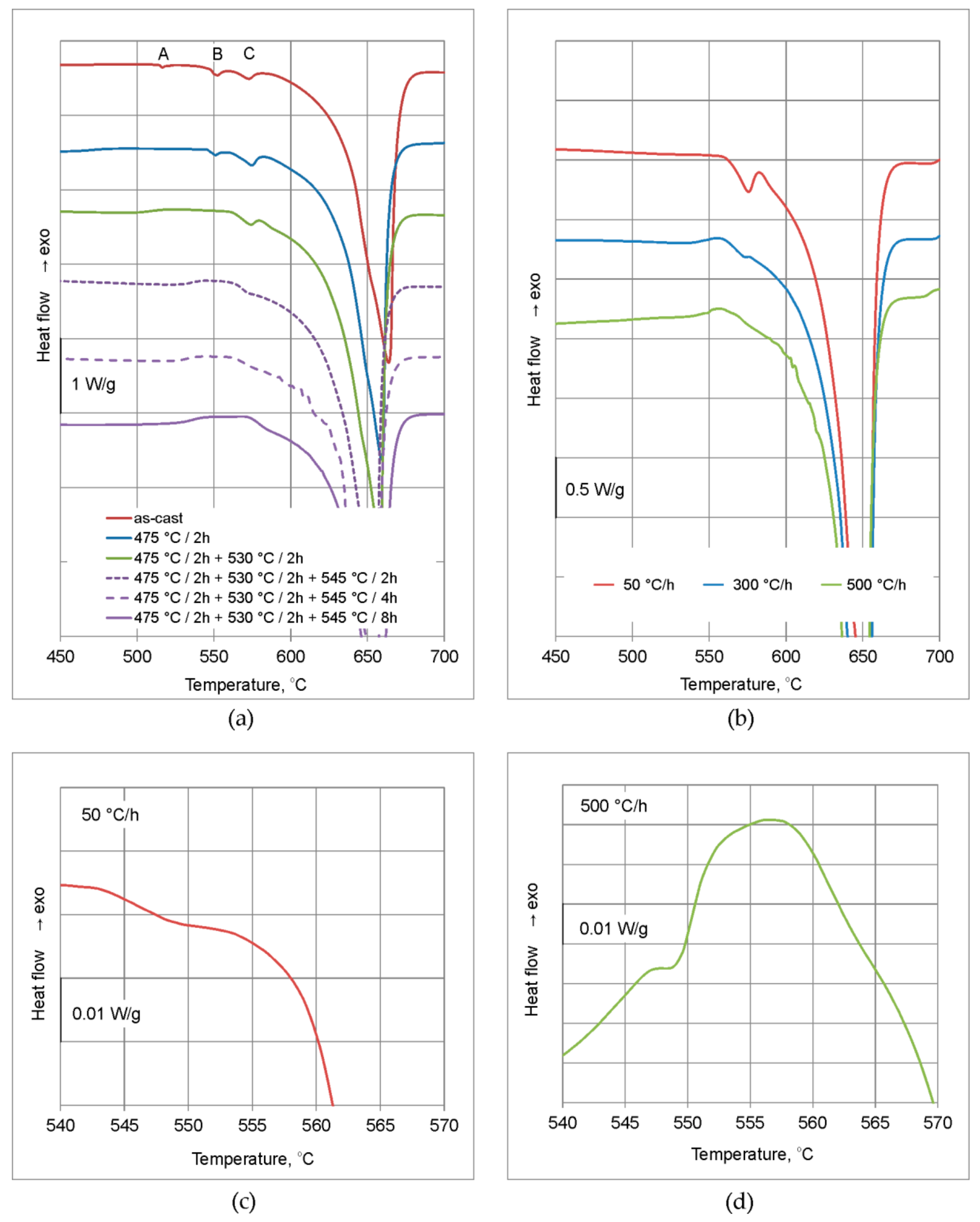
3.1. DSC
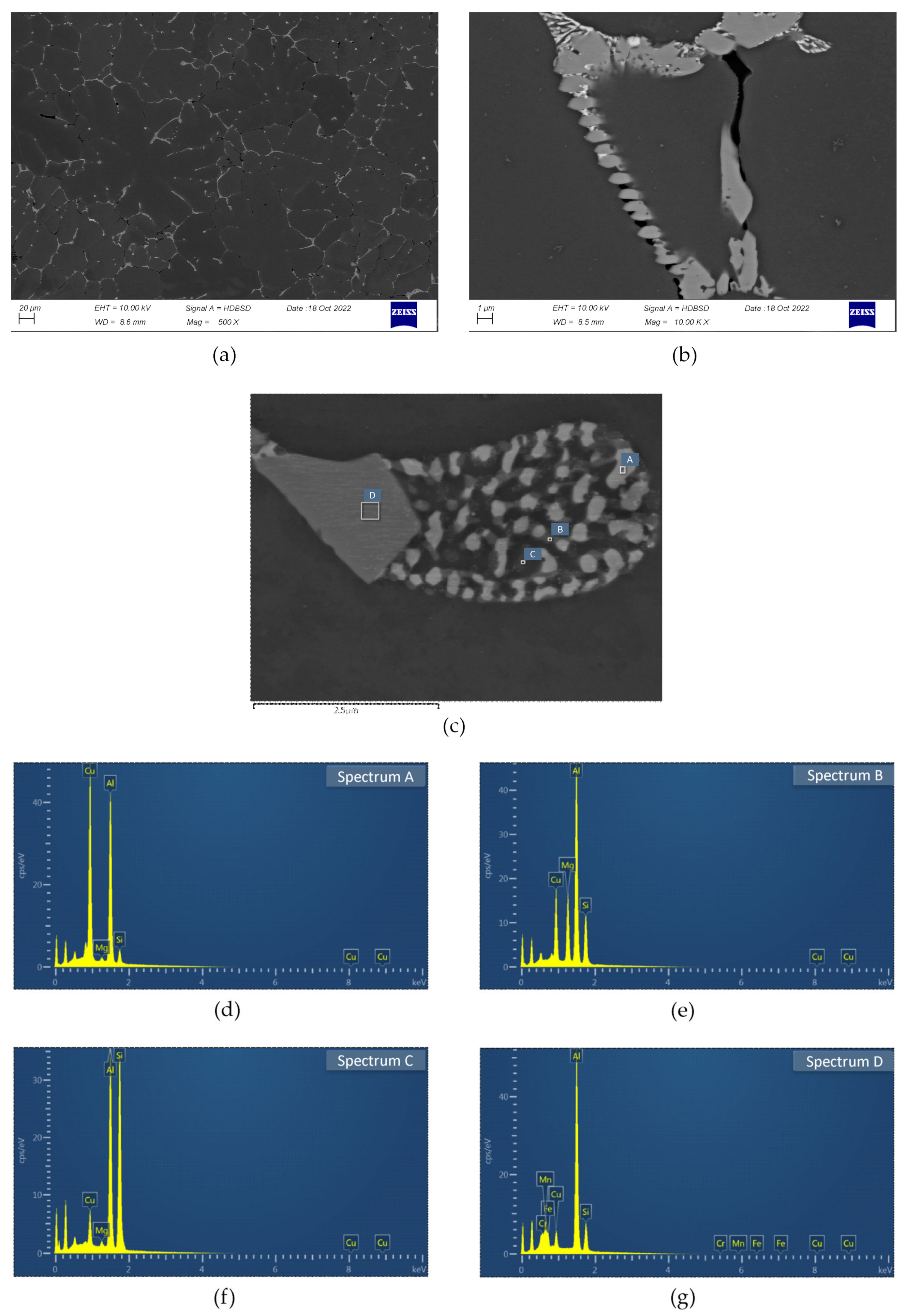
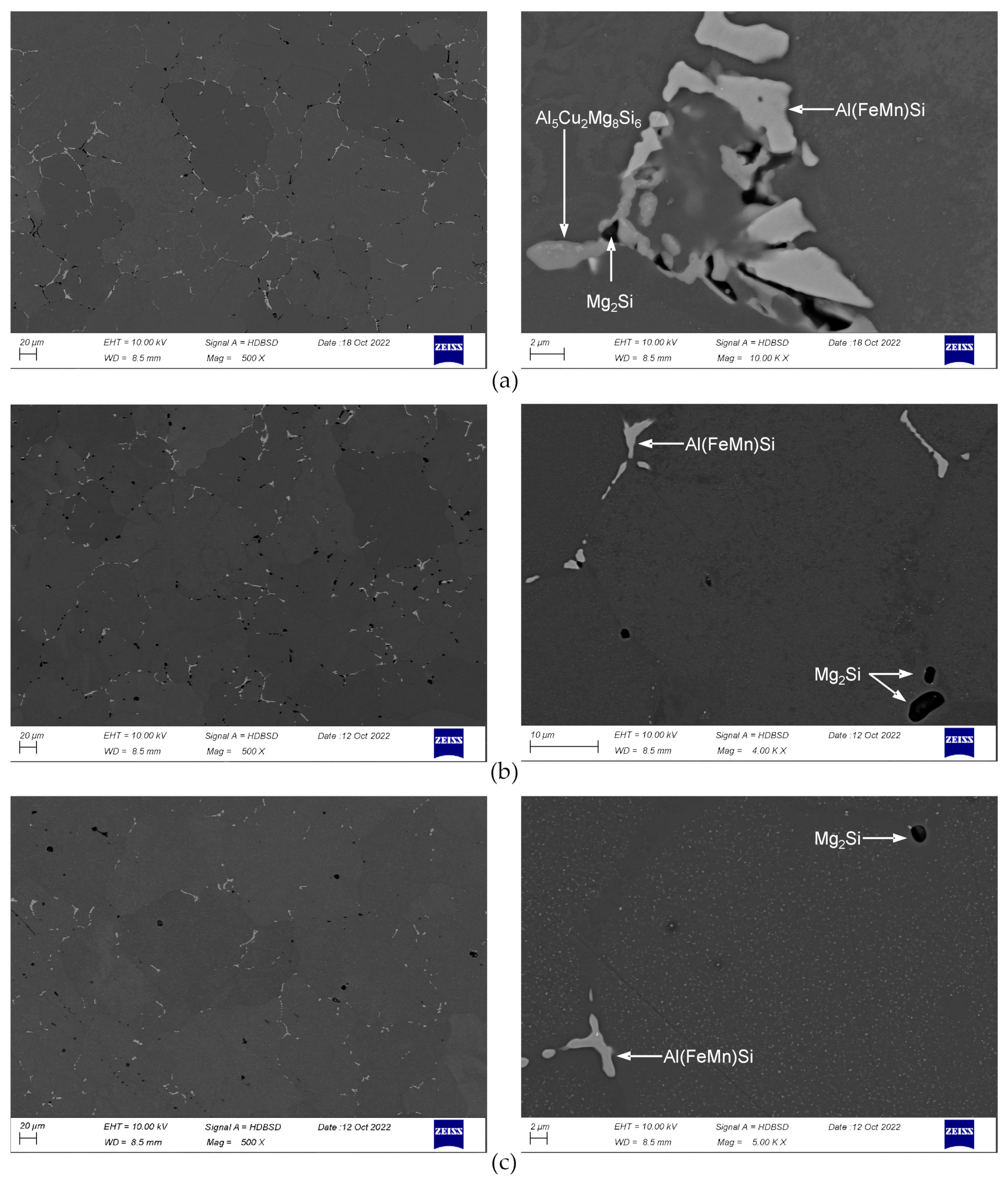
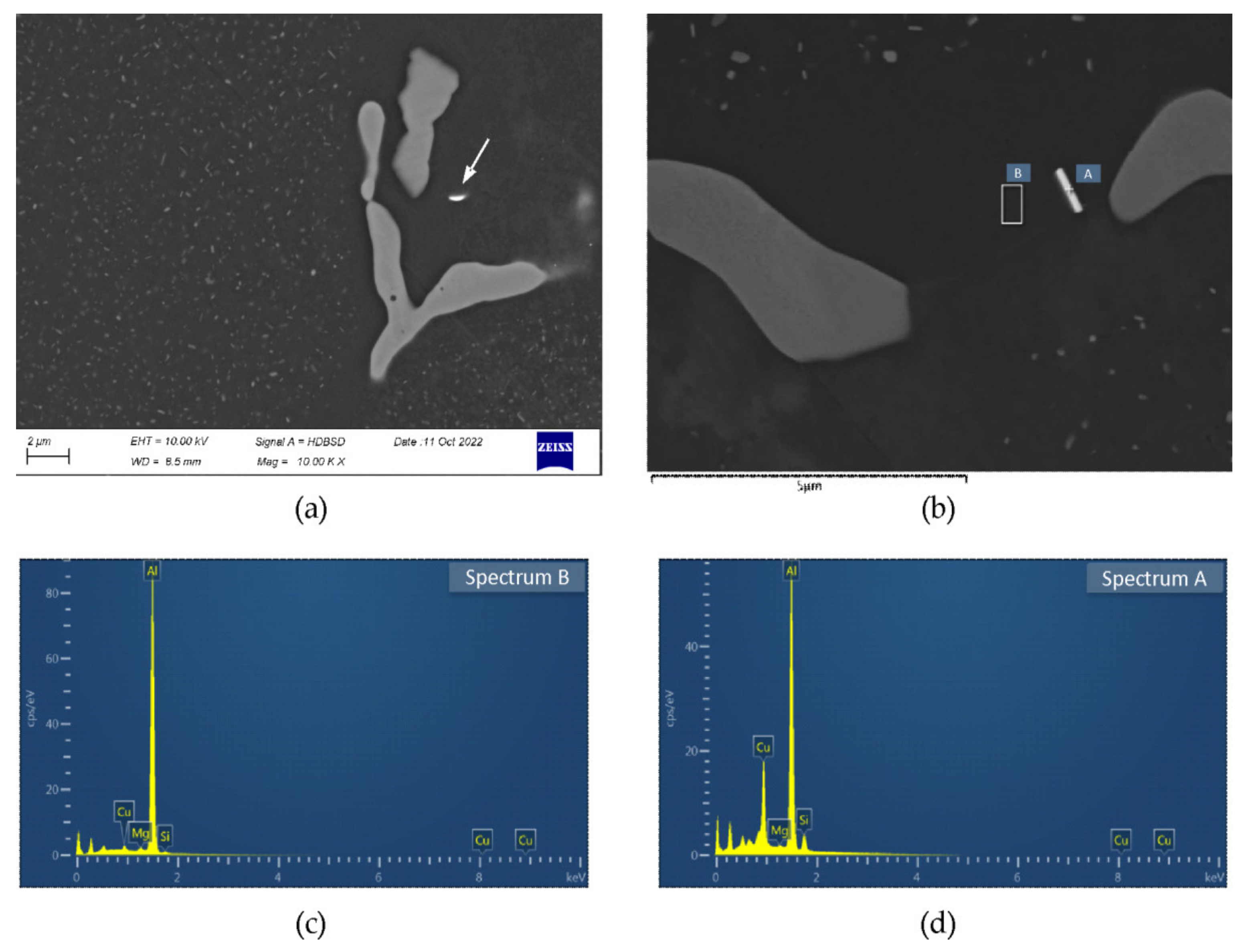
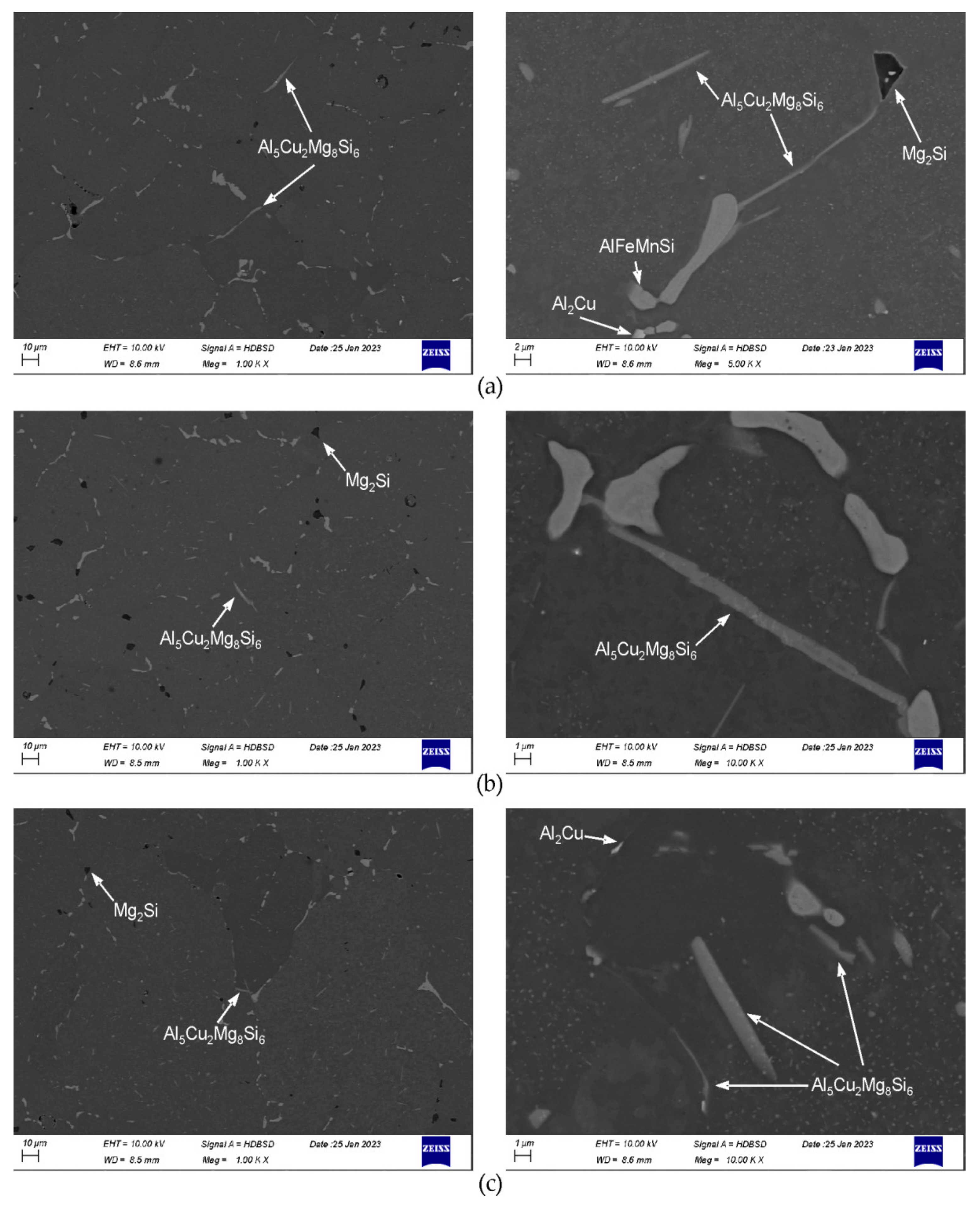
3.2. SEM/EDS
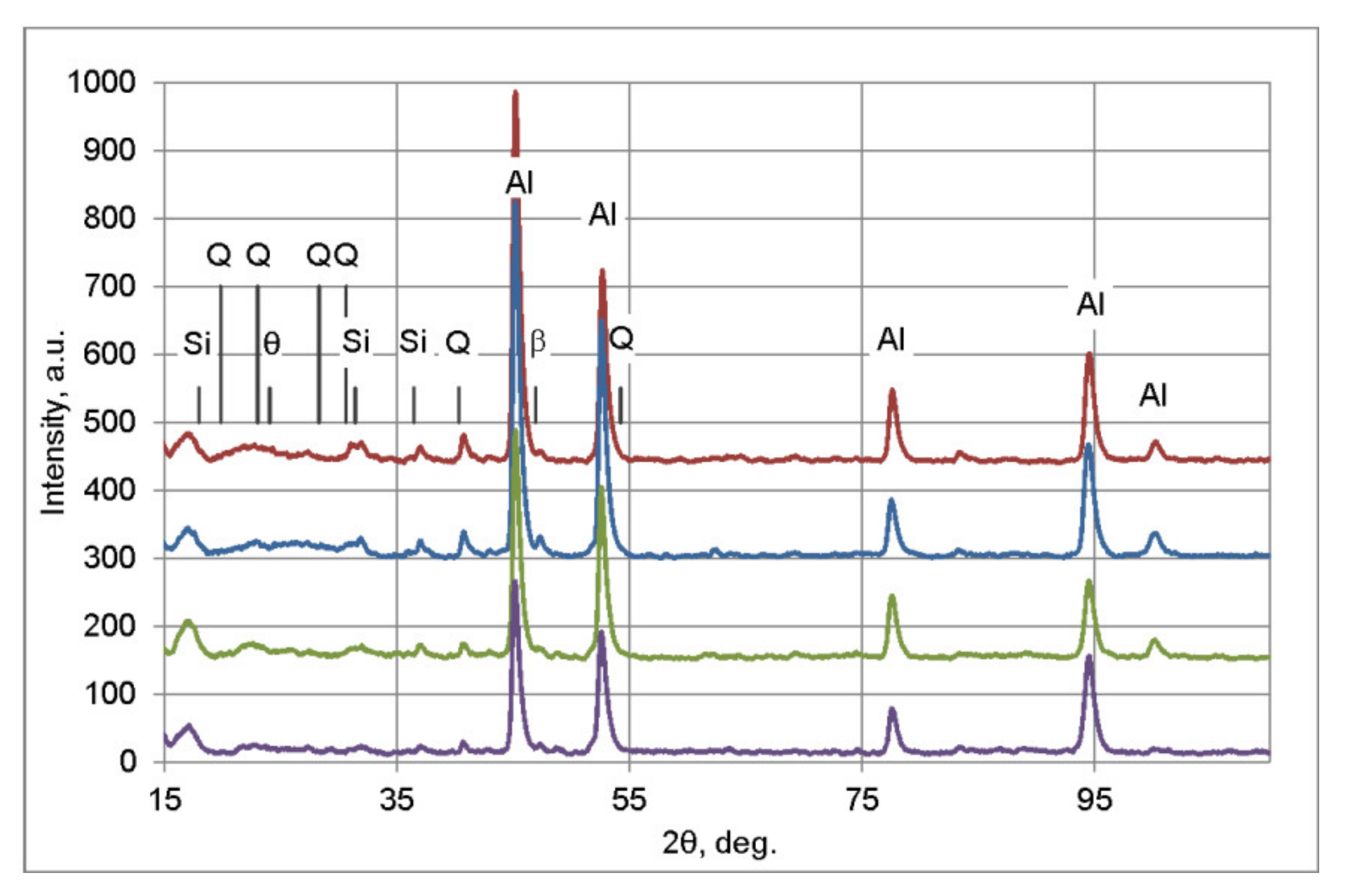
3.3. XRD
4. Discussion
5. Conclusions
- The examined alloy in as-cast state was characterized by dendritic microstructure with large amount of particles and eutectic areas at the dendrites boundaries. Besides (Al) solid solution, in the microstructure phases β-Mg2Si, Q-Al5Cu2Mg8Si6, θ-Al2Cu, (Si) and Al(FeMn)Si were found. On the DSC curves three peaks resulting from incipient melting reactions were observed, solidus temperature of the alloy in as-cast state was 513 °C.
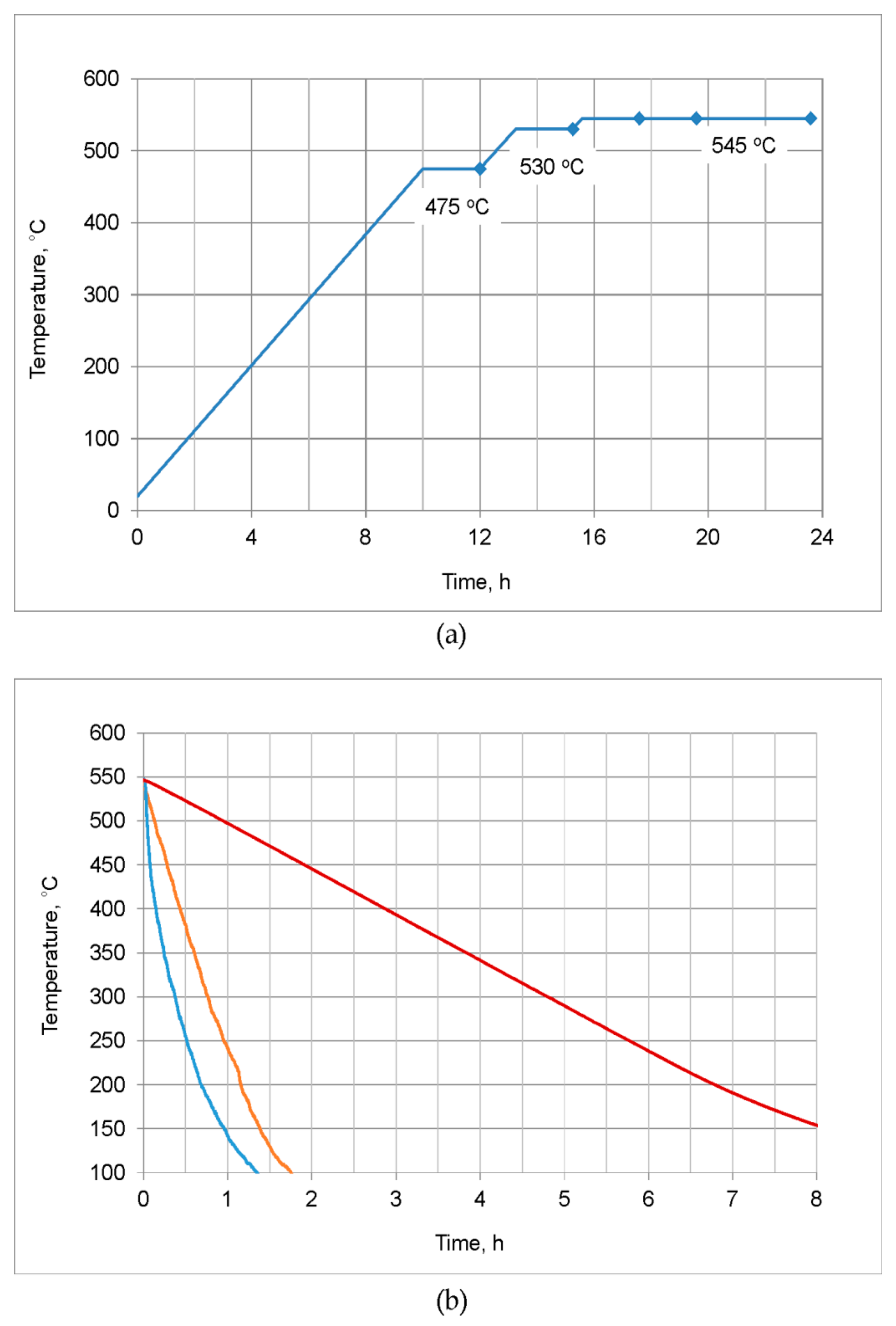
- In order to avoid incipient melting, the homogenization scheme with three soaking stages: at 475, 530, and 545 °C was applied. During soaking, full dissolution of Q-Al5Cu2Mg8Si6 and θ-Al2Cu phases took place. The β-Mg2Si phase did not dissolve completely during soaking, but its amount was significantly reduced.
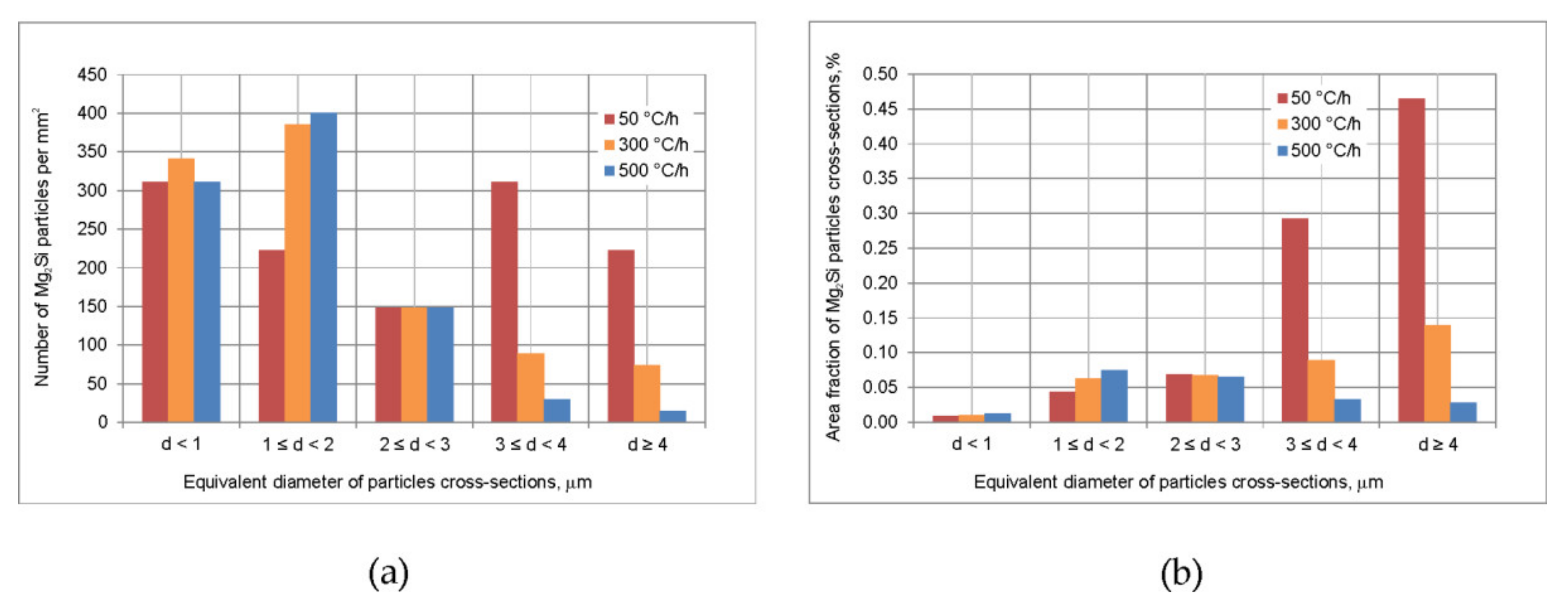
- The alloy needs to be fast cooled from homogenization temperature, at least at 500 °C/h. The β-Mg2Si and θ-Al2Cu particles precipitated during cooling were sufficiently refined. Despite such fast cooling, in the microstructure coarse Q-Al5Cu2Mg8Si6 phase particles were found and rapid billets heating may lead to incipient melting at the temperature of about 545 °C. Thus, the careful selection of billets preheating and extrusion conditions was found to be necessary.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fuel Types of New Passenger Cars in the EU. Available online: https://www.acea.auto/figure/fuel-types-of-new-passenger-cars-in-eu/ (accessed on 29 December 2022).
- Scamans, G. Electric Vehicles Spike Demand for High Strength Aluminum Extrusions. Light Met. Age 2018, 76, 6–9. [Google Scholar]
- Brown, L.; Nelson, R. The Coming BEV Wave: Implications for Extrusion. Light Met. Age 2020, 78, 22–24. [Google Scholar]
- International Alloy Designations and Chemical Composition Limits for Wrought Aluminum and Wrought Aluminum Alloys. Available online: https://www.aluminum.org/sites/default/files/2021-11/TealSheet.pdf (accessed on 31 December 2022).
- Chakrabarti, D.J.; Laughlin, D.E. Phase relations and precipitation in Al-Mg-Si alloys with Cu additions. Prog. Mater. Sci. 2004, 49, 389–410. [Google Scholar] [CrossRef]
- Ding, L.; Jia, Z.; Nie, J.-F.; Weng, Y.; Cao, L.; Chen, H.; Wu, X.; Liu, Q. The structural and compositional evolution of precipitates in Al-Mg-Si-Cu alloy. Acta Mater. 2018, 145, 437–450. [Google Scholar] [CrossRef]
- Bergsma, S.C.; Kassner, M.E.; Li, X.; Delos-Reyes, M.A.; Hayes, T.A. The Optimized Mechanical Properties of the New Aluminum Alloy AA 6069. J. Mater. Eng. Perform. 1996, 5, 111–116. [Google Scholar] [CrossRef]
- Guo, S.; Yu, H.; Wang, Z.; Yu, W.; Cheng, W.; Huang, L.; Liu, C.; Yin, F.; Zhao, W.; Qin, C. Microstructural Evolution and Mechanical Properties of Pure Aluminum upon Multi-Pass Caliber Rolling. Materials 2022, 15, 1206. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, Z.; Yu, H.; Chen, T.; Wang, X.; Ye, L. Performances of Al-xLi alloy anodes for Al-air batteries in alkaline electrolyte. J. Alloys Compd. 2021, 889, 161677. [Google Scholar] [CrossRef]
- Liu, H.L.; Li, P.; Xue, K.M.; Qian, C.H.; Hua, R. Microstructural evolution and fracture mechanism of a new Al-Zn-Mg-Cu-Zr alloy processed by equal-channel angular pressing. Rare Met. 2022, 41, 3546–3551. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, X.; Qin, J.; Zhou, W.; Ling, J.; Dong, Q.; Yu, J.; Nagaumi, H.; Zhang, B. Effect of Mn/Cr ratio on precipitation behaviors of α-Al(FeMnCr)Si dispersoids and mechanical properties of Al-Mg-Si-Cu alloys. Mater. Sci. Eng. A-Struct. 2022, 860, 144269. [Google Scholar] [CrossRef]
- Zupanič, F.; Steinacher, M.; Žist, S.; Bončina, T. Microstructure and Properties of a Novel Al-Mg-Si Alloy AA 6086. Metals 2021, 11, 368. [Google Scholar] [CrossRef]
- Žist, S.; Steinacher, M.; Bončina, T.; Albu, M.; Burja, J.; Vončina, M.; Zupanič, F. The Effect of Scandium on the Microstructure of the Aluminium Alloy AA 6086. Crystals 2022, 12, 973. [Google Scholar] [CrossRef]
- Xue, G.; Zhong, G.; Lin, S.; Li, H.-T.; Gui, X.; Zhang, L. Study on Microstructure and Mechanical Properties of Al-Mg-Si-Cu Aluminium Alloy with High Ductility. In Proceedings of the MATEC Web of Conferences Vol. 326 the 17th International Conference on Aluminium Alloys 2020 (ICAA17), Grenoble, France, 26–29 October 2020. [Google Scholar] [CrossRef]
- Chen, X.G.; Langlais, J. Solidification behavior of AA6111 Automotive Alloy. Mater. Sci. Forum 2000, 331–337, 215–222. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, B.; Zhang, Y.; Li, X.; Wang, F.; Li, Z.; Liu, H. The Microstructural Evolution of AA6111 Aluminum Alloy during Homogenization Treatment. Mater. Sci. Forum 2013, 749, 223–228. [Google Scholar] [CrossRef]
- Li, K.; Song, M.; Dua, Y.; Tang, Y.; Dong, H.; Ni, S. Investigation of the as-solidified microstructure of an Al-Mg-Si-Cu alloy. J. Alloys Compd. 2014, 602, 312–321. [Google Scholar] [CrossRef]
- Wu, Y.; Xiong, J.; Lai, R.; Zhang, X.; Guo, Z. The microstructure evolution of an Al-Mg-Si-Mn-Cu-Ce alloy during homogenization. J. Alloys Compd. 2009, 475, 332–338. [Google Scholar] [CrossRef]
- Lodgaard, L.; Ryum, N. Precipitation of dispersoids containing Mn and/or Cr in Al-Mg-Si alloys. Mater. Sci. Eng. A-Struct. 2000, 283, 144–152. [Google Scholar] [CrossRef]
- Han, Y.; Ma, K.; Wang, C.; Nagaumi, H. Precipitation behavior of dispersoids in Al-Mg-Si-Cu-Mn-Cr alloy during homogenization annealing. In Proceedings of the 13th International Conference on Aluminium Alloys ICAA13, Pittsburgh, PA, USA, 3–7 June 2012. [Google Scholar] [CrossRef]
- Li, Z.; Qin, J.; Zhang, H.; Wang, X.; Zhang, B.; Nagaumi, H. Improved Distribution and Uniformity of α-Al(Mn,Cr)Si Dispersoids in Al-Mg-Si-Cu-Mn (6xxx) Alloys by Two-Step Homogenization. Metall. Mater. Trans. A 2021, 52, 3204–3220. [Google Scholar] [CrossRef]
- Elasheri, A.; Elgallad, E.M.; Parson, N.; Chen, X.G. Improving the dispersoid distribution and recrystallization resistance of a Zr-containing 6xxx alloy using two-step homogenization. Philos. Mag. A 2022, 102, 2345–2361. [Google Scholar] [CrossRef]
- Mochugovskiy, A.G.; Mosleh, A.O.; Kotov, A.D.; Khokhlov, A.V.; Kaplanskaya, L.Y.; Mikhaylovskaya, A.V. Microstructure Evolution, Constitutive Modelling, and Superplastic Forming of Experimental 6XXX-Type Alloys Processed with Different Thermomechanical Treatments. Materials 2023, 16, 445. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, C.; Zhang, J. Microstructural characteristics and aging response of Zn-containing Al-Mg-Si-Cu alloy. International. J. Miner. Metall. Mater. 2013, 20, 659–664. [Google Scholar] [CrossRef]
- Kuijpers, N.C.W.; Kool, W.H.; Koenis, P.; Nilsen, K.; Todd, I.; Zwaag, S. Assessment of different techniques for quantification of α-Al(FeMn)Si and β-AlFeSi intermetallics in AA 6xxx alloys. Mater. Charact. 2002, 49, 409–420. [Google Scholar] [CrossRef]
- Bayat, N.; Carlberg, T.; Cieslar, M. In-situ study of phase transformations during homogenization of 6005 and 6082 Al alloys. J. Alloy. Compd. 2017, 725, 504–509. [Google Scholar] [CrossRef]
- Reiso, O.; Hafsås, J.E.; Sjothun, O.; Tundal, U. The Effect of Cooling Rate After Homogenization and Billet Preheating Practice on Extrudability and Section Properties. Part 1: Extrudability and Mechanical Properties. In Proceedings of the 6th International Aluminum Extrusion Technology Seminar, Chicago, IL, USA, 14–17 May 1996. [Google Scholar]
- Lefstad, M.; Reiso, O. Metalurgical Speed Limitations During the Extrusion of AlMgSi-Alloys. In Proceedings of the 6th International Aluminum Extrusion Technology Seminar, Chicago, IL, USA, 14–17 May 1996. [Google Scholar]
- Ricks, R.A.; Parson, N.C.; Yiu, H.L.; Court, S.A. Microstructural Optimisation for Extrusion Of 6063 Alloys, In Proceedings of the 5th International Aluminum Extrusion Technology Seminar, Chicago, IL, USA, 19–22 May 1992.
- Zając, S.; Bengtsson, B.; Jönsson, C. Influence of cooling after homogenization and reheating to extrusion on extrudability and final properties of AA6063 and AA6082 alloys. Mater. Sci. Forum 2002, 396–402, 399–404. [Google Scholar] [CrossRef]
- Birol, Y. Homogenization of direct chill cast AlSi1MgMn billets. Int. J. Mater. Res. (Z. Metallkd.) 2014, 105, 75–82. [Google Scholar] [CrossRef]
- Qin, J.; Nagaumi, H.; Yu, C.; Liu, F.; Li, Y.; Wang, L. Coarsening behavior of Mg2Si precipitates during post homogenization cooling process in Al-Mg-Si alloy. J. Alloy. Compd. 2022, 902, 162851. [Google Scholar] [CrossRef]
- Belov, N.A.; Eskin, D.G.; Aksenov, A.A. Multicomponent Phase Diagrams, Applications for Commercial Aluminum Alloys; Elsevier: London, UK, 2005. [Google Scholar]

| Si | Fe | Cu | Mn | Mg | Cr | Ti | Zr |
|---|---|---|---|---|---|---|---|
| 1.22 | 0.05 | 1.41 | 0.62 | 0.80 | 0.38 | 0.02 | 0.15 |
| State | Peak A | Peak B | Peak C | Bulk Melting | |||
|---|---|---|---|---|---|---|---|
| Onset, °C | Heat, J/g | Onset, °C | Heat, J/g | Onset, °C | Heat, J/g | Onset, °C | |
| As-cast | 513.4 | 0.47 | 539.7 | 1.48 | 561.3 | 2.12 | |
| 475 °C/2 h–wq | 544.8 | 0.90 | 560.4 | 3.77 | |||
| 475 °C/2 h + 530 °C/2 h–wq | 561.3 | 2.36 | |||||
| 475 °C/2 h + 530 °C/2 h + 545 °C/2 h–wq | 562.2 | 0.79 | |||||
| 475 °C/2 h + 530 °C/2 h + 545 °C/4 h–wq | 561.6 | 1 | |||||
| 475 °C/2 h + 530 °C/2 h + 545 °C/8 h–wq | 573.4 | ||||||
| 475 °C/2 h + 530 °C/2 h + 545 °C/8 h–cooling 50 °C/h | 542.7 | 0.02 | 560.3 | 5.95 | |||
| 475 °C/2 h + 530 °C/2 h + 545 °C/8 h–cooling 300 °C/h | 558.9 | 0.78 | |||||
| 475 °C/2 h + 530 °C/2 h + 545 °C/8 h–cooling 500 °C/h | 546.7 | 0.10 | 562.9 | 0.14 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźnicki, A.; Leszczyńska-Madej, B.; Włoch, G.; Madura, J.; Bogusz, M.; Leśniak, D. Homogenization of Extrusion Billets of a Novel Al-Mg-Si-Cu Alloy with Increased Copper Content. Materials 2023, 16, 2091. https://doi.org/10.3390/ma16052091
Woźnicki A, Leszczyńska-Madej B, Włoch G, Madura J, Bogusz M, Leśniak D. Homogenization of Extrusion Billets of a Novel Al-Mg-Si-Cu Alloy with Increased Copper Content. Materials. 2023; 16(5):2091. https://doi.org/10.3390/ma16052091
Chicago/Turabian StyleWoźnicki, Antoni, Beata Leszczyńska-Madej, Grzegorz Włoch, Jacek Madura, Marek Bogusz, and Dariusz Leśniak. 2023. "Homogenization of Extrusion Billets of a Novel Al-Mg-Si-Cu Alloy with Increased Copper Content" Materials 16, no. 5: 2091. https://doi.org/10.3390/ma16052091
APA StyleWoźnicki, A., Leszczyńska-Madej, B., Włoch, G., Madura, J., Bogusz, M., & Leśniak, D. (2023). Homogenization of Extrusion Billets of a Novel Al-Mg-Si-Cu Alloy with Increased Copper Content. Materials, 16(5), 2091. https://doi.org/10.3390/ma16052091








