Analysis of Different Early Strength Agents on the Performance of Prefabricated UHPC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Design
2.3. Specimen Molding and Maintenance
2.4. Testing Methods
2.4.1. Workability and Setting Time
2.4.2. Compressive and Flexural Strength
2.4.3. Hydration Heat
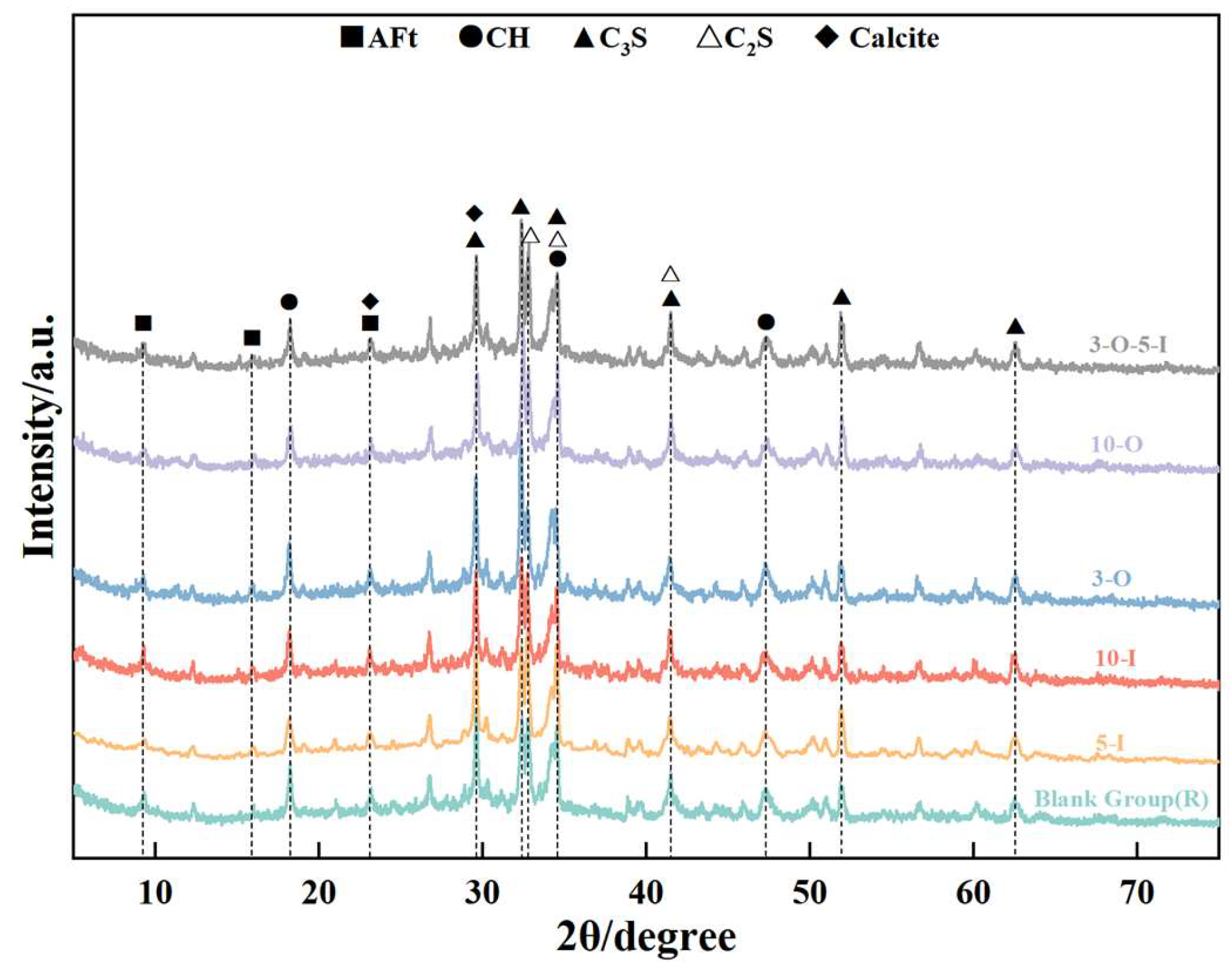
2.4.4. X-ray Powder Diffraction
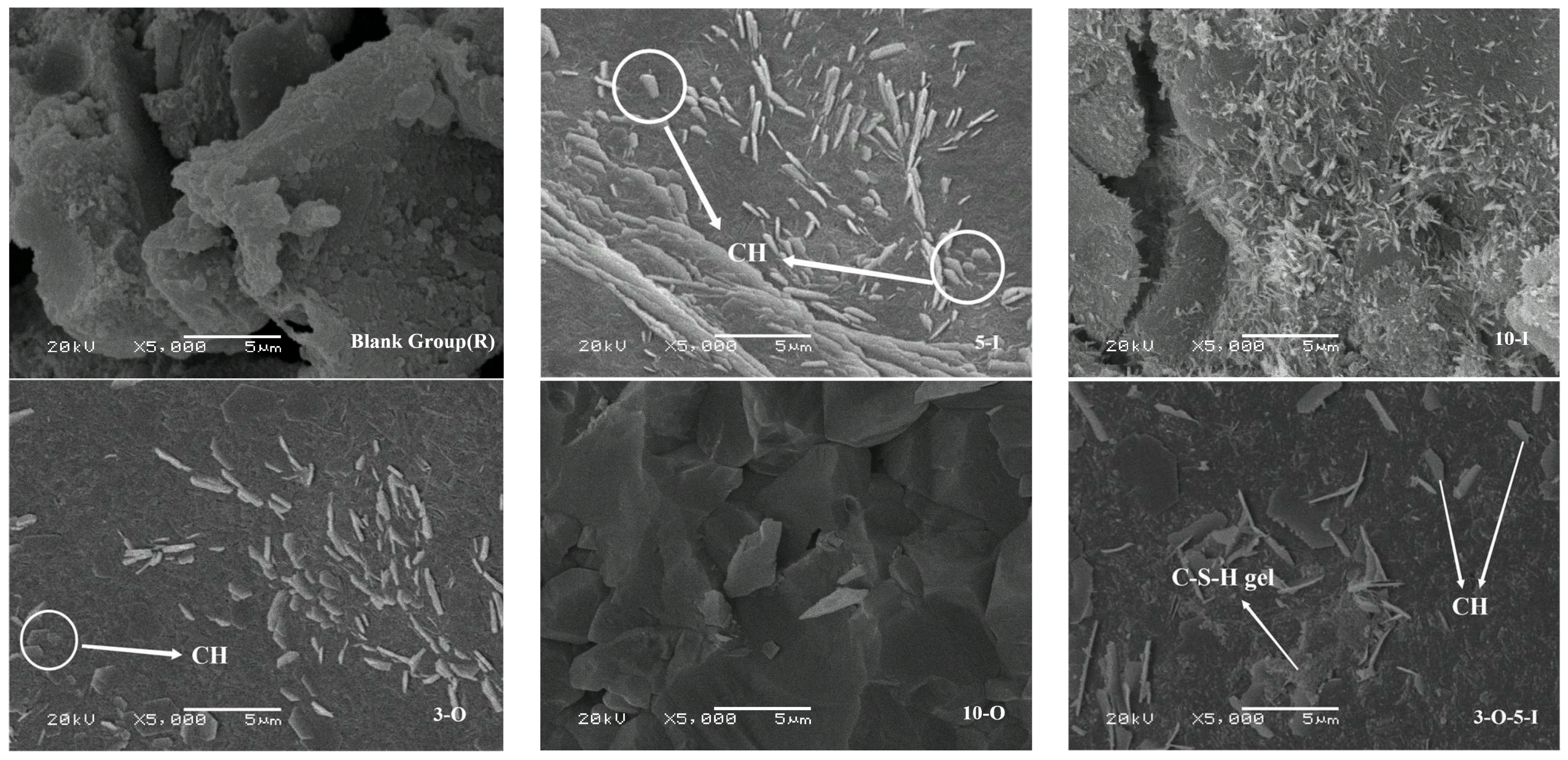
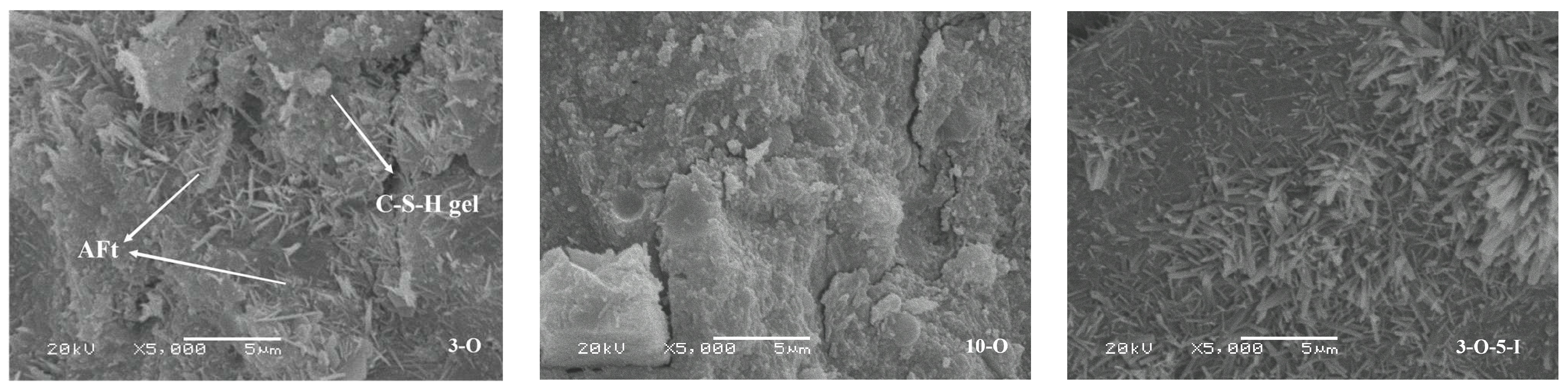
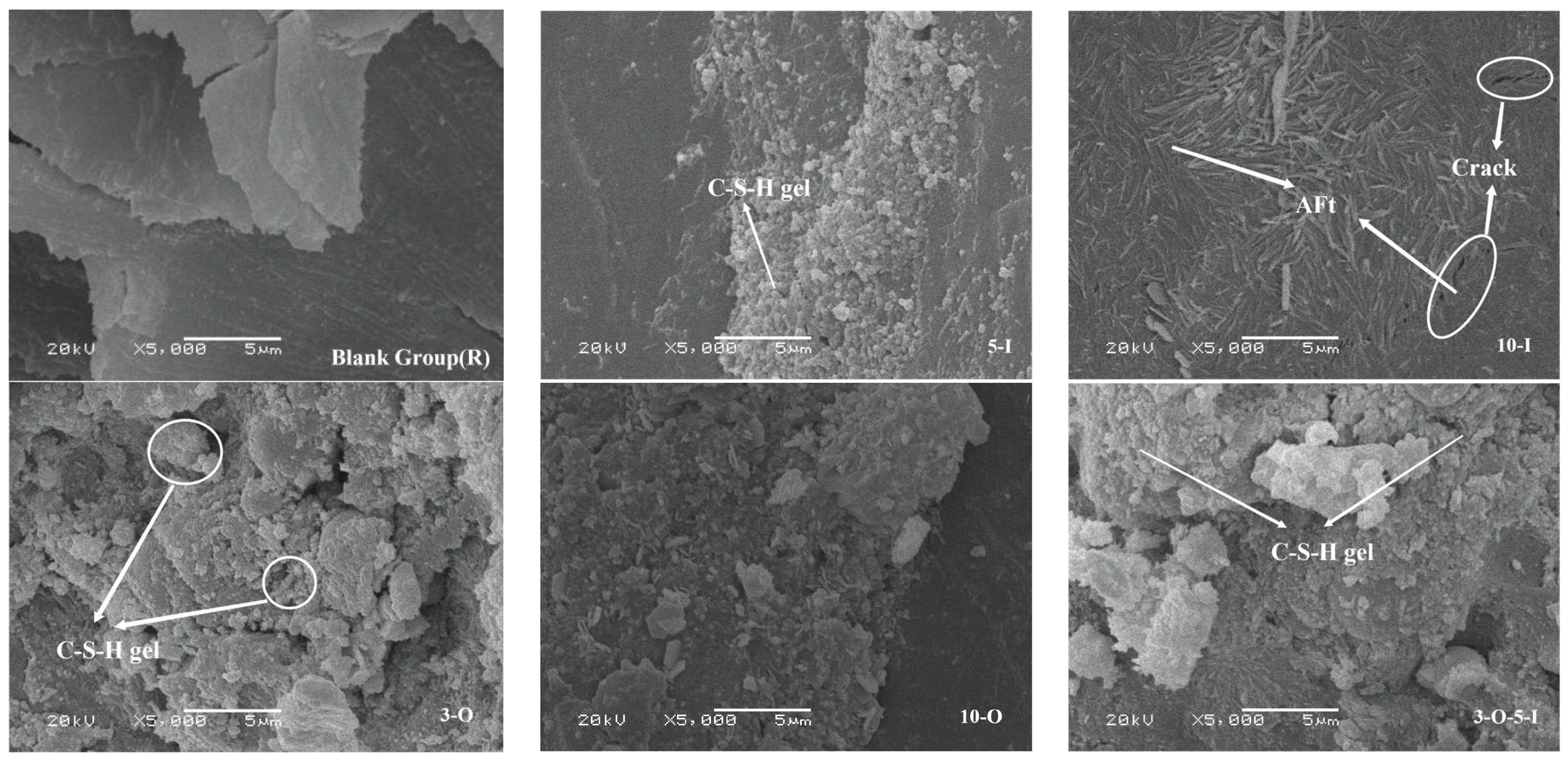
2.4.5. Scanning Electron Microscopy
3. Results and Analysis
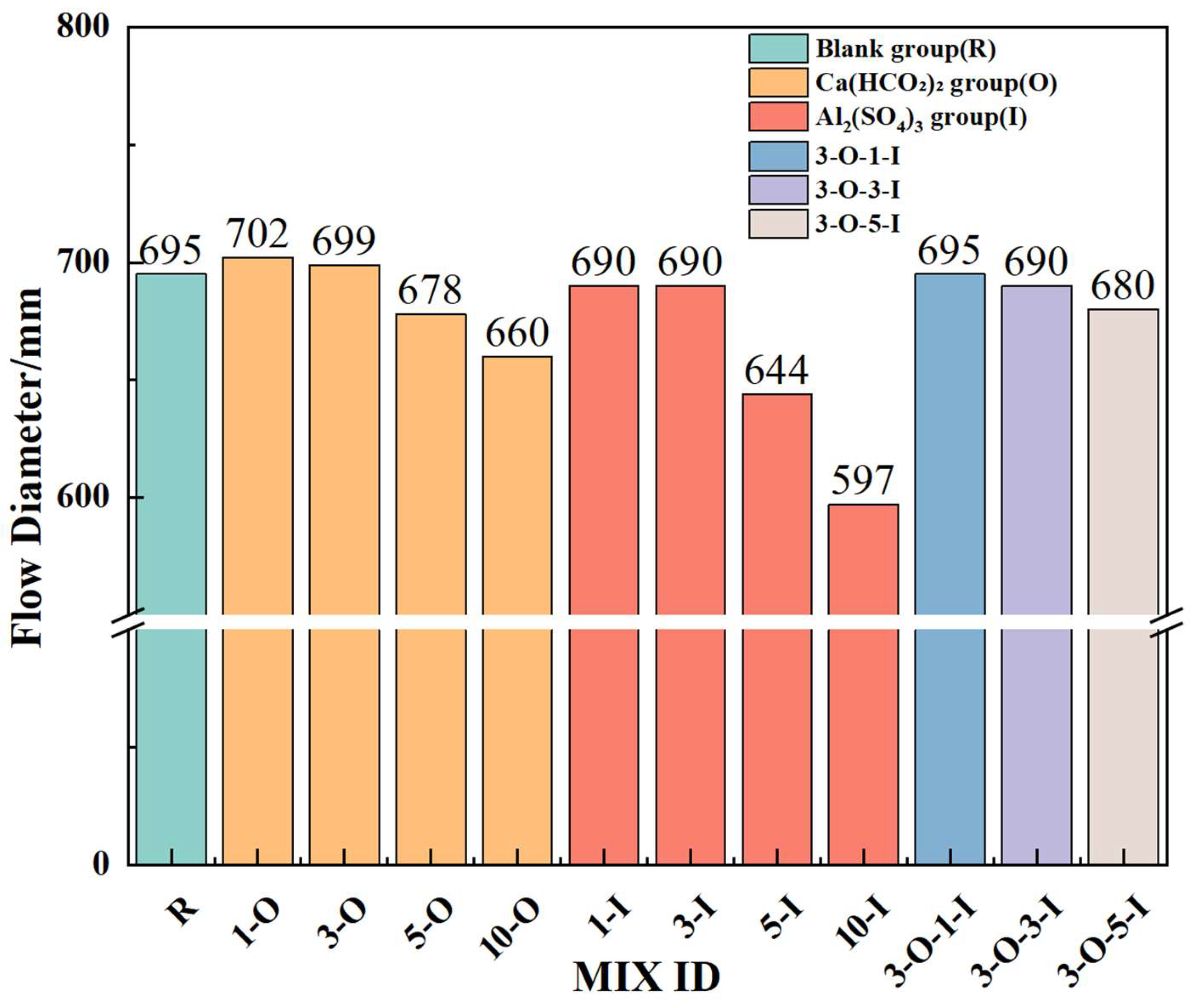
3.1. Impact of Early Strength Agents on Workability
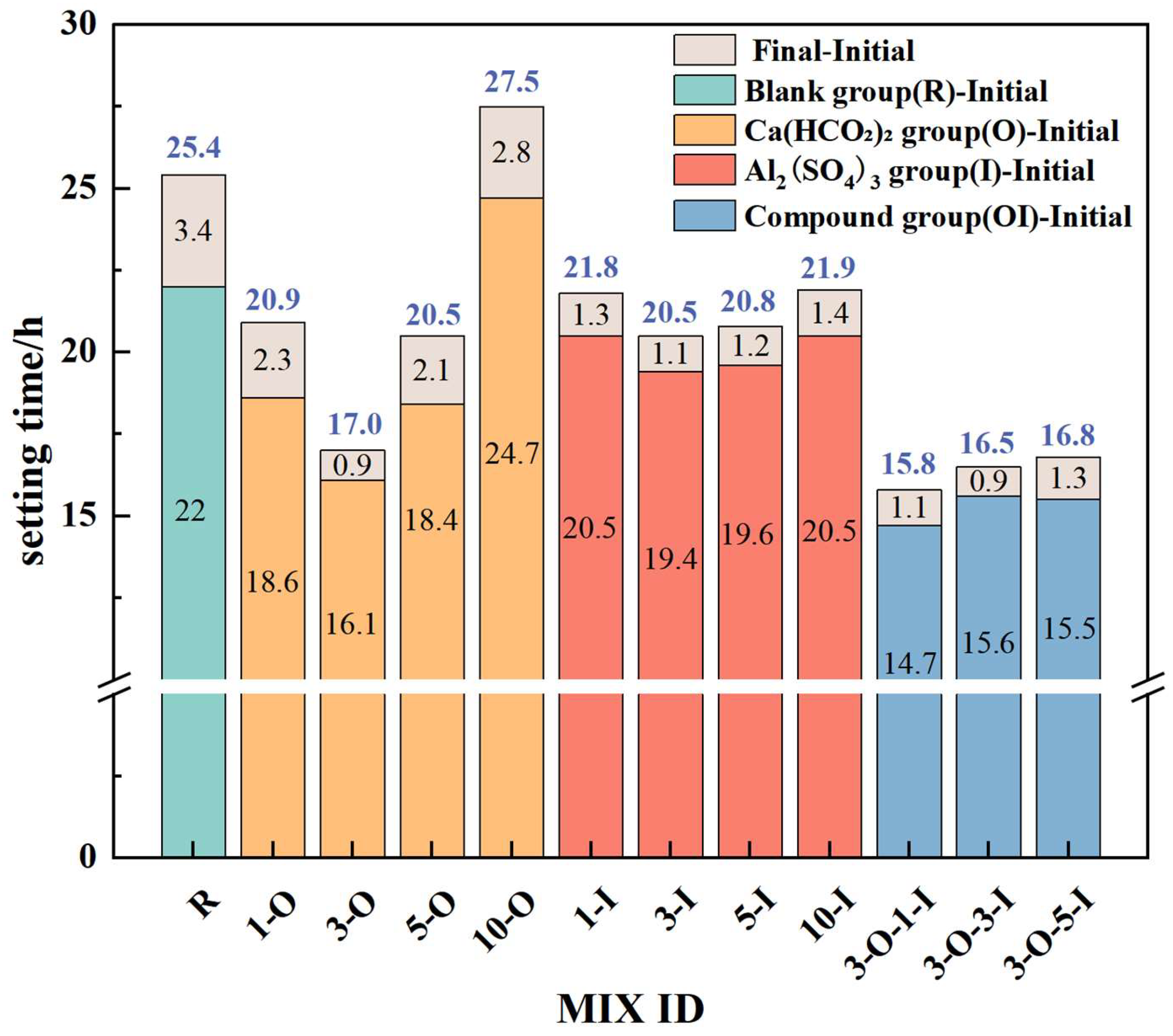
3.2. Impact of Early Strength Agents on Setting Time
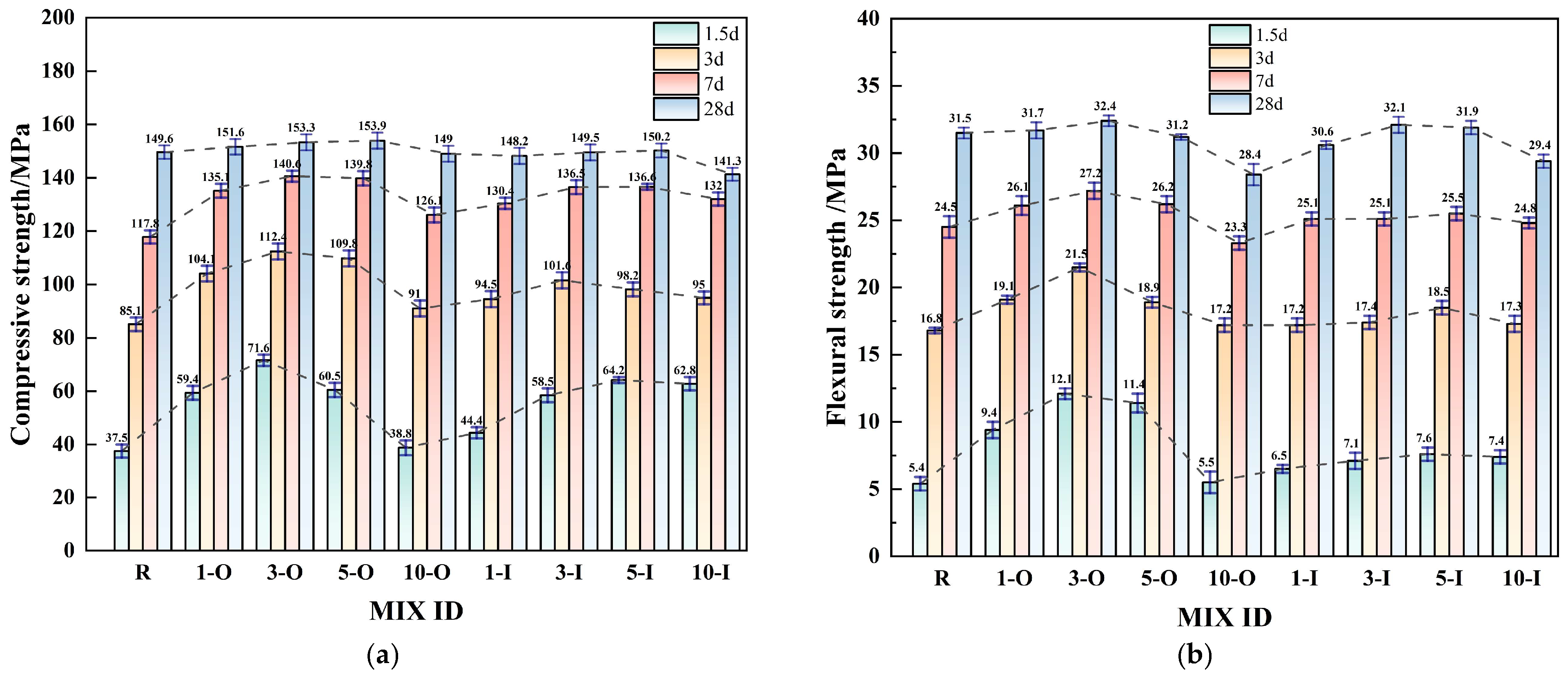
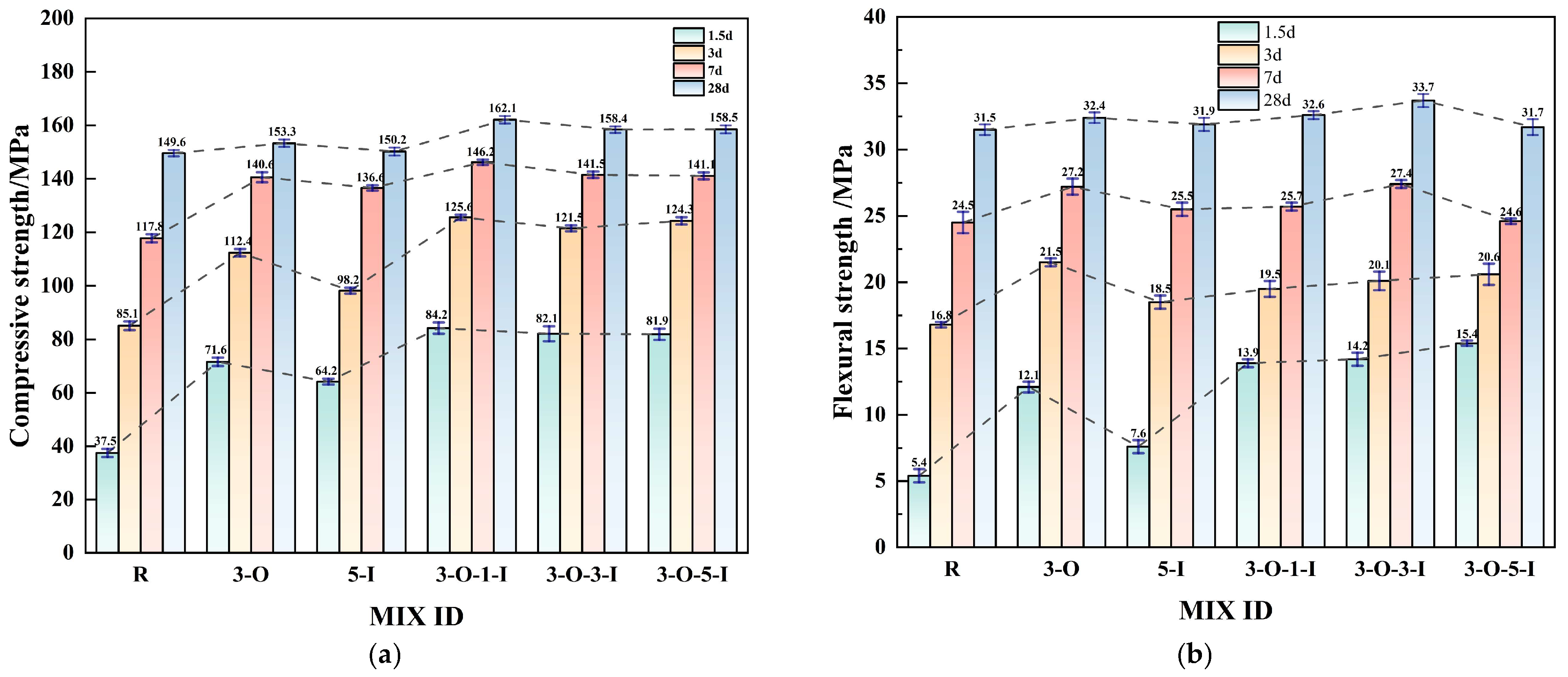
3.3. Impact of Early Strength Agents on Compressive and Flexural Strength
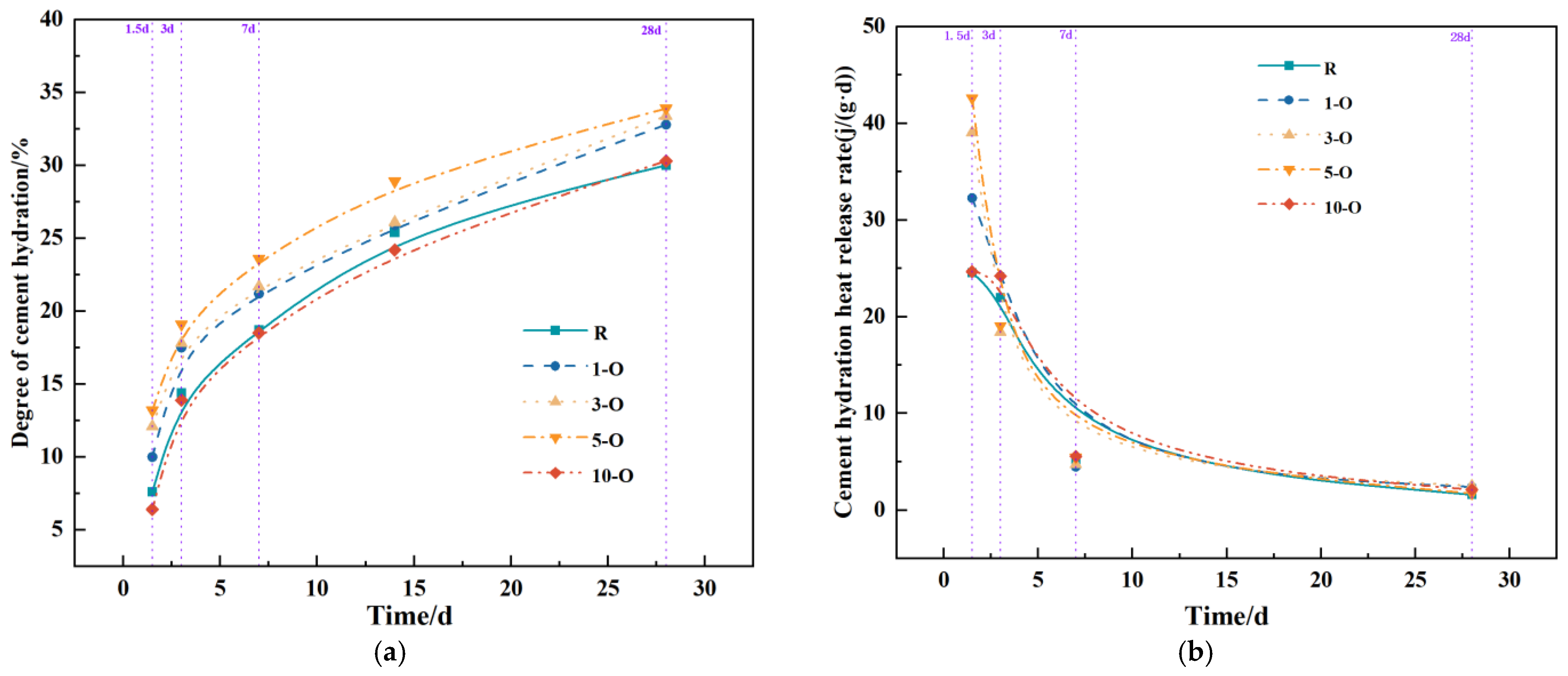
3.4. Impacts of Early Strength Agents on Hydration Reaction Process
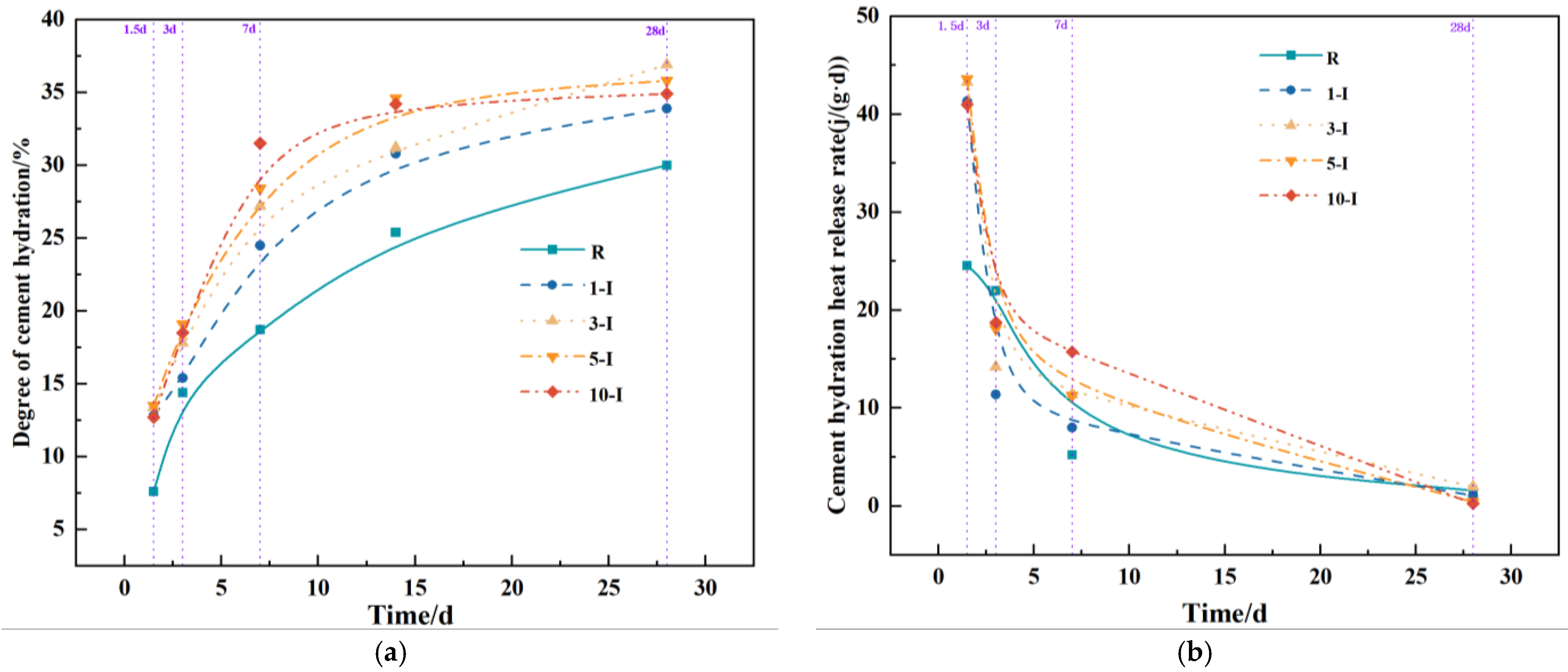
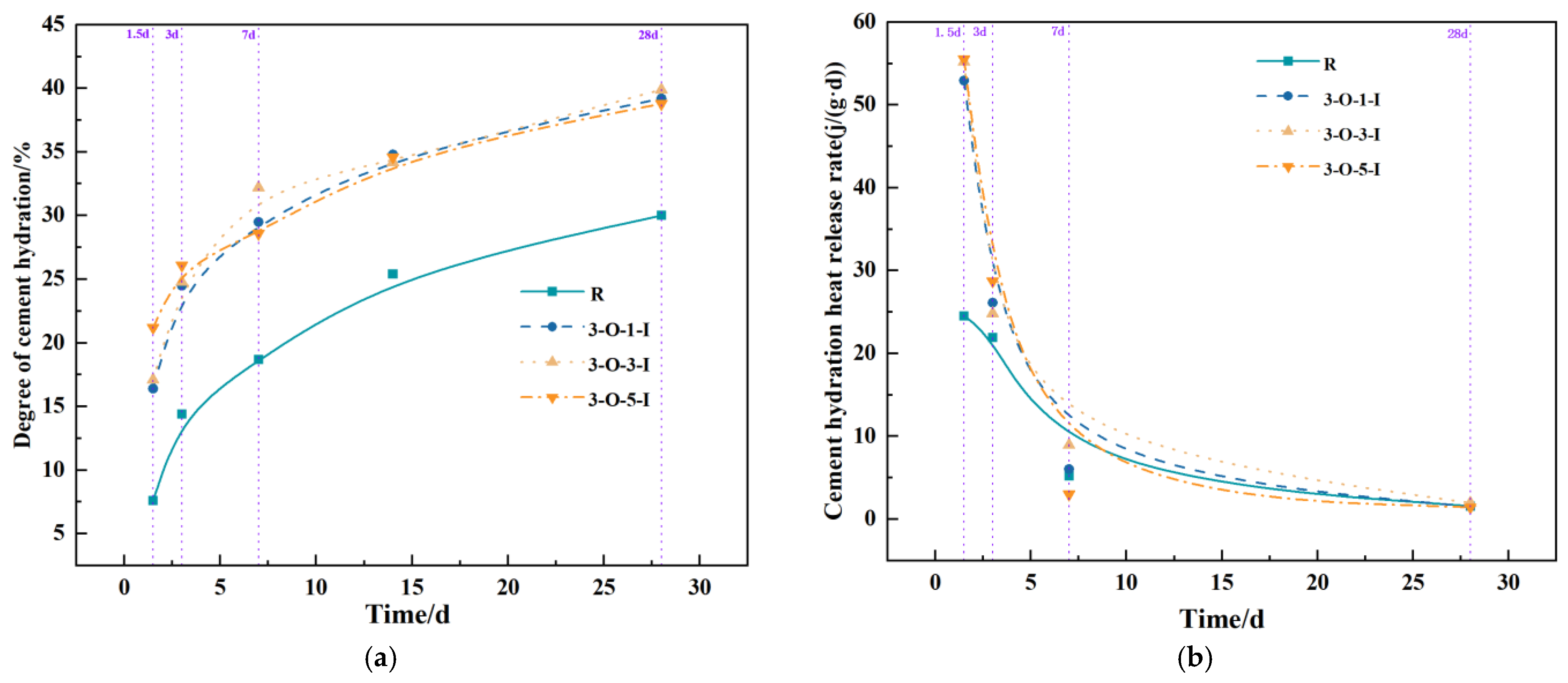
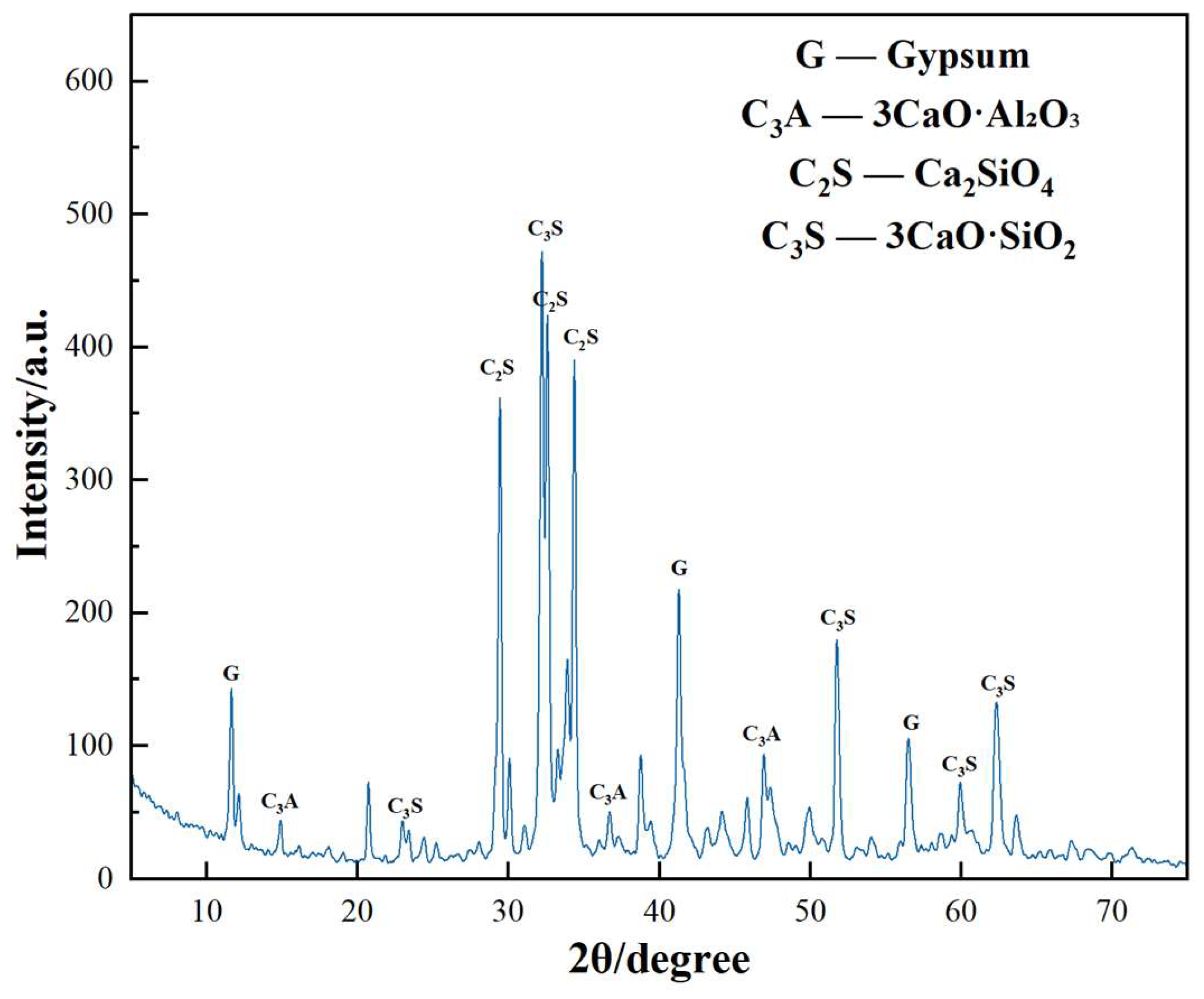
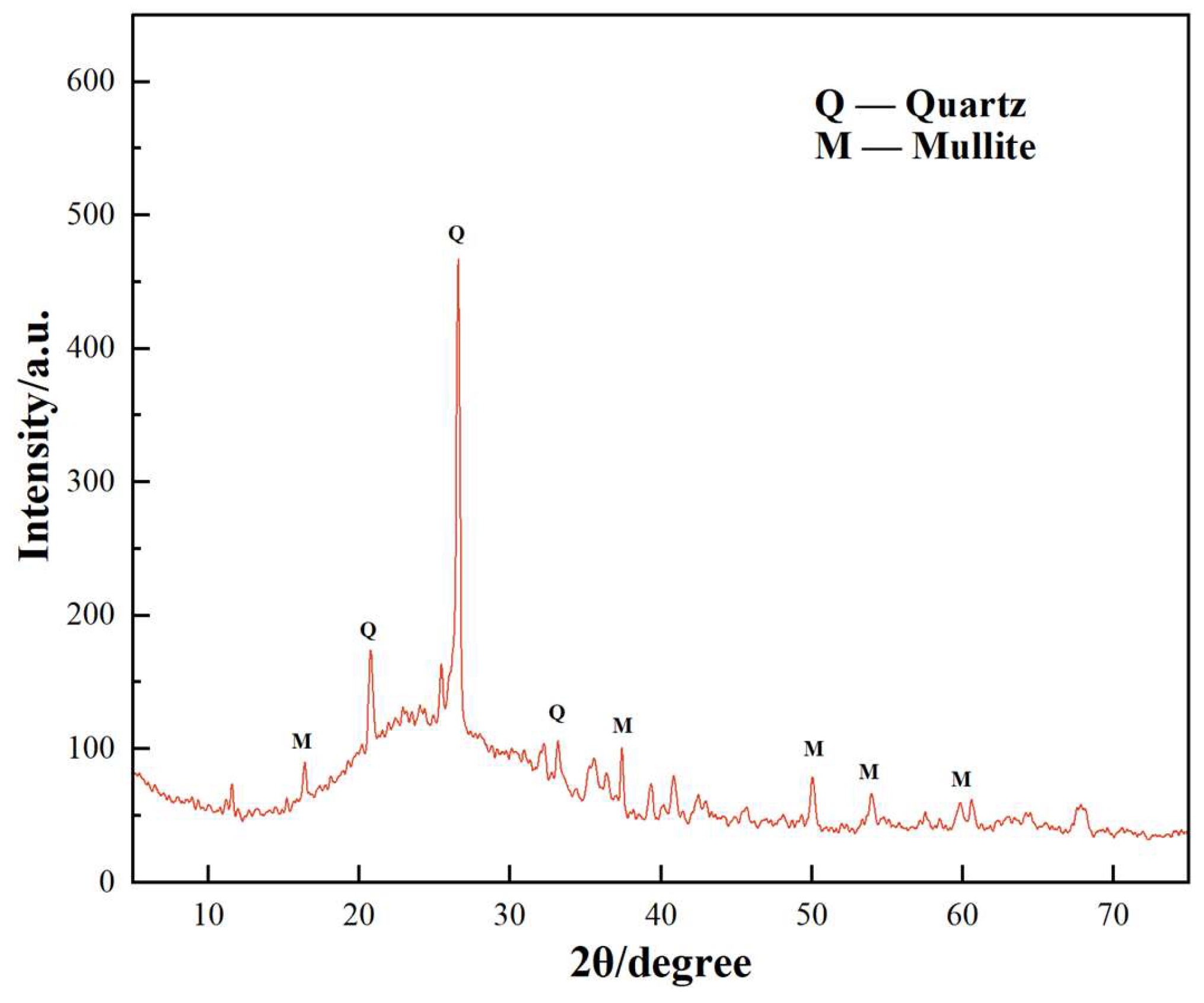
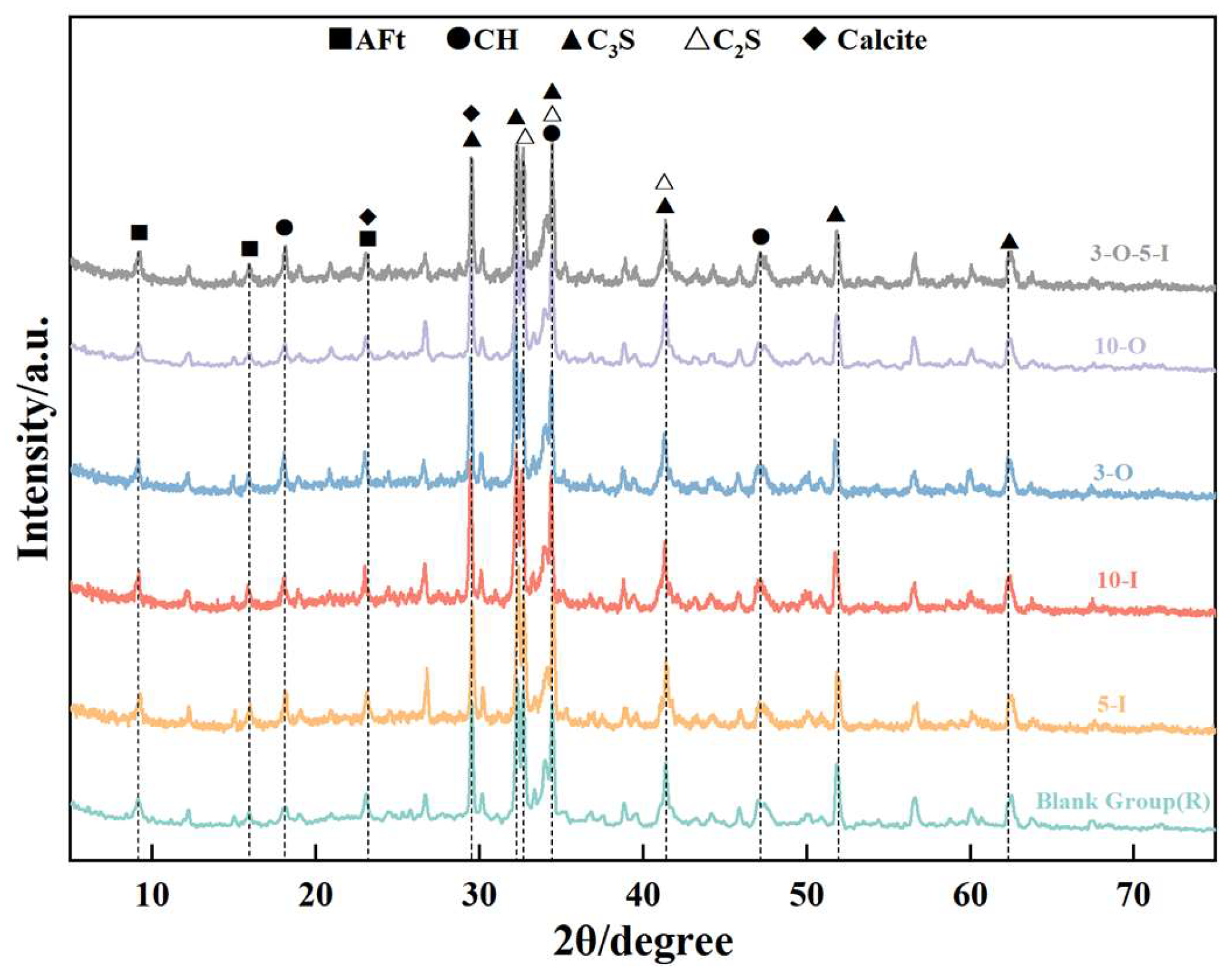
3.5. Analysis of X-ray Powder Diffraction
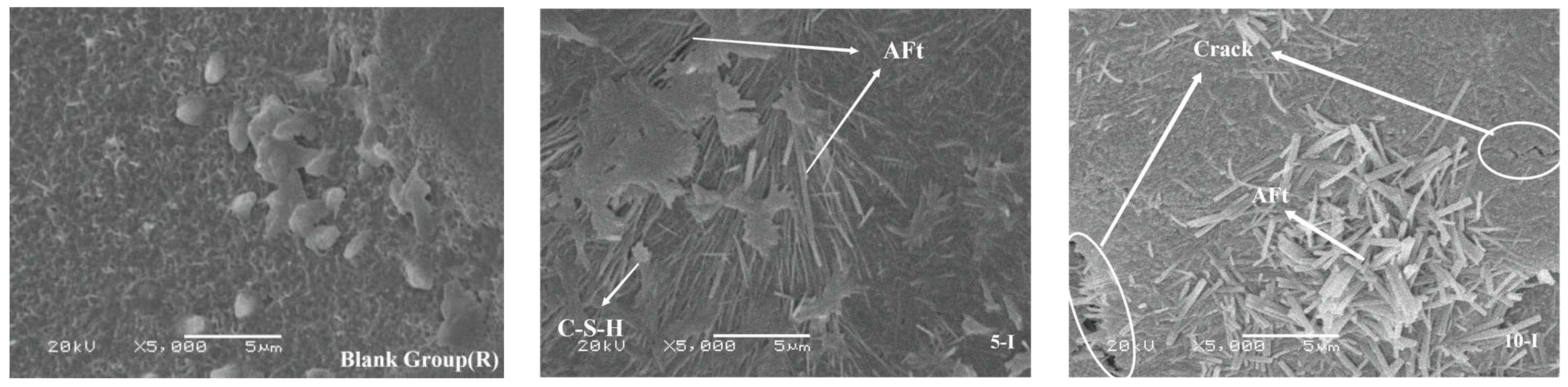
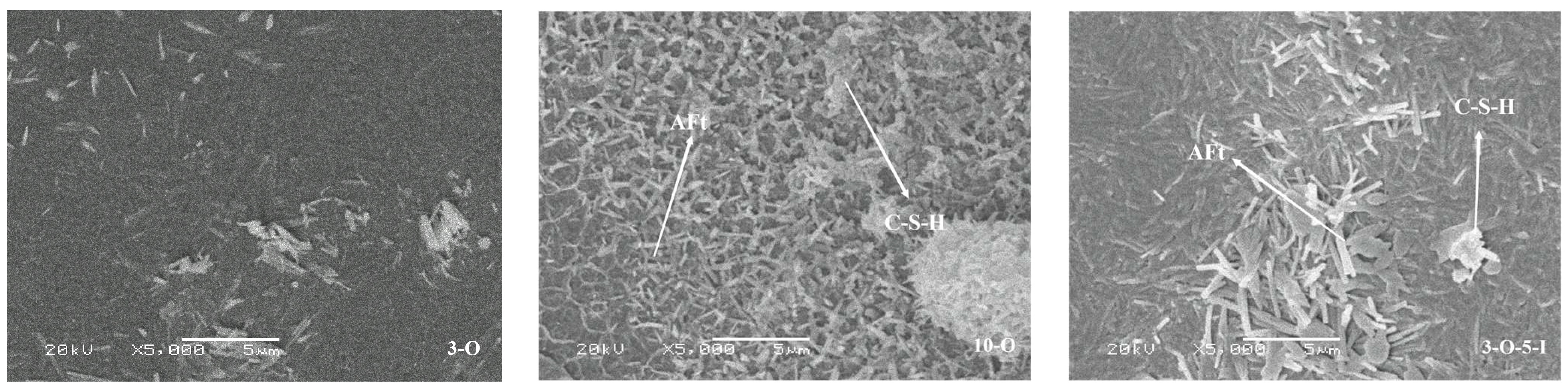
3.6. Analysis of Scanning Electron Microscopy
4. Conclusions
- (1)
- Within a reasonable dosage range, both early strength agents, Ca(HCO2)2 and Al2(SO4)3, can substantially reduce the curing time and enhance the mechanical properties of UHPC. The optimal dosages of Ca(HCO2)2 and Al2(SO4)3 are approximately 0.3% and 0.5%, respectively.
- (2)
- With the exception of 10-O, all other groups significantly enhanced hydration during the early and middle stages. The heat release rate of Al2(SO4)3 during these stages exceeds that of Ca(HCO2)2.
- (3)
- The two early strength agents did not change the type of hydration products but changed the shape and quantity of the hydration products, especially in the early stage of hydration, which greatly increased the content of AFt. In addition, the two early strength agents improved the microstructure and matrix density at reasonable dosages.
- (4)
- Adding calcium formate or calcium sulfate alone does not meet the expected early strength effect. The sole addition of calcium formate exhibits the drawback of sluggish mid-term hydration, whereas the exclusive use of aluminum sulfate fails to adequately enhance initial strength. Moreover, using a large dosage of a single early strength agent poses risks to UHPC. However, when these two agents are combined, they exhibit a synergistic effect, resulting in better performance than that obtained using either agent alone.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Su, Y.; Luo, B.; Luo, Z.; Huang, H.; Li, J.; Wang, D. Effect of Accelerators on the Workability, Strength, and Microstructure of Ultra-High-Performance Concrete. Materials 2022, 15, 159. [Google Scholar] [CrossRef] [PubMed]
- He, Z.-H.; Shen, M.-L.; Shi, J.-Y.; Yalçınkaya, Ç.; Du, S.-G.; Yuan, Q. Recycling coral waste into eco-friendly UHPC: Mechanical strength, microstructure, and environmental benefits. Sci. Total Environ. 2022, 836, 155424. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, M.; Shafei, B. Internal curing capabilities of natural zeolite to improve the hydration of ultra-high performance concrete. Constr. Build. Mater. 2022, 340, 127452. [Google Scholar] [CrossRef]
- Lee, N.K.; Koh, K.; Kim, M.O.; Ryu, G. Uncovering the role of micro silica in hydration of ultra-high performance concrete (UHPC). Cem. Concr. Res. 2018, 104, 68. [Google Scholar] [CrossRef]
- Wu, Z.; Khayat, K.H.; Shi, C.; Tutikian, B.F.; Chen, Q. Mechanisms underlying the strength enhancement of UHPC modified with nano-SiO2 and nano-CaCO3. Cem. Concr. Compos. 2021, 119, 103992. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, X.; Shen, H.; Guo, S.; Liu, C. Analysis of Structural Parameters of Steel-NC-UHPC Composite Beams. Materials 2023, 16, 5586. [Google Scholar] [CrossRef] [PubMed]
- Yalçınkaya, Ç.; Çopuroğlu, O. Hydration heat, strength and microstructure characteristics of UHPC containing blast furnace slag. J. Build. Eng. 2021, 34, 101915. [Google Scholar] [CrossRef]
- Abdulkareem, O.M.; Fraj, A.B.; Bouasker, M.; Khelidj, A. Mixture design and early age investigations of more sustainable UHPC. Constr. Build. Mater. 2018, 163, 235. [Google Scholar] [CrossRef]
- De la Varga, I.; Graybeal, B.A. Dimensional stability of grout-type materials used as connections between prefabricated concrete elements. J. Mater. Civ. Eng. 2015, 27, 04014246. [Google Scholar] [CrossRef]
- Meng, Q.; Wu, C.; Li, J.; Liu, Z.; Wu, P.; Yang, Y.; Wang, Z. Steel/basalt rebar reinforced Ultra-High Performance Concrete components against methane-air explosion loads. Compos. Part B Eng. 2020, 198, 108215. [Google Scholar] [CrossRef]
- Abouhussien, A.A.; Hassan, A.A. Monitoring early age strength gain of SCC with different supplementary cementitious materials using acoustic emission sensors. Constr. Build. Mater. 2019, 229, 116858. [Google Scholar] [CrossRef]
- Hussein, H.H.; Walsh, K.K.; Sargand, S.M.; Steinberg, E.P. Interfacial properties of ultrahigh-performance concrete and high-strength concrete bridge connections. J. Mater. Civ. Eng. 2016, 28, 04015208. [Google Scholar] [CrossRef]
- Yu, R.; Spiesz, P.; Brouwers, H. Development of an eco-friendly Ultra-High Performance Concrete (UHPC) with efficient cement and mineral admixtures uses. Cem. Concr. Compos. 2015, 55, 383–394. [Google Scholar] [CrossRef]
- Bahmani, H.; Mostofinejad, D. Microstructure of ultra-high-performance concrete (UHPC)—A review study. J. Build. Eng. 2022, 50, 104118. [Google Scholar] [CrossRef]
- Won, J.-P.; Hwang, U.-J.; Lee, S.-J. Enhanced long-term strength and durability of shotcrete with high-strength C12A7 mineral-based accelerator. Cem. Concr. Res. 2015, 76, 121–129. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, S.; Liu, Z.; Wang, F. Utilization of steel slag in ultra-high performance concrete with enhanced eco-friendliness. Constr. Build. Mater. 2019, 214, 28–36. [Google Scholar] [CrossRef]
- Yang, R.; He, T. Influence of liquid accelerators combined with mineral admixtures on early hydration of cement pastes. Constr. Build. Mater. 2021, 295, 123659. [Google Scholar] [CrossRef]
- Arora, A.; Yao, Y.; Mobasher, B.; Neithalath, N. Fundamental insights into the compressive and flexural response of binder-and aggregate-optimized ultra-high performance concrete (UHPC). Cem. Concr. Compos. 2019, 98, 1–13. [Google Scholar] [CrossRef]
- Alkaysi, M.; El-Tawil, S.; Liu, Z.; Hansen, W. Effects of silica powder and cement type on durability of ultra high performance concrete (UHPC). Cem. Concr. Compos. 2016, 66, 47. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.; Liu, F.; Wan, C.; Pu, X. Preparation of ultra-high performance concrete with common technology and materials. Cem. Concr. Compos. 2012, 34, 538. [Google Scholar] [CrossRef]
- Lam, L.; Wong, Y.; Poon, C.S. Degree of hydration and gel/space ratio of high-volume fly ash/cement systems. Cem. Concr. Res. 2000, 30, 747. [Google Scholar] [CrossRef]
- Rong, Z.; Sun, W.; Xiao, H.; Wang, W. Effect of silica fume and fly ash on hydration and microstructure evolution of cement based composites at low water–binder ratios. Constr. Build. Mater. 2014, 51, 446. [Google Scholar] [CrossRef]
- Park, S.; Wu, S.; Liu, Z.; Pyo, S. The role of supplementary cementitious materials (SCMs) in ultra high performance concrete (UHPC): A review. Materials 2021, 14, 1472. [Google Scholar] [CrossRef] [PubMed]
- Li, P.P.; Yu, Q.L.; Brouwers, H.J.H. Effect of PCE-type superplasticizer on early-age behaviour of ultra-high performance concrete (UHPC). Constr. Build. Mater. 2017, 153, 740. [Google Scholar] [CrossRef]
- Qasim, O.A. Experimental investigation on autogenous shrinkage of high and ultra-high strength concrete. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454, 012067. [Google Scholar] [CrossRef]
- T/CECS 864-2021; Standard for Test Method of Ultra-High Performance Concrete. China Construction Industry Press: Beijing, China, 2021.
- DB62/T 4690-2023; Ultra-High Performance Concrete Application Technical Specification. Gansu Provincial Market Supervision Administration: Lanzhou, China, 2023.
- GB/T 12959 2024; Cement Hydration Heat Determination Method. Standardization Administration of China: Beijing, China, 2024.
- Snellings, R.; Salze, A.; Scrivener, K. Use of X-ray diffraction to quantify amorphous supplementary cementitious materials in anhydrous and hydrated blended cements. Cem. Concr. Res. 2014, 64, 89. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Argiz, C.; Gálvez, J.C.; Moragues, A. Effect of silica fume fineness on the improvement of Portland cement strength performance. Constr. Build. Mater. 2015, 96, 55–64. [Google Scholar] [CrossRef]
- Zhou, M.; Lu, W.; Song, J.; Lee, G.C. Application of Ultra-High Performance Concrete in bridge engineering. Constr. Build. Mater. 2018, 186, 1256–1267. [Google Scholar] [CrossRef]
- Tan, H.; Li, M.; Ren, J.; Deng, X.; Zhang, X.; Nie, K.; Zhang, J.; Yu, Z. Effect of aluminum sulfate on the hydration of tricalcium silicate. Constr. Build. Mater. 2019, 205, 414. [Google Scholar] [CrossRef]
- Pang, C.; Tang, Z.; Yang, Z.; Huang, P. Early Strengthening Agent in Cementitious Composites and Its Function Mechanism: A Review. Mater. Rep. 2023, 37, 21110247. [Google Scholar]
- Li, G.; Zhang, J.; Niu, M.; Song, Z. The mechanism of alkali-free liquid accelerator on the hydration of cement pastes. Constr. Build. Mater. 2020, 233, 117296. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Zhao, P.; Lu, L.; Cheng, X. Effect of early-strength-enhancing agents on setting time and early mechanical strength of belite–barium calcium sulfoaluminate cement. J. Therm. Anal. Calorim. 2018, 131, 2337. [Google Scholar] [CrossRef]
- Matusinović, T.; Čurlin, D. Lithium salts as set accelerators for high alumina cement. Cem. Concr. Res. 1993, 23, 885–895. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.; Ye, W.; Chang, Y.; Liu, Q.; Song, Z. Effects of ordinary Portland cement on the early properties and hydration of calcium sulfoaluminate cement. Constr. Build. Mater. 2018, 186, 1144–1153. [Google Scholar] [CrossRef]
- Liu, M.; Tan, H.; He, X. Effects of nano-SiO2 on early strength and microstructure of steam-cured high volume fly ash cement system. Constr. Build. Mater. 2019, 194, 350–359. [Google Scholar] [CrossRef]
- Yu, R.; Spiesz, P.; Brouwers, H. Effect of nano-silica on the hydration and microstructure development of Ultra-High Performance Concrete (UHPC) with a low binder amount. Constr. Build. Mater. 2014, 65, 140–150. [Google Scholar] [CrossRef]
- Huang, W.; Kazemi-Kamyab, H.; Sun, W.; Scrivener, K. Effect of cement substitution by limestone on the hydration and microstructural development of ultra-high performance concrete (UHPC). Cem. Concr. Compos. 2017, 77, 86–101. [Google Scholar] [CrossRef]
- Mostafa, S.A.; Faried, A.S.; Farghali, A.A.; El-Deeb, M.M.; Tawfik, T.A.; Majer, S.; Abd Elrahman, M. Influence of nanoparticles from waste materials on mechanical properties, durability and microstructure of UHPC. Materials 2020, 13, 4530. [Google Scholar] [CrossRef] [PubMed]

| Materials | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO2 | CO2 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement/% | 13.58 | 5.37 | 3.51 | 57.05 | 1.12 | 0.82 | 0.62 | 16.25 | 0.00 | 98.32 |
| Fly ash/% | 56.30 | 27.99 | 4.99 | 6.38 | 0.75 | 1.18 | 0.82 | 0.00 | 0.22 | 98.63 |
| Silica fume/% | 96.25 | 0.33 | 0.54 | 0.52 | 0.31 | 1.87 | 0.18 | 0.00 | 0.00 | 100.00 |
| Particle Size Range/mm | SiO2 Content/% | Fe2O3 Content/% | Moisture Content/% |
|---|---|---|---|
| 0.15–1.00 | 99.0–99.6 | 0.03–0.10 | 1.5 |
| Water Reduction/% | Methane Content/% | Solid Content/% | Fluidity of Cement Mortar/mm |
|---|---|---|---|
| ≥35 | ≤6.0 | 30% | ≥240 |
| Numbering | Cement | Flyash | Silica Fume | Quartz Sand | Steel Fiber | Water | Accelerating Admixture/% | |
|---|---|---|---|---|---|---|---|---|
| O | I | |||||||
| R | 780 | 220 | 150 | 950 | 188 | 171 | / | / |
| 1-O | 780 | 220 | 150 | 950 | 188 | 171 | 0.1 | / |
| 3-O | 780 | 220 | 150 | 950 | 188 | 171 | 0.3 | / |
| 5-O | 780 | 220 | 150 | 950 | 188 | 171 | 0.5 | / |
| 10-O | 780 | 220 | 150 | 950 | 188 | 171 | 1.0 | / |
| 1-I | 780 | 220 | 150 | 950 | 188 | 171 | / | 0.1 |
| 3-I | 780 | 220 | 150 | 950 | 188 | 171 | / | 0.3 |
| 5-I | 780 | 220 | 150 | 950 | 188 | 171 | / | 0.5 |
| 10-I | 780 | 220 | 150 | 950 | 188 | 171 | / | 1.0 |
| 3-O-1-I | 780 | 220 | 150 | 950 | 188 | 171 | 0.3 | 0.1 |
| 3-O-3-I | 780 | 220 | 150 | 950 | 188 | 171 | 0.3 | 0.3 |
| 3-O-5-I | 780 | 220 | 150 | 950 | 188 | 171 | 0.3 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Hu, L.; Guo, F.; Li, X. Analysis of Different Early Strength Agents on the Performance of Prefabricated UHPC. Materials 2024, 17, 2481. https://doi.org/10.3390/ma17112481
Wu X, Hu L, Guo F, Li X. Analysis of Different Early Strength Agents on the Performance of Prefabricated UHPC. Materials. 2024; 17(11):2481. https://doi.org/10.3390/ma17112481
Chicago/Turabian StyleWu, Xiaohu, Lien Hu, Fucheng Guo, and Xiaomin Li. 2024. "Analysis of Different Early Strength Agents on the Performance of Prefabricated UHPC" Materials 17, no. 11: 2481. https://doi.org/10.3390/ma17112481







