SBA-15 Type Mesoporous Silica Modified with Vanadium as a Catalyst for Oxidative and Extractive Oxidative Desulfurization Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SBA-15 Support
2.3. Modification of SBA-15 with Vanadium Species
2.4. Catalyst Characterization
2.5. Catalytic Tests
2.5.1. Catalytic Oxidative Desulphurization (CODS)
2.5.2. Extractive Catalytic Oxidative Desulphurization (ECODS)
2.5.3. Determination of Catalytic Activity
3. Results and Discussion
3.1. Characterization of the Catalysts
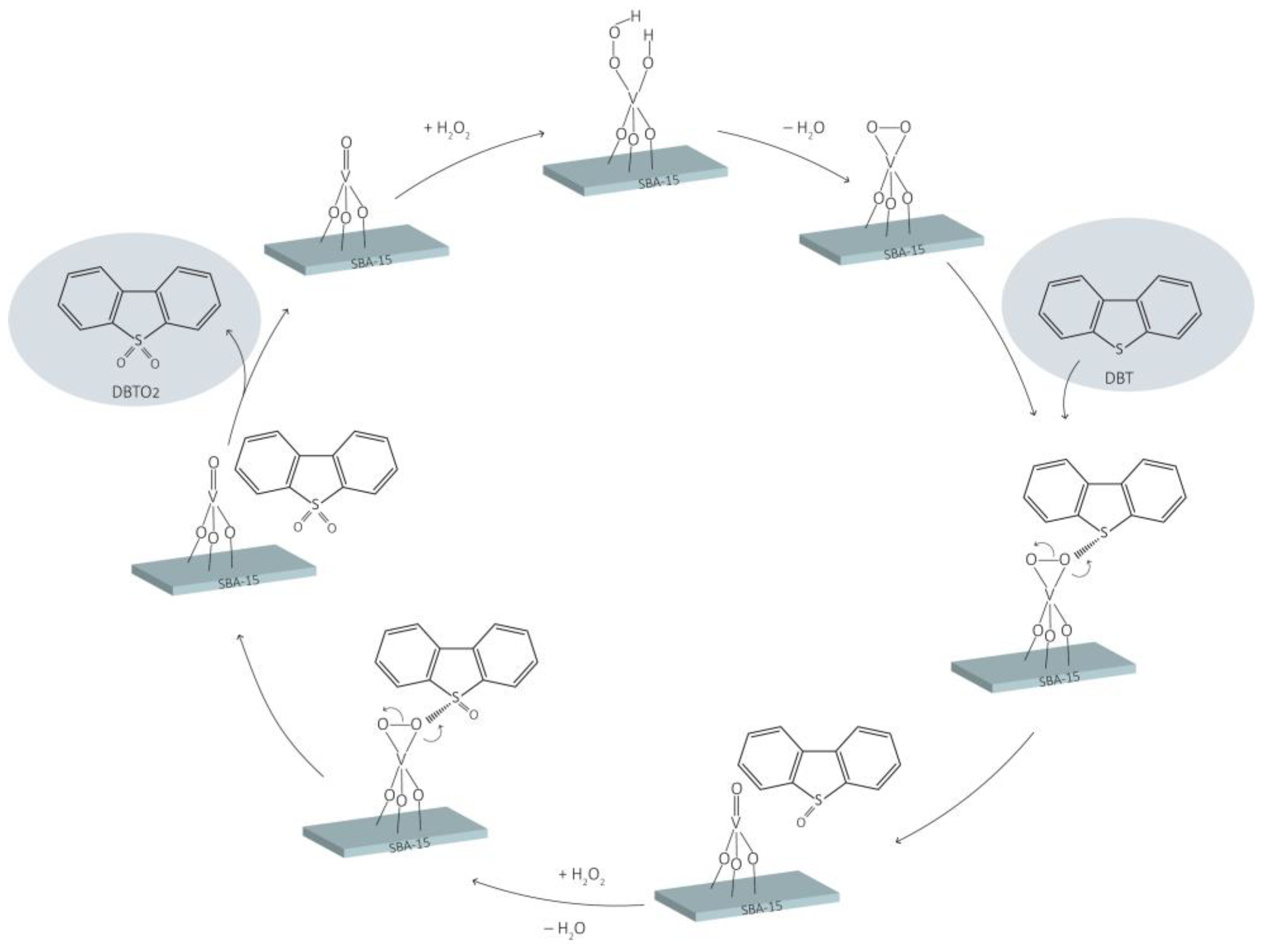
3.2. Catalytic Oxidative Desulphurization of Dibenzothiophene
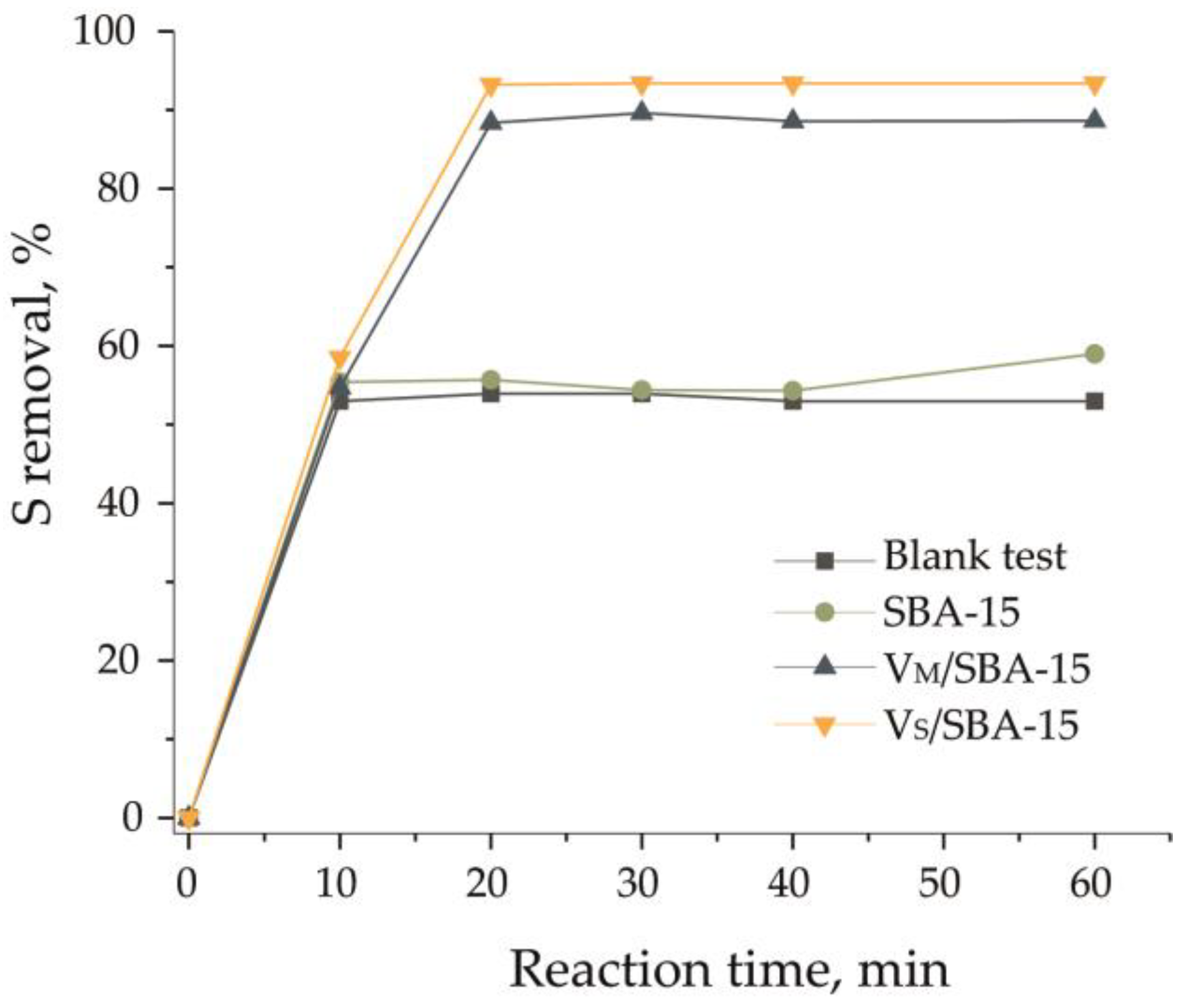
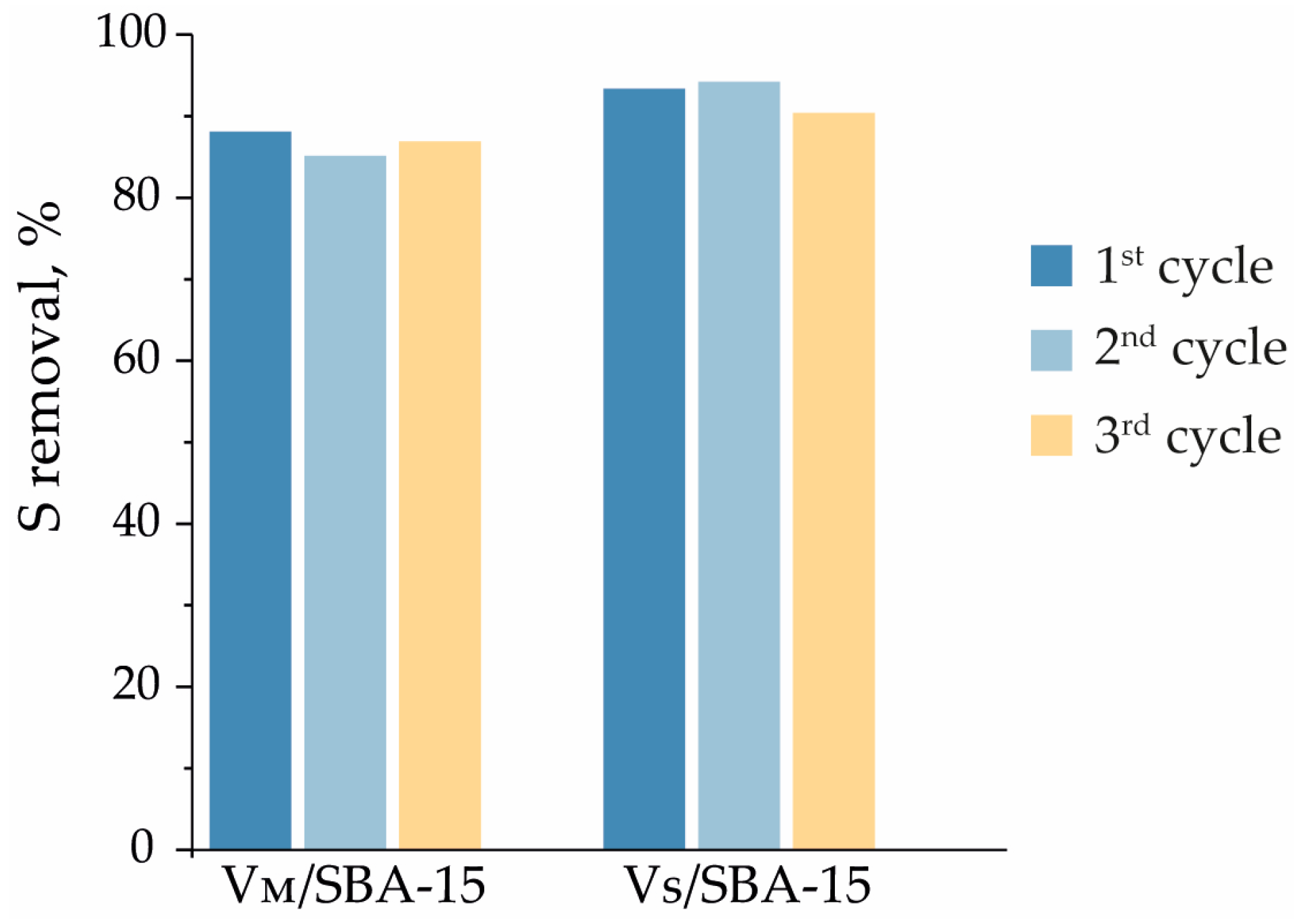
3.3. Extractive Catalytic Oxidative Desulfurization of Dibenzothiophene
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartholomew, C.H.; Farrauto, R.J. Fundamentals of Industrial Catalytic Processes; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-0-471-45713-8. [Google Scholar]
- Appaturi, J.N.; Ratti, R.; Phoon, B.L.; Batagarawa, S.M.; Din, I.U.; Selvaraj, M.; Ramalingam, R.J. A Review of the Recent Progress on Heterogeneous Catalysts for Knoevenagel Condensation. Dalton Trans. 2021, 50, 4445–4469. [Google Scholar] [CrossRef] [PubMed]
- Testa, M.L.; La Parola, V. Sulfonic Acid-Functionalized Inorganic Materials as Efficient Catalysts in Various Applications: A Minireview. Catalysts 2021, 11, 1143. [Google Scholar] [CrossRef]
- Arias, K.S.; Climent, M.J.; Corma, A.; Iborra, S. Chemicals from Biomass: Synthesis of Biologically Active Furanochalcones by Claisen–Schmidt Condensation of Biomass-Derived 5-Hydroxymethylfurfural (HMF) with Acetophenones. Top. Catal. 2016, 59, 1257–1265. [Google Scholar] [CrossRef]
- Bui, T.V.; Umbarila, S.J.; Wang, B.; Sooknoi, T.; Li, G.; Chen, B.; Resasco, D.E. High-Temperature Grafting Silylation for Minimizing Leaching of Acid Functionality from Hydrophobic Mesoporous Silicas Used as Catalysts in the Liquid Phase. Langmuir 2019, 35, 6838–6852. [Google Scholar] [CrossRef] [PubMed]
- Estephane, G.; Lancelot, C.; Blanchard, P.; Dufaud, V.; Chambrey, S.; Nuns, N.; Toufaily, J.; Hamiye, T.; Lamonier, C. W-SBA Based Materials as Efficient Catalysts for the ODS of Model and Real Feeds: Improvement of Their Lifetime through Active Phase Encapsulation. Appl. Catal. A Gen. 2019, 571, 42–50. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Margolese, D.; Melero, J.A.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D. Direct Syntheses of Ordered SBA-15 Mesoporous Silica Containing Sulfonic Acid Groups. Chem. Mater. 2000, 12, 2448–2459. [Google Scholar] [CrossRef]
- Javadli, R.; De Klerk, A. Desulfurization of Heavy Oil-Oxidative Desulfurization (ODS) as Potential Upgrading Pathway for Oil Sands Derived Bitumen. Energy Fuels 2012, 26, 594–602. [Google Scholar] [CrossRef]
- Nurwita, A.; Trejda, M. The Effect of Mesoporous Structure of the Support on the Oxidation of Dibenzothiophene. Int. J. Mol. Sci. 2023, 24, 16957. [Google Scholar] [CrossRef]
- Polikarpova, P.; Akopyan, A.; Shlenova, A.; Anisimov, A. New Mesoporous Catalysts with Brønsted Acid Sites for Deep Oxidative Desulfurization of Model Fuels. Catal. Commun. 2020, 146, 106123. [Google Scholar] [CrossRef]
- Wang, Y.; Du, F.; Wang, C.; Zhao, J.; Sun, H.; Sun, C. The Synthesis and Oxidation Desulfurization Performance of Ti-Modified Hierarchical Cheese-like ZSM-5 Zeolite. J. Chem. Res. 2022, 46, 1–8. [Google Scholar] [CrossRef]
- Rivoira, L.P.; Ledesma, B.C.; Juárez, J.M.; Beltramone, A.R. Novel and Simple One-Pot Method for the Synthesis of TiO2 Modified-CMK-3 Applied in Oxidative Desulfurization of Refractory Organosulfur Compounds. Fuel 2018, 226, 498–507. [Google Scholar] [CrossRef]
- Polikarpova, P.; Akopyan, A.; Shigapova, A.; Glotov, A.; Anisimov, A.; Karakhanov, E. Oxidative Desulfurization of Fuels Using Heterogeneous Catalysts Based on MCM-41. Energy Fuels 2018, 32, 10898–10903. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Q.; Tian, Y.; Cui, J.; Wang, G. Ultra-Deep Oxidative Desulfurization of Fuel with H2O2 Catalyzed by Mesoporous Silica-Supported Molybdenum Oxide Modified by Ce. Appl. Sci. 2021, 11, 2018. [Google Scholar] [CrossRef]
- Teimouri, A.; Mahmoudsalehi, M.; Salavati, H. Catalytic Oxidative Desulfurization of Dibenzothiophene Utilizing Molybdenum and Vanadium Oxides Supported on MCM-41. Int. J. Hydrogen Energy 2018, 43, 14816–14833. [Google Scholar] [CrossRef]
- Akopyan, A.; Polikarpova, P.; Gul, O.; Anisimov, A.; Karakhanov, E. Catalysts Based on Acidic SBA-15 for Deep Oxidative Desulfurization of Model Fuels. Energy Fuels 2020, 34, 14611–14619. [Google Scholar] [CrossRef]
- Rivoira, L.P.; Cussa, J.; Martínez, M.L.; Beltramone, A.R. Experimental Design Optimization of the ODS of DBT Using Vanadium Oxide Supported on Mesoporous Ga-SBA-15. Catal. Today 2020, 349, 68–80. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Guan, T.; Xu, F.; Wu, J.; Zhou, E.; Wang, J.; Li, K. Ultra-deep Oxidative Desulfurization of Model Oil Catalyzed by in Situ Carbon-supported Vanadium Oxides Using Cumene Hydroperoxide as Oxidant. ChemistrySelect 2020, 5, 2148–2156. [Google Scholar] [CrossRef]
- Ramos, J.M.; Wang, J.A.; Flores, S.O.; Chen, L.; Arellano, U.; Noreña, L.E.; González, J.; Navarrete, J. Ultrasound-Assisted Hydrothermal Synthesis of V2O5/Zr-SBA-15 Catalysts for Production of Ultralow Sulfur Fuel. Catalysts 2021, 11, 408. [Google Scholar] [CrossRef]
- Stawicka, K.; Gierada, M.; Gajewska, J.; Tielens, F.; Ziolek, M. The Importance of Residual Water for the Reactivity of MPTMS with Silica on the Example of SBA-15. Appl. Surf. Sci. 2020, 513, 145802. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.; Wan, H.; Kong, Y.; Wu, X.; Dong, L.; Li, B.; Chen, Y. The States of Vanadium Species in V-SBA-15 Synthesized under Different PH Values. Microporous Mesoporous Mater. 2008, 110, 508–516. [Google Scholar] [CrossRef]
- Bulánek, R.; Čapek, L.; Setnička, M.; Čičmanec, P. DR UV-Vis Study of the Supported Vanadium Oxide Catalysts. J. Phys. Chem. C 2011, 115, 12430–12438. [Google Scholar] [CrossRef]
- Fornés, V.; López, C.; López, H.H.; Martínez, A. Catalytic Performance of Mesoporous VOx/SBA-15 Catalysts for the Partial Oxidation of Methane to Formaldehyde. Appl. Catal. A Gen. 2003, 249, 345–354. [Google Scholar] [CrossRef]
- Keller, D.E.; Visser, T.; Soulimani, F.; Koningsberger, D.C.; Weckhuysen, B.M. Hydration Effects on the Molecular Structure of Silica-Supported Vanadium Oxide Catalysts: A Combined IR, Raman, UV-Vis and EXAFS Study. Vib. Spectrosc. 2007, 43, 140–151. [Google Scholar] [CrossRef]
- Held, A.; Kowalska-Kuś, J.; Nowińska, K.; Góra-Marek, K. MCF Material as an Attractive Support for Vanadium Oxide Applied as a Catalyst for Propene Epoxidation with N2O. Catal. Lett. 2018, 148, 2058–2068. [Google Scholar] [CrossRef]
- Nitsche, D.; Hess, C. Structure of Isolated Vanadia and Titania: A Deep UV Raman, UV-Vis, and IR Spectroscopic Study. J. Phys. Chem. C 2016, 120, 1025–1037. [Google Scholar] [CrossRef]
- Zhao, W.; Zhong, Q.; Pan, Y.; Zhang, R. Systematic Effects of S-Doping on the Activity of V2O5/TiO2 Catalyst for Low-Temperature NH3-SCR. Chem. Eng. J. 2013, 228, 815–823. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Popova, G.Y.; Chesalov, Y.A.; Saraev, A.A.; Zemlyanov, D.Y.; Beloshapkin, S.A.; Knop-Gericke, A.; Schlögl, R.; Andrushkevich, T.V.; Bukhtiyarov, V.I. Selective Oxidation of Methanol to Form Dimethoxymethane and Methyl Formate over a Monolayer V2O5/TiO2 Catalyst. J. Catal. 2014, 311, 59–70. [Google Scholar] [CrossRef]
- Gao, Y.; Thevuthasan, S.; Mccready, D.E.; Engelhard, M. MOCVD Growth and Structure of Nb-and V-Doped TiO Films on Sapphire. J. Cryst. Growth 2000, 212, 178–190. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS Binding Energies for Different Vanadium Oxidation States (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Vieira, L.H.; Lopez-Castillo, A.; Jones, C.W.; Martins, L. Exploring the Multifunctionality and Accessibility of Vanadosilicates to Produce Acrylic Acid in One-Pot Glycerol Oxydehydration. Appl. Catal. A Gen. 2020, 602, 117687. [Google Scholar] [CrossRef]
- Zou, J.; Lin, Y.; Yang, C. Covalency Triggers High Catalytic Activity of Amorphous Molybdenum Oxides for Oxidative Desulfurization. Sci. China Chem. 2023, 66, 1211–1220. [Google Scholar] [CrossRef]
- Caero, L.C.; Hernández, E.; Pedraza, F.; Murrieta, F. Oxidative Desulfurization of Synthetic Diesel Using Supported Catalysts: Part I. Study of the Operation Conditions with a Vanadium Oxide Based Catalyst. Catal. Today 2005, 107–108, 564–569. [Google Scholar] [CrossRef]
- Hosseini, A.; Alavi, S.M.; Bazyari, A.; Valaei, A. Exploring the Impact of Competitive Compounds and Catalyst Synthesis Method in DBT Oxidative Desulfurization Using MoO3-V2O5/Al2O3 Catalyst. Environ. Sci. Pollut. Res. Int. 2024, 31, 6332–6349. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Najafi Chermahini, A.; Kasiri Baboukani, Z. Oxidative Desulfurization of Real and Model Fuel Using Vanadium–Chromium Bimetallic Catalysts Supported on KIT-6. Res. Chem. Intermed. 2024, 50, 597–624. [Google Scholar] [CrossRef]
- Ogunlaja, A.S.; Hosten, E.C.; Tshentu, Z.R. The Oxidation of Dibenzothiophene Using Oxidovanadium(IV)-Containing Nanofibres as Catalyst. S. Afr. J. Chem. 2015, 68, 172–180. [Google Scholar] [CrossRef]
- Rivoira, L.; Martínez, M.L.; Anunziata, O.; Beltramone, A. Vanadium Oxide Supported on Mesoporous SBA-15 Modified with Al and Ga as a Highly Active Catalyst in the ODS of DBT. Microporous Mesoporous Mater. 2017, 254, 96–113. [Google Scholar] [CrossRef]

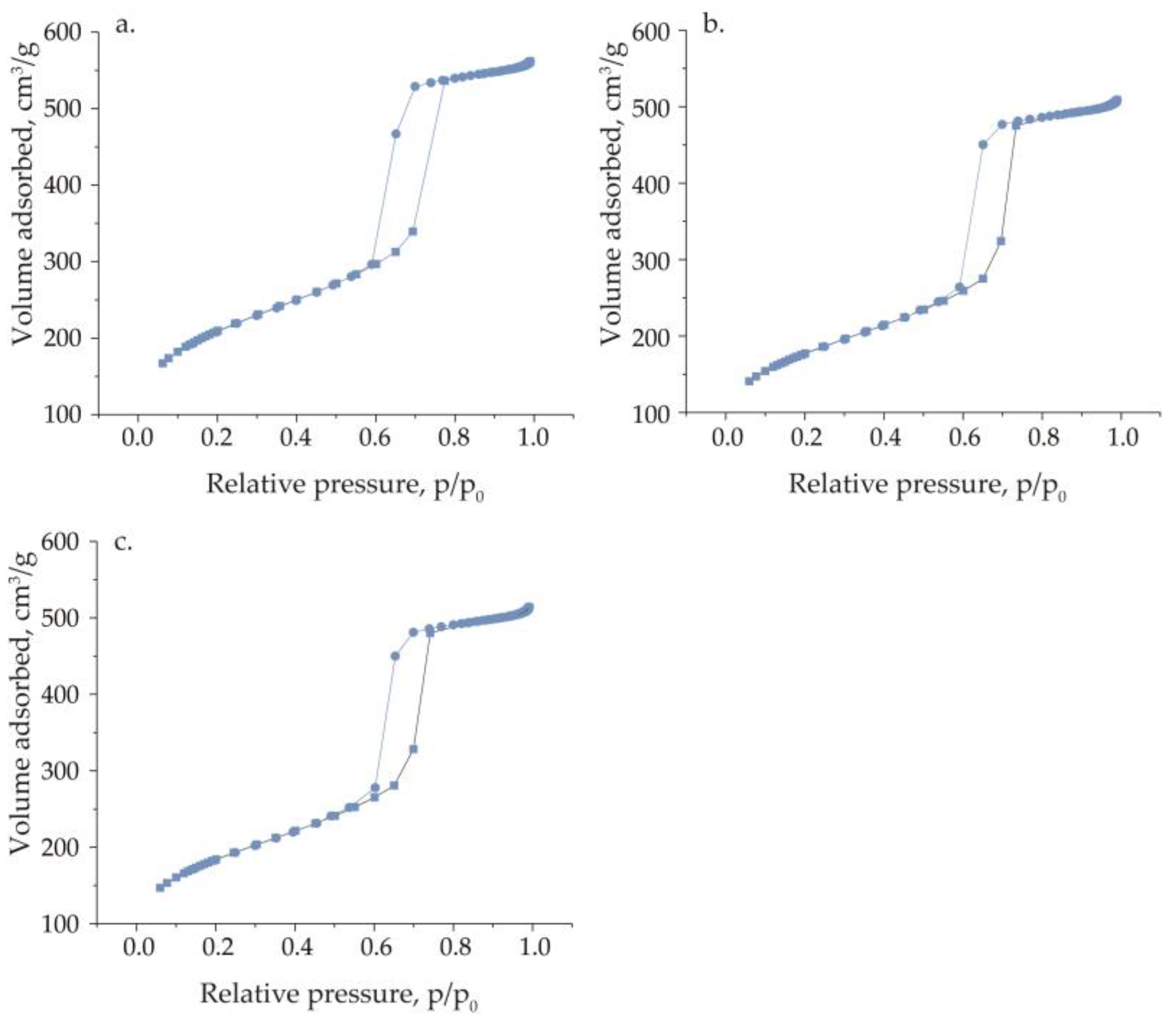
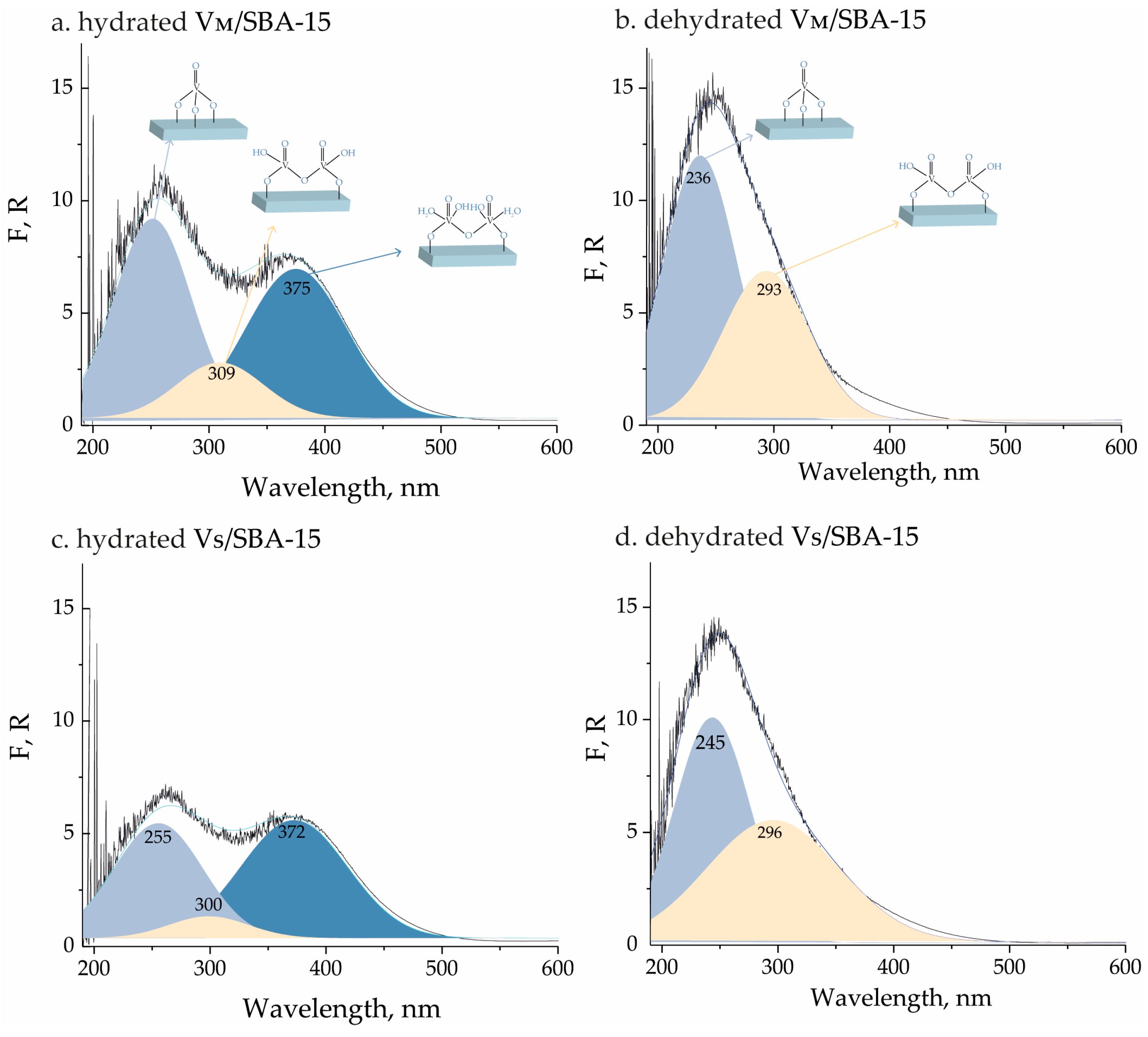
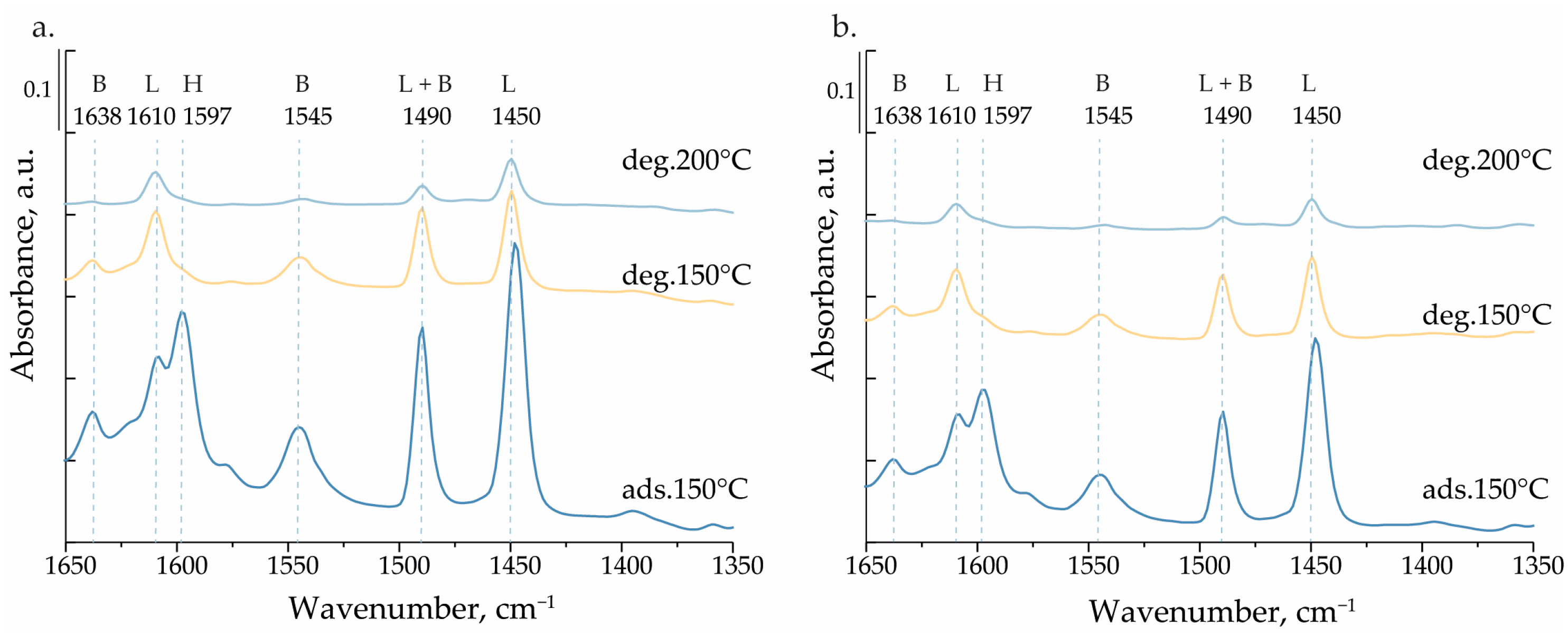

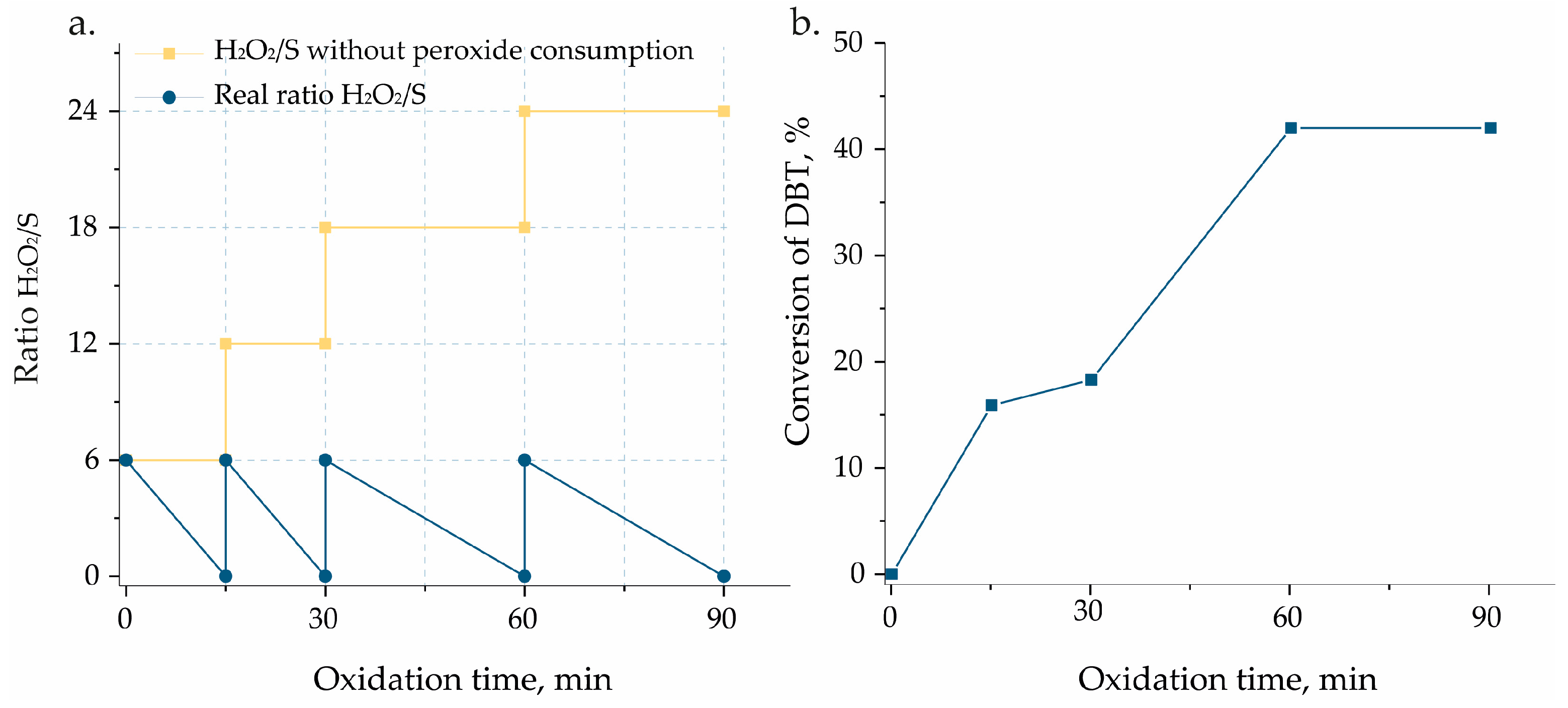
| Catalyst | BET, (m² g−1) | Pore Volume, (cm³ g−1) | SMicro, (m² g−1) a | PSDDFT, (nm) b | V Content, (wt.%) c |
|---|---|---|---|---|---|
| SBA-15 | 762 | 0.83 | 176 | 10.1 | - |
| VM/SBA-15 | 642 | 0.77 | 141 | 9.6 | 1.42 |
| VS/SBA-15 | 667 | 0.77 | 157 | 9.6 | 1.12 |
| BE, eV | VM/SBA-15 | VS/SBA-15 |
|---|---|---|
| V5+ | - | 517.7 (10.8%) |
| V4+ | 516.9 (58.4%) | 516.6 (89.2%) |
| V3+ | 515.0 (41.6%) | - |
| LAS, μmol/g | BAS, μmol/g | |||
|---|---|---|---|---|
| 150 °C | 200 °C | 150 °C | 200 °C | |
| VM/SBA-15 | 49 | 21 | 35 | 6 |
| VS/SBA-15 | 40 | 14 | 26 | 4 |
| Catalyst | CODS Catalyst + H2O2 | EODS Catalysts + MeCN | ECODS Catalyst + MeCN + H2O2 |
|---|---|---|---|
| Blank | 3 | 53 | 53 |
| SBA-15 | 0 | 55 | 55 |
| VM/SBA-15 | 26 | 55 | 88 |
| VS/SBA-15 | 18 | 59 | 90 |
| Catalyst | V Source | H2O2/S | Time, min | Temp., °C | S Removal, % | Ref. |
|---|---|---|---|---|---|---|
| VOx-Ga-SBA-15 (4 wt.% Ga, 6 wt.% V) | VCl3 | 5 | 15 | 60 | 98 | [18] |
| VOx-Ga-SBA-15 (0 wt.% Ga, 6 wt.% V) | VCl3 | 5 | 15 | 60 | 65 | [18] |
| 10% V2O5/SBA-15 | NH4VO3 | 10 | 20 | 60 | 75 | [20] |
| 10% V2O5/Zr-SBA-15 | NH4VO3 | 10 | 20 | 60 | 90 | [20] |
| VOx-Ga-SBA-15 (1/30) | VCl3 | 6 | 15 | 60 | 99 | [39] |
| VOx-SBA-15 (1/30) | VCl3 | 6 | 15 | 60 | 99 | [39] |
| V-SBA-15 (1/30) | VCl3 | 6 | 15 | 60 | 85 | [39] |
| VM/SBA-15 | NH4VO3 | 6 | 20 | 60 | 88 | This work |
| VS/SBA-15 | VOSO4 | 6 | 20 | 60 | 93 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurwita, A.; Stawicka, K.; Trejda, M. SBA-15 Type Mesoporous Silica Modified with Vanadium as a Catalyst for Oxidative and Extractive Oxidative Desulfurization Processes. Materials 2024, 17, 4041. https://doi.org/10.3390/ma17164041
Nurwita A, Stawicka K, Trejda M. SBA-15 Type Mesoporous Silica Modified with Vanadium as a Catalyst for Oxidative and Extractive Oxidative Desulfurization Processes. Materials. 2024; 17(16):4041. https://doi.org/10.3390/ma17164041
Chicago/Turabian StyleNurwita, Ardian, Katarzyna Stawicka, and Maciej Trejda. 2024. "SBA-15 Type Mesoporous Silica Modified with Vanadium as a Catalyst for Oxidative and Extractive Oxidative Desulfurization Processes" Materials 17, no. 16: 4041. https://doi.org/10.3390/ma17164041








