Evaluation of the Impact of Parylene C Deposition Method on the Functional Properties of Fabrics
Abstract
:1. Introduction
- surface properties (especially hydrophobicity), which may be important when using textiles in special clothing (e.g., jackets, coats, uniforms for firefighters, rescue), special equipment (e.g., backpacks, tarpaulins), packaging used in high-humidity conditions (e.g., during transport).
- barrier properties protecting against the harmful effects of liquid chemicals (e.g., acids and bases), which may be important when using textiles in protective clothing or packaging used in places where these substances occur (e.g., chemical or medical laboratories).
- electrostatic properties, which is important when using these textiles in areas where there is a risk of explosion by means of uncontrolled charge transfer in the form of a spark (e.g., gas stations, mills, sawmills, mines and others).
- properties affecting heat and mass transfer through them, affecting the use of textiles as a barrier between environments with different temperatures and pressures. When used in clothing and footwear, these properties have a significant impact on ensuring the heat balance between a person and the environment in which he or she lives and protecting him or her from hypothermia (in cold environments) or from overheating (in hot environments). In other applications (e.g., packaging), these properties may contribute to ensuring the thermal safety of packaged products.
- flammability properties that may be important for textiles that may come into direct contact with flame (e.g., firefighting, rescue and clothing and equipment for metallurgical industry).
2. Materials and Methods
2.1. Materials
2.2. Methods
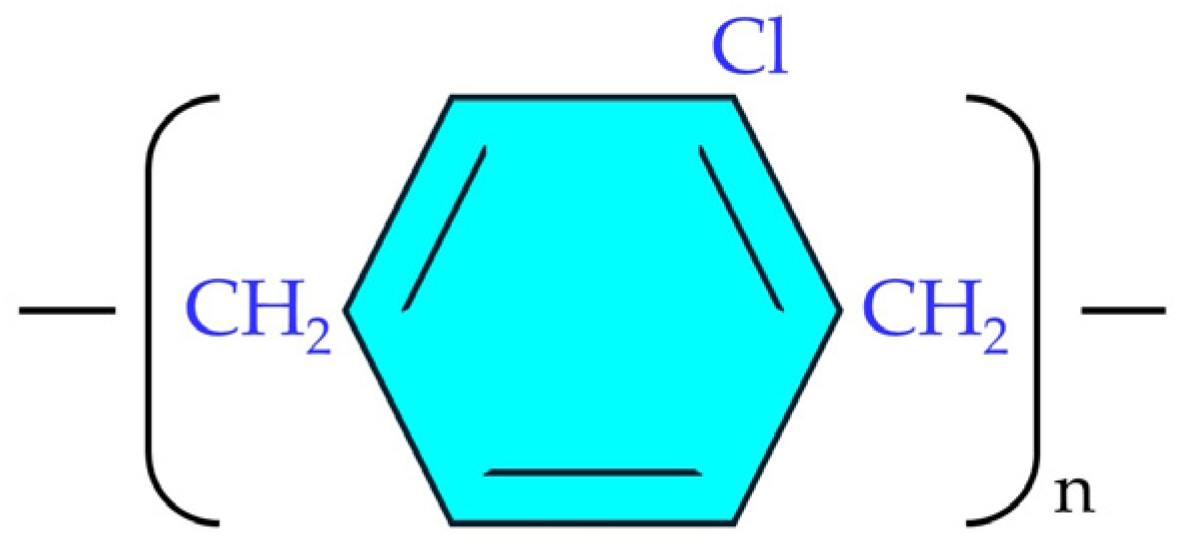
2.2.1. Parylene C Coating Method
2.2.2. The Influence of Coating on Structural Properties of Fabrics
X-ray Computed Tomography, Micro-CT
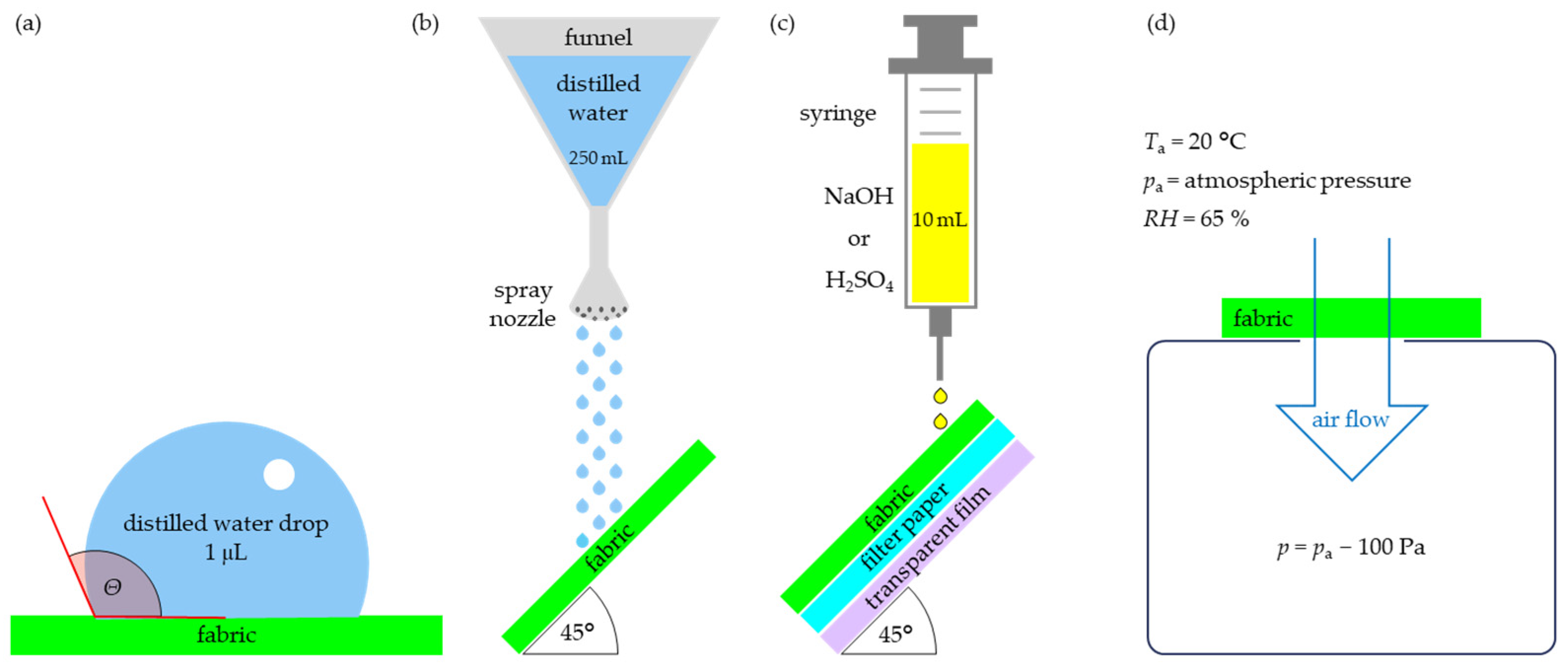
2.2.3. The Influence of Coating on the Wettability and Tightness of Fabrics
Contact Angle
Spray Test
Chemical Liquids Penetration Test
Air Permeability
2.2.4. The Influence of Coating on Electrostatic Properties of Fabrics
Charge Decay, Surface Resistance and Vertical Resistance
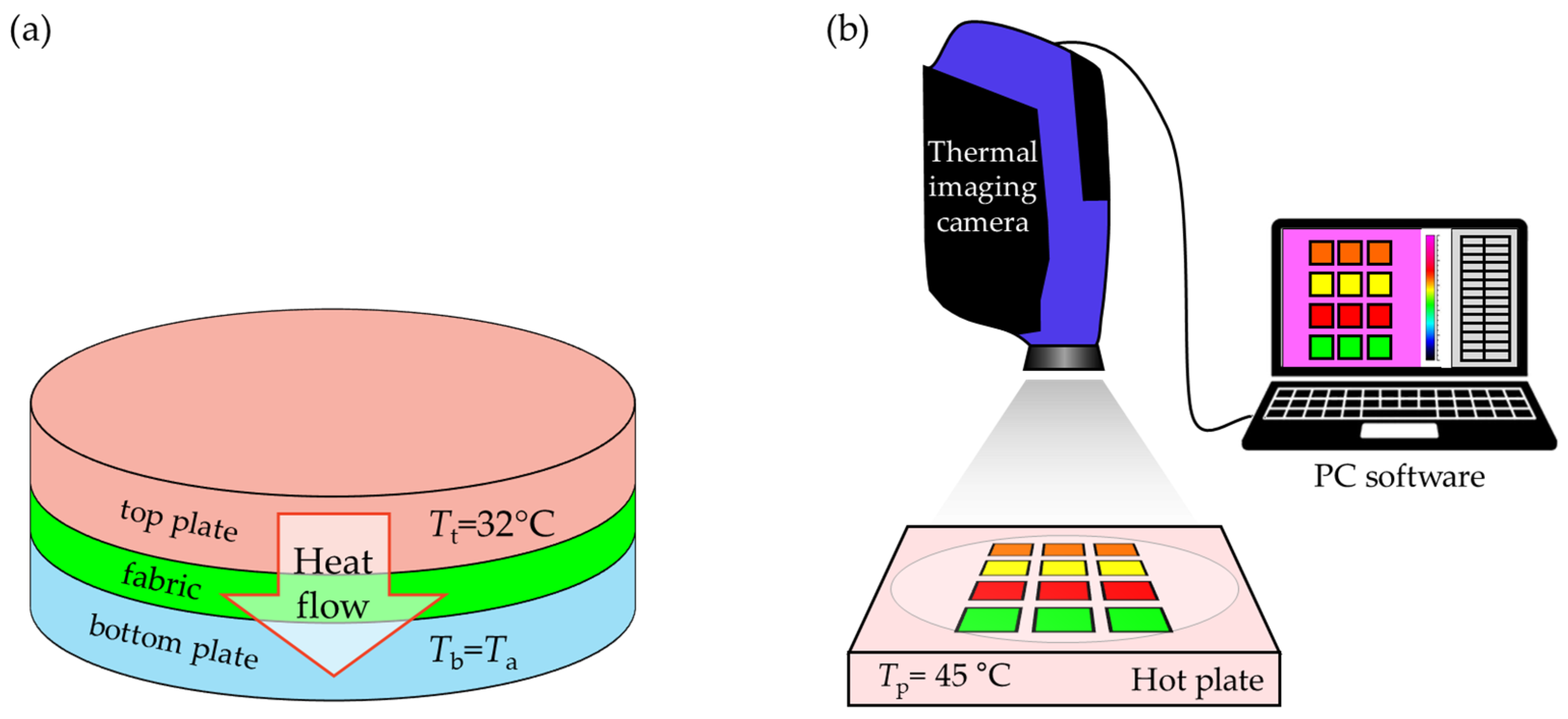
2.2.5. The Influence of Coating on Thermal Insulation Properties of Fabrics
Thermal Conductivity Coefficient
Thermal Imaging
2.2.6. The Influence of Coating on Flammability of Fabrics
Limiting Oxygen Index
3. Results and Discussion
3.1. The Influence of Coating on Structural Properties of Fabrics
3.2. The Influence of Coating on the Wettability and Tightness of Fabrics
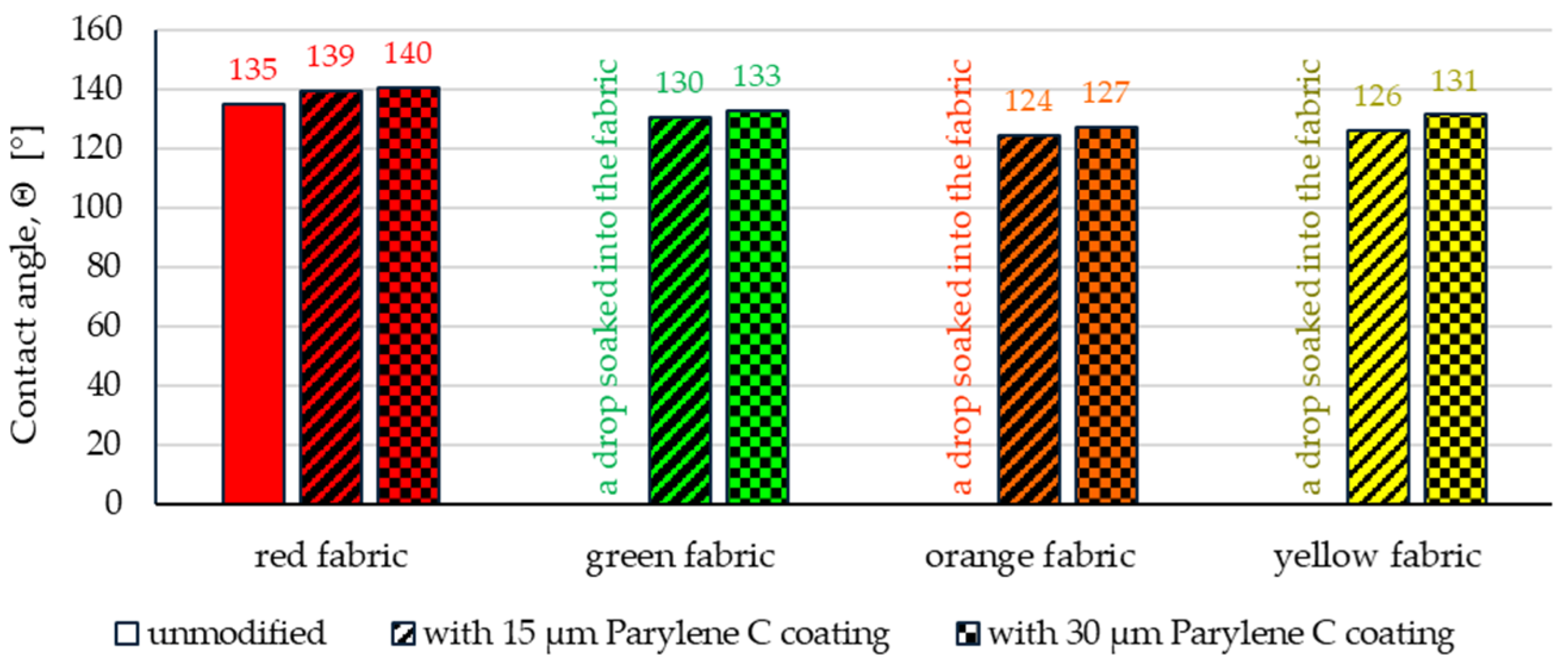
3.2.1. Contact Angle
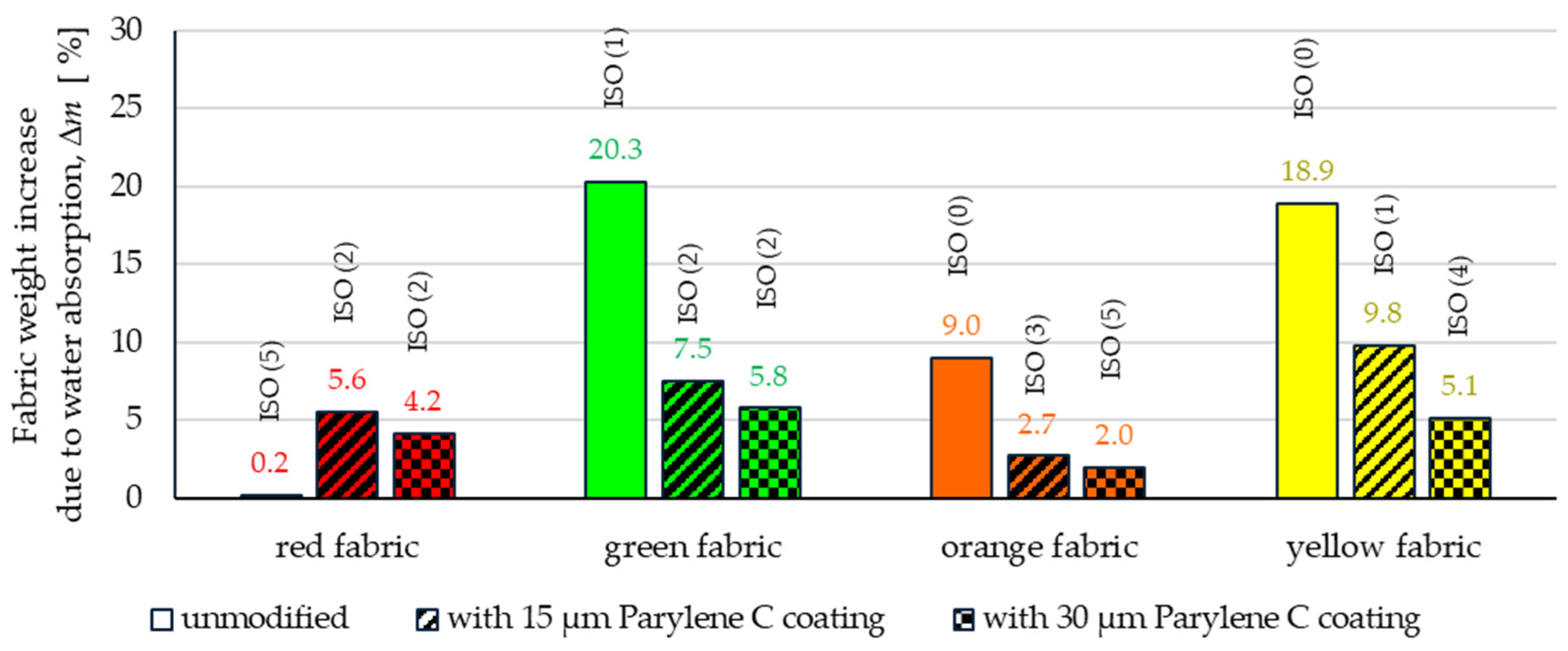
3.2.2. Spray Test
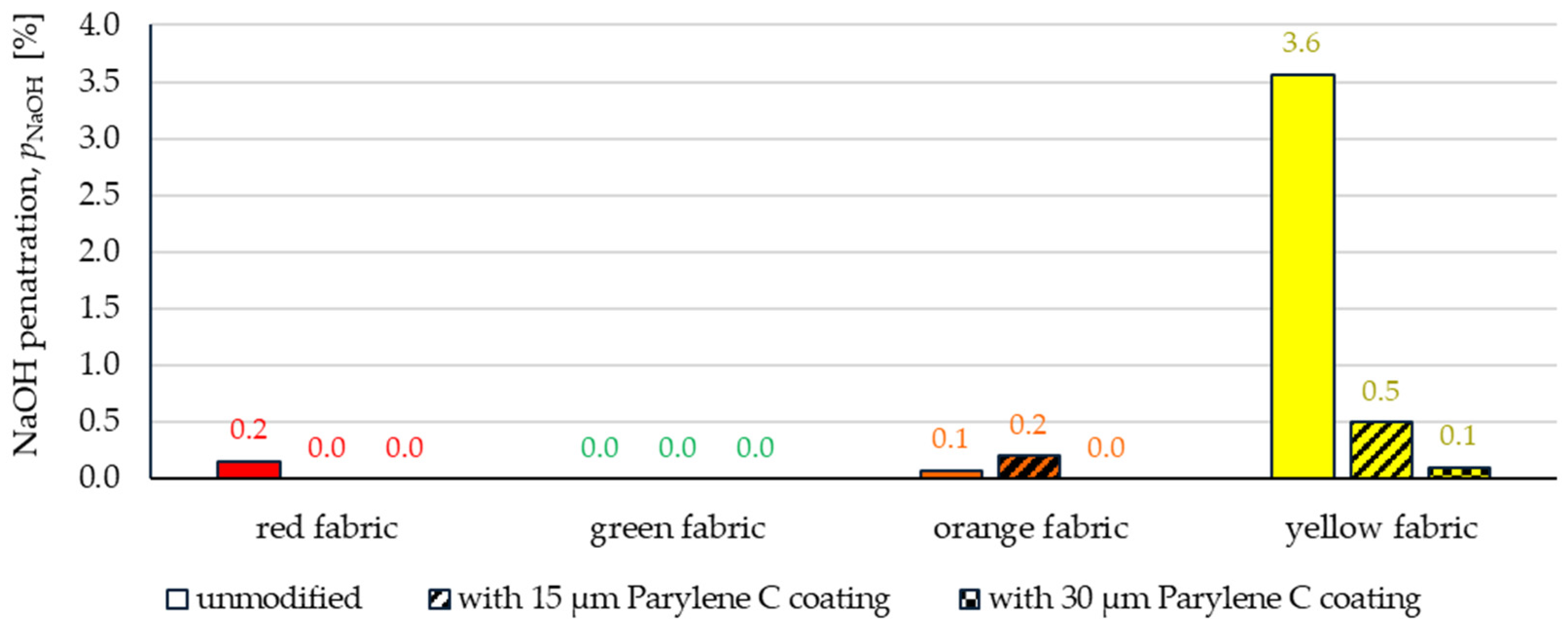
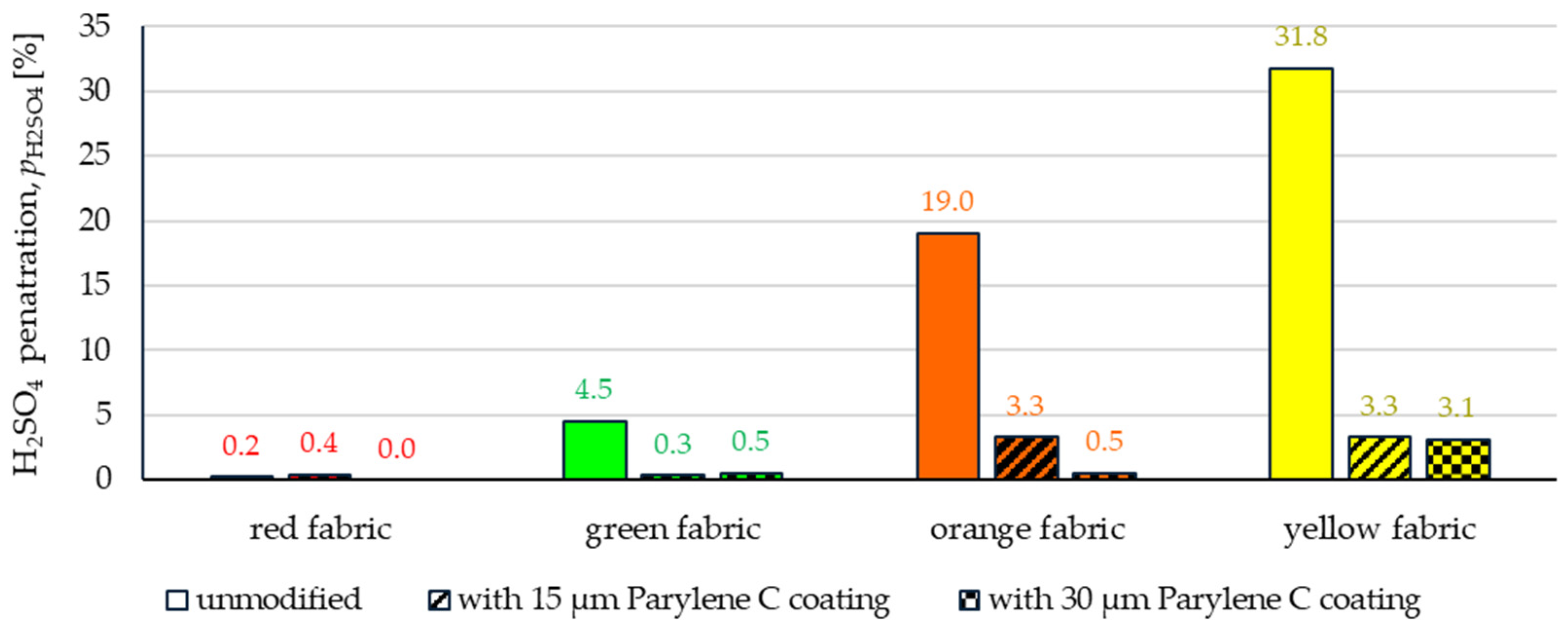
3.2.3. Chemical Liquids Penetration Test
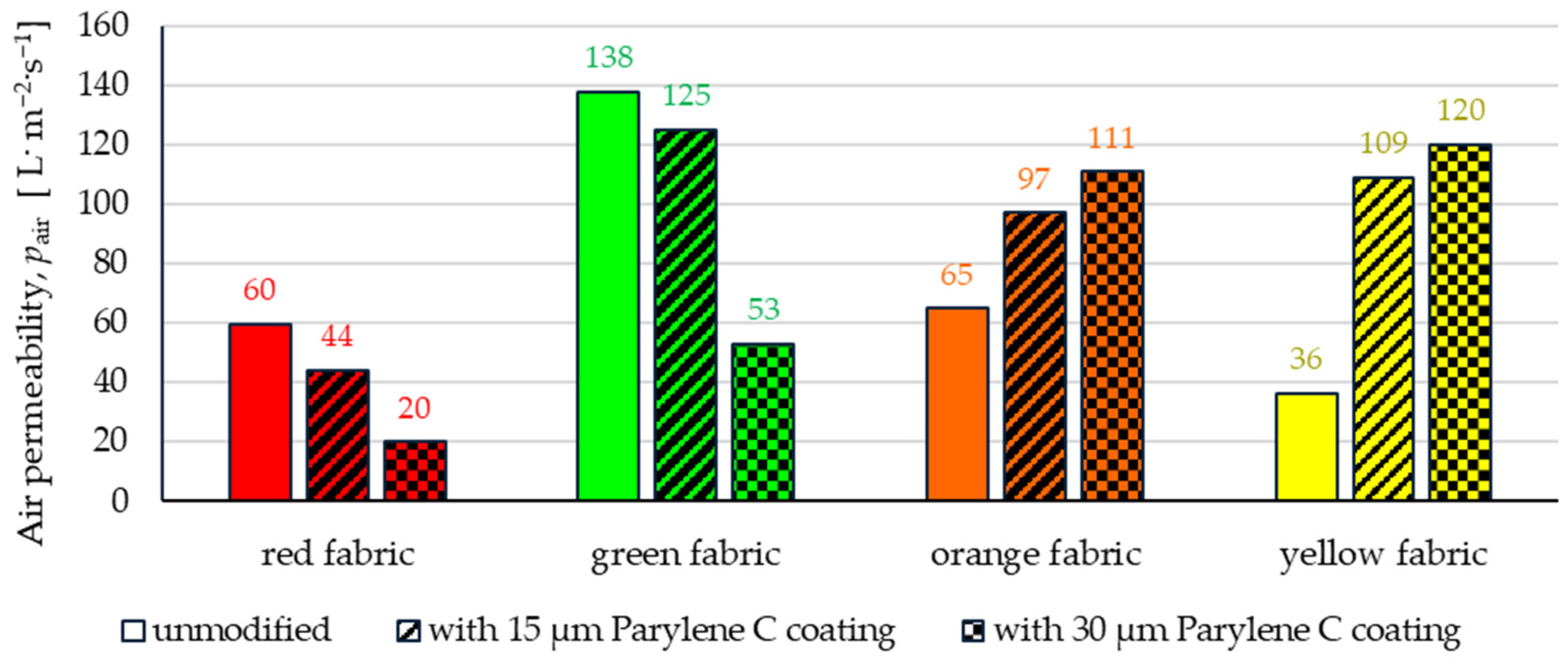
3.2.4. Air Permeability
3.3. The Influence of Coating on Electrostatic Properties of Fabrics
3.3.1. Charge Decay
3.3.2. Surface and Vertical Resistance
3.4. The Influence of Coating on Thermal Insulation Properties of Fabrics
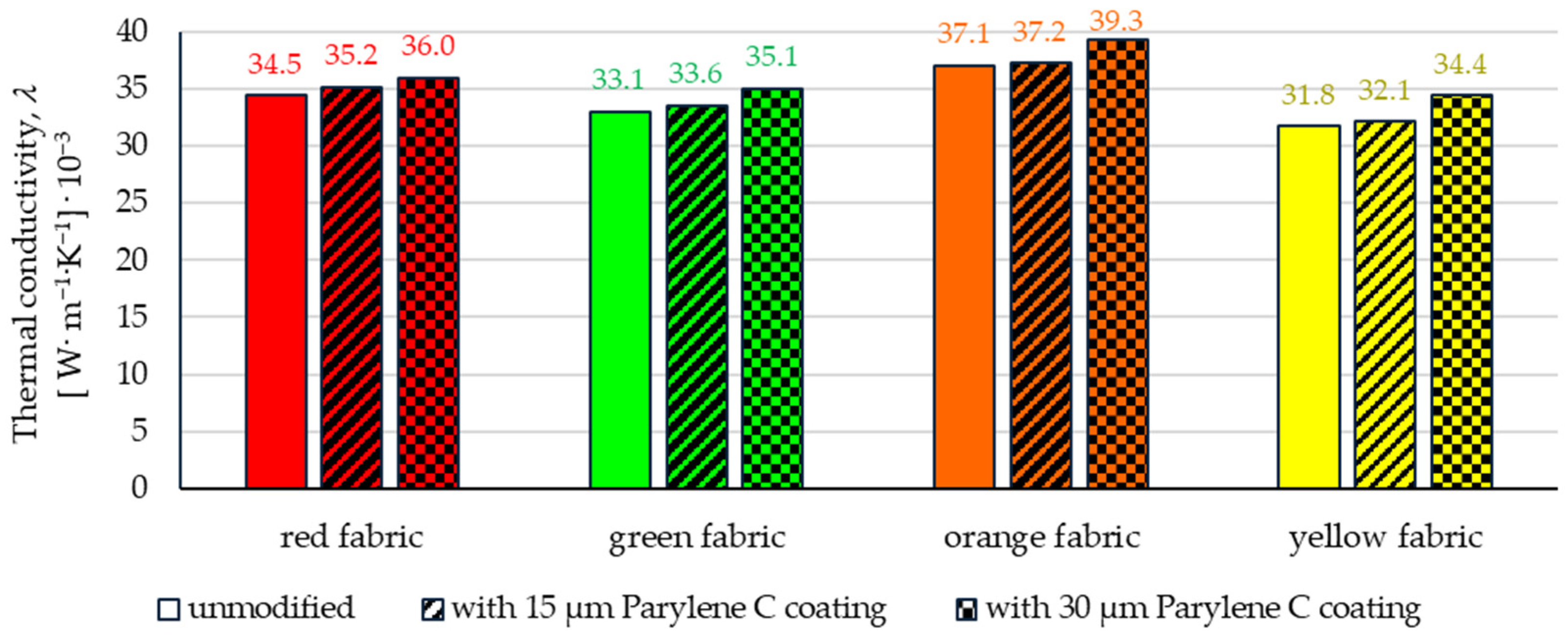
3.4.1. Thermal Conductivity Coefficient
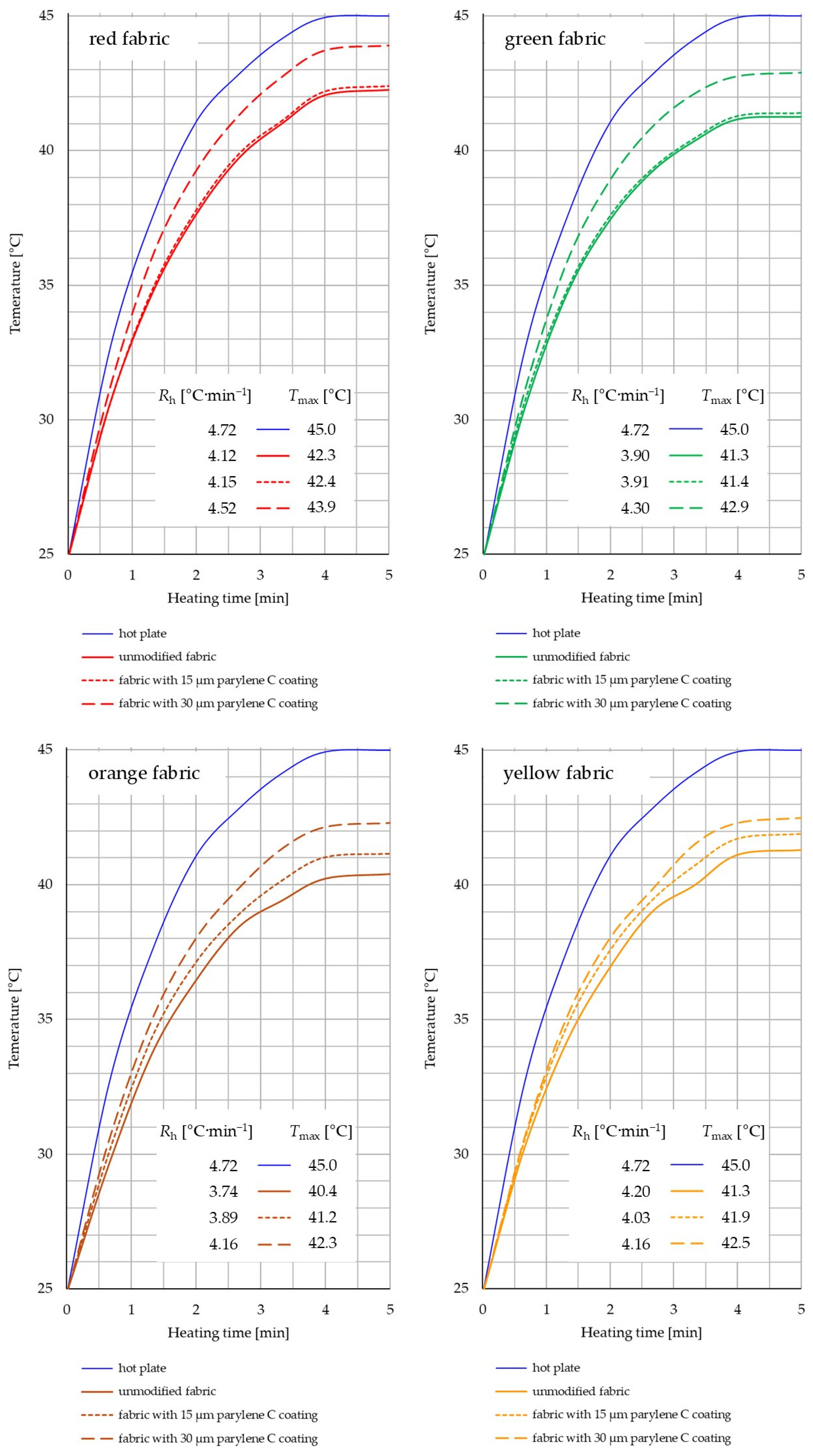
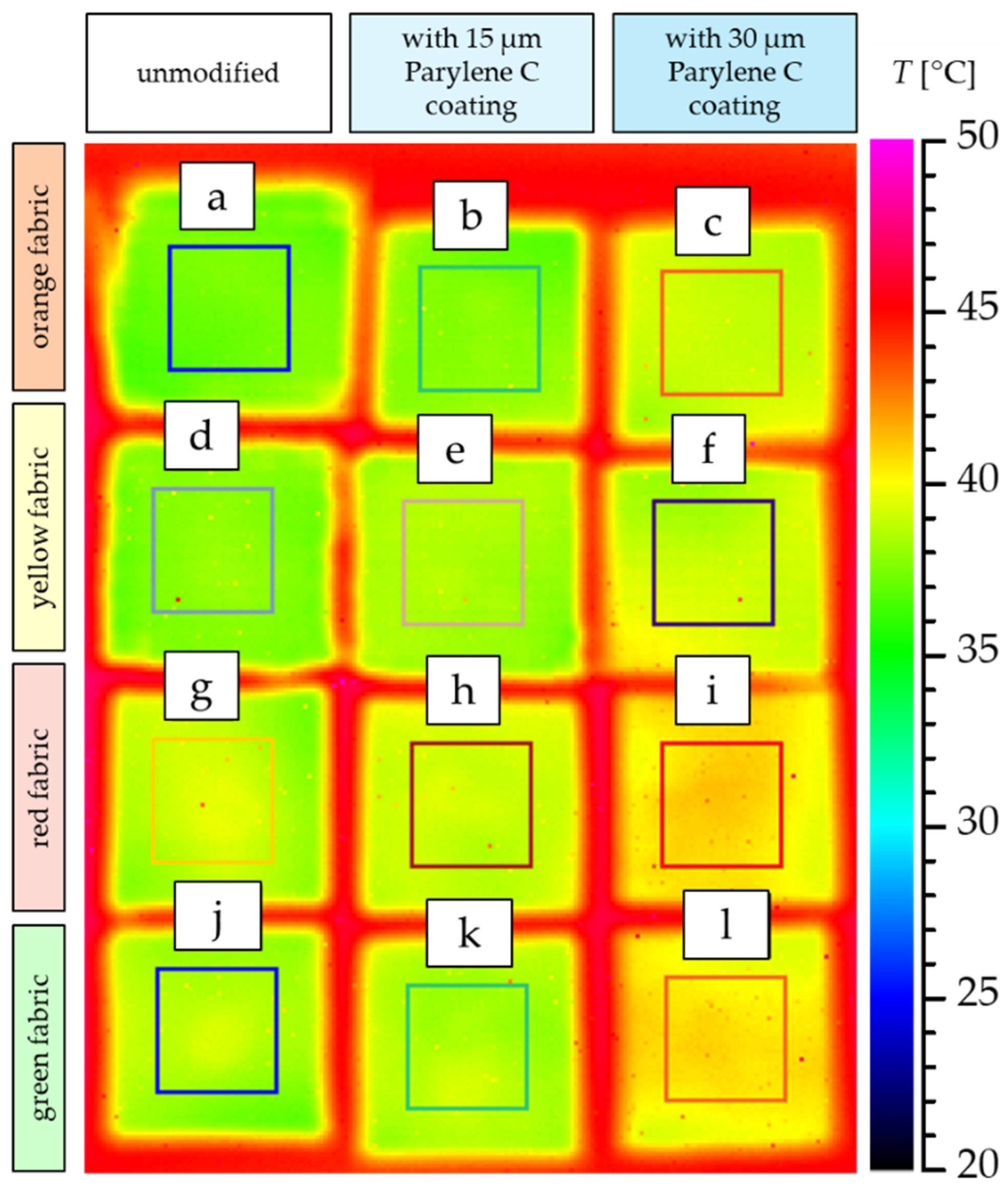
3.4.2. Thermal Imaging
3.5. The Influence of Coating on Flammability of Fabrics
4. Conclusions
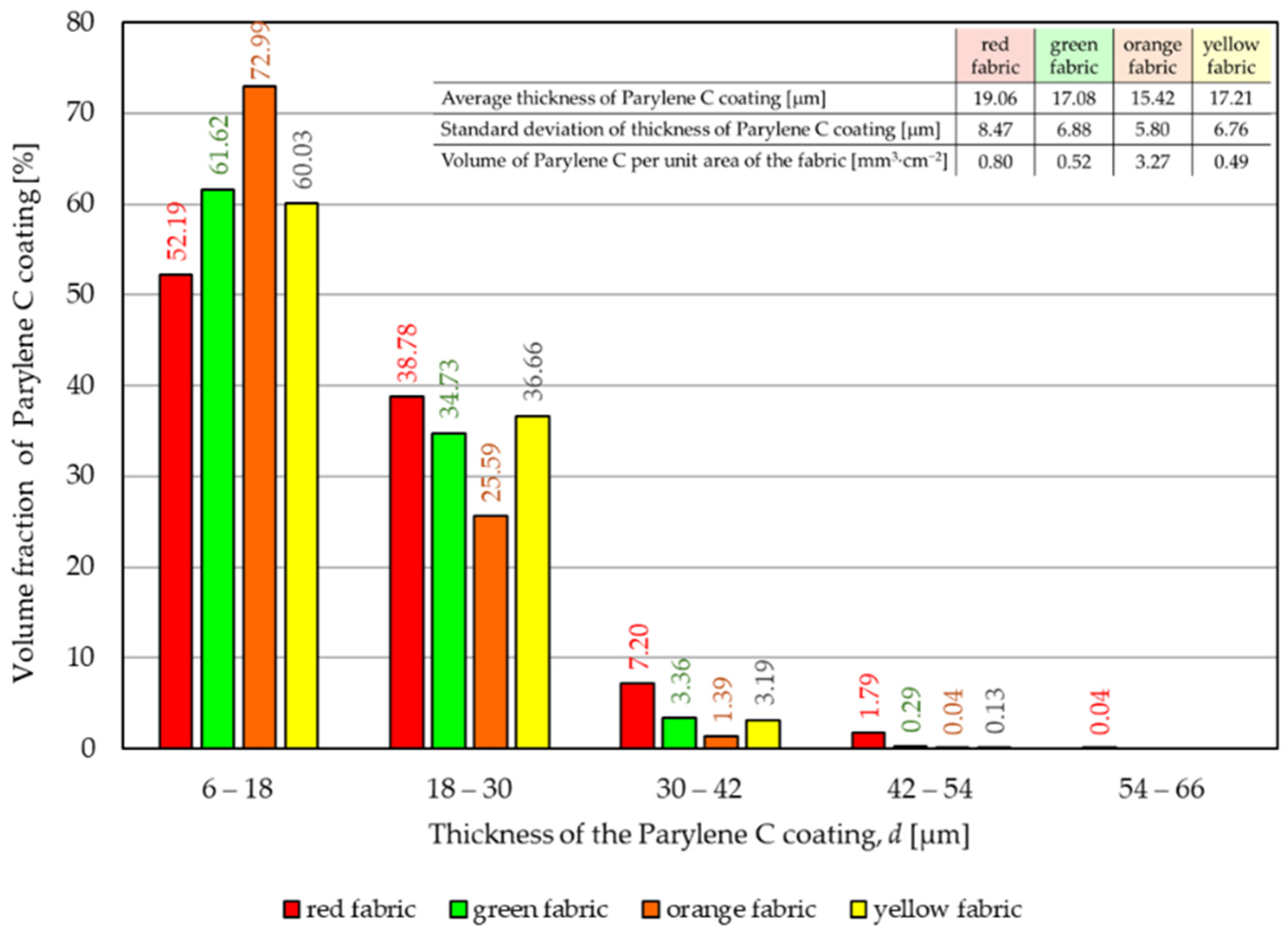
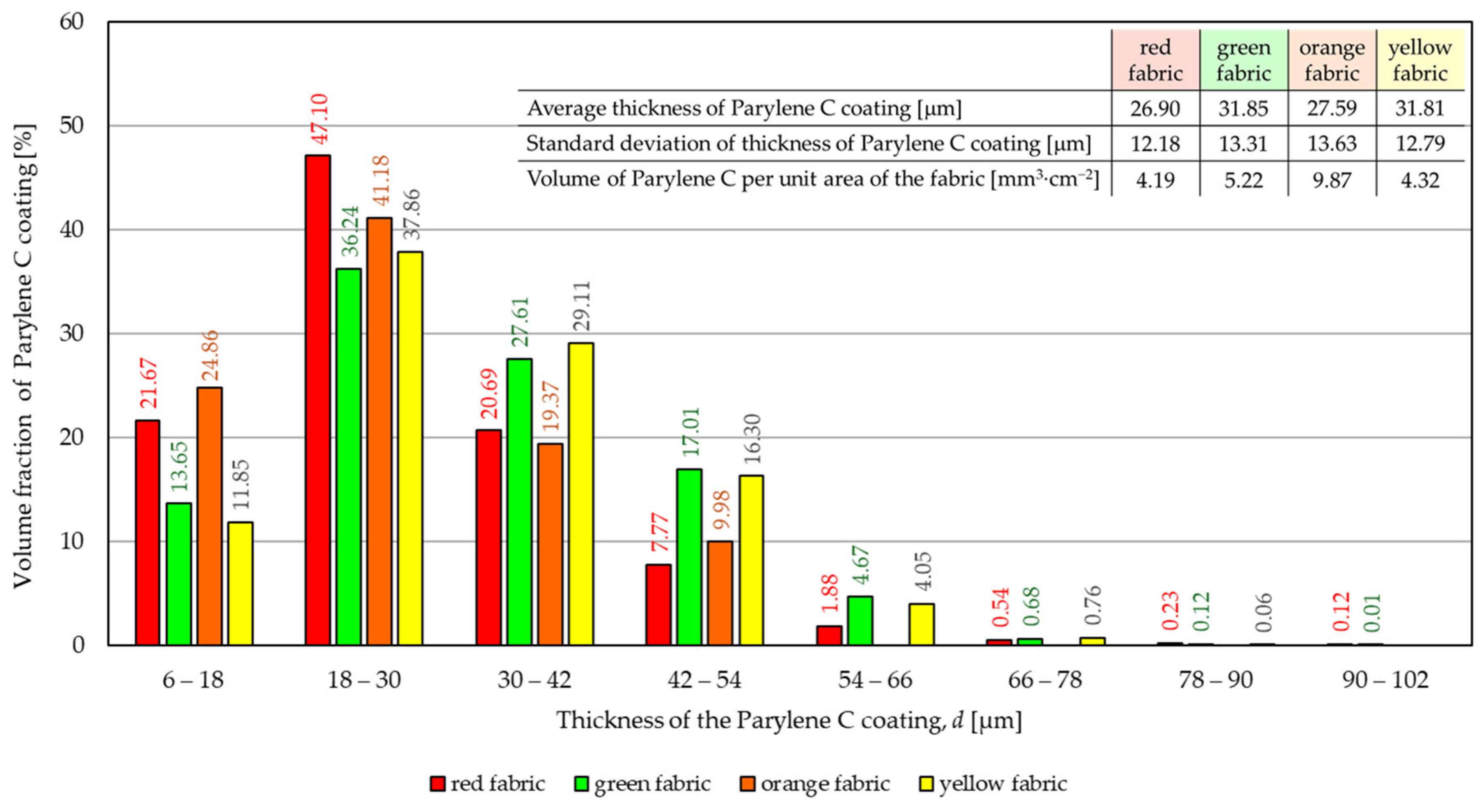
- According to the results of micro-CT analysis, the Parylene C deposition method used is a controlled process in terms of coating thickness on textile substrates. Despite the complicated geometric structure of textile substrates, the results confirmed that both assumed average thicknesses of Parylene C coatings on fabrics (15 μm and 30 μm) were similar to the actual ones. Larger differences between the assumed and actual average thickness of the Parylene C coating were observed in the case of deposition of the thinner coating.
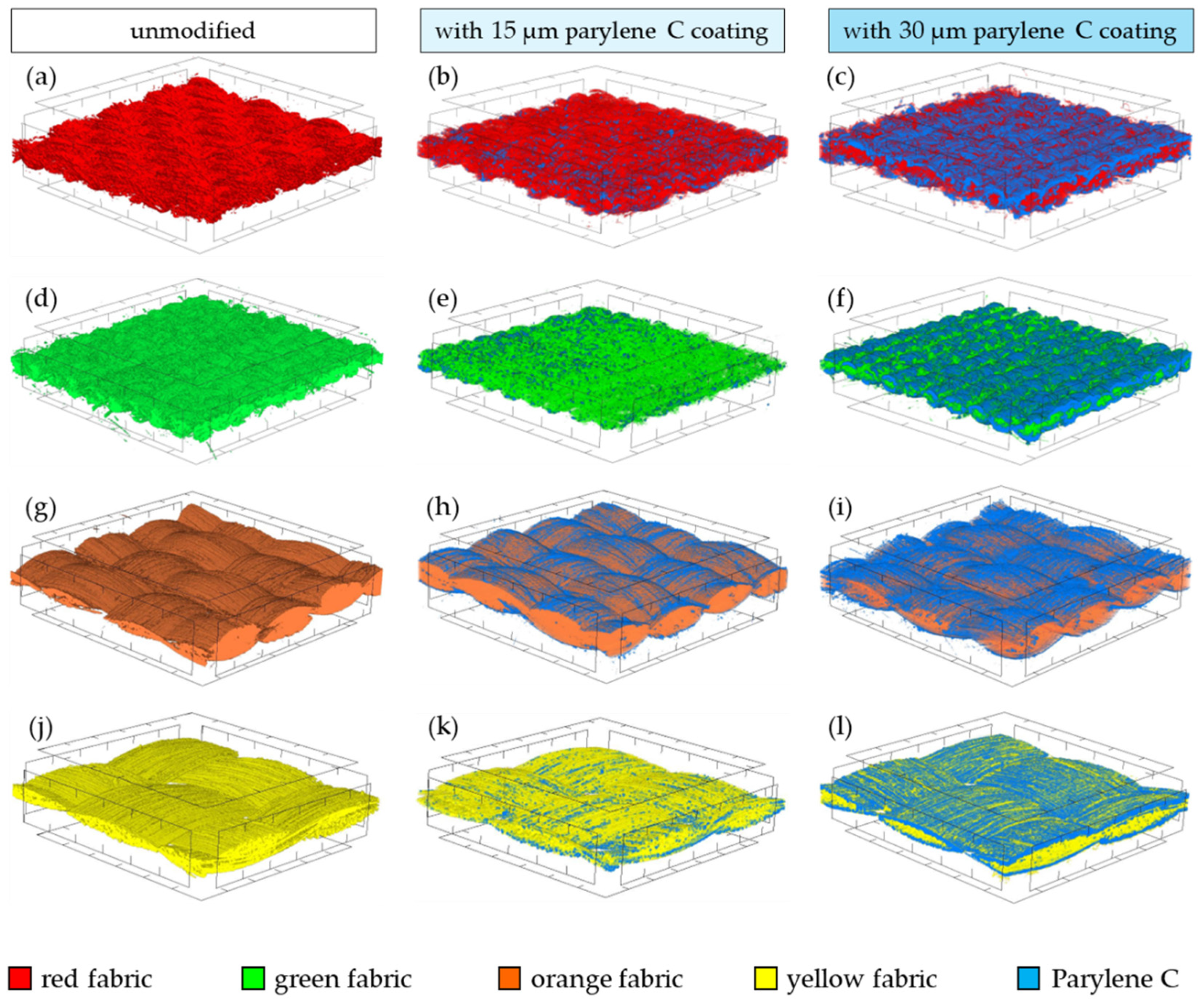
- Micro-CT 3D visualizations of fabrics (Figure 11) showed that in the case of deposition of a 15 μm Parylene C coating on the fabric surface, the coating is discontinuous because a significant part of Parylene C penetrates the fabrics’ structure, especially those characterized by higher porosity (Figure 9). Deposition of a thicker coating of Parylene C (30 μm) results in the formation of a more extensive and continuous layer on the surface of fabrics, especially those made of filament yarns.
- Wetting tests showed that the deposition of even a thinner coating of Parylene C (15 μm) gives the fabrics’ surface a hydrophobic character (contact angle values range from 124° to 139°). The highest contact angle (139°) was found for the red fabric, which is due to the fact that it was hydrophobic-finished before modification with Parylene C. The spray test results (Figure 14) showed that with the exception of this red hydrophobic-finished fabric, the deposited Parylene C coatings cause significantly lower water absorption by fabrics compared to unmodified fabrics (water absorption is lower by 71% to 78% depending on the fabric). Generally speaking, applied deposition of Parylene C is an effective method of hydrophobizing the fabrics’ surfaces, including fabrics with a high water wettability. The results of chemical liquids penetration through fabrics correlate with the results of the spray test. Deposited coatings of Parylene C cause much less acid penetration through fabrics compared to unmodified fabrics (H2SO4 penetration is lower from 83% to 97% depending on the fabric). This confirms the high chemical resistance of Parylene C applied to fabrics.
- Depending on the type of yarn the fabrics were made of (continuous or staple fibres and more or less porous), the Parylene C deposition had a different effect on the air permeability of the textiles (Figure 17). In the case of fabrics made of continuous fibres (orange and yellow), the factor determining the increase in air permeability of modified fabrics was the phenomenon of yarn compression, which increased the air flow through the fabrics (from 49% to 231% depending on the fabric and coating thickness). In the case of fabrics made of staple fibres (red and green), the twisted and more stiff yarns did not undergo compression during modification with Parylene C. The main factor determining the decrease in air permeability of these fabrics due to their modification was the deposition of Parylene C in the yarns’ structure, reducing free spaces between the fibres, increasing flow resistance and ultimately reducing their air permeability (from 9% to 66% depending on the fabric and coating thickness).
- Deposited coatings of Parylene C significantly influenced the electrostatic properties of only the orange fabric (made of basalt fibres), causing the decay half-time of the electric charge to drop below 4 s (T50 < 4 s qualifies the products for use in areas where there is a risk of explosion). The remaining three fabrics in the unmodified version and those with a Parylene C coating meet this safety criterion. The results of surface and vertical resistance do not change the category of fabrics as electrical insulators (both resistances for unmodified fabrics and fabrics with a Parylene C coating reached values above 106 Ω).
- Based on the results of thermal insulation properties tests, fabrics coated with Parylene C are characterized by a higher thermal conductivity coefficient than unmodified fabrics (the coefficient increases with the increase in coating thickness). These results were confirmed by thermal imaging, according to which, in particular, the deposition of a thicker coating of Parylene C (30 μm) contributes to a significantly greater increase in the fabric heating rate Rh compared to unmodified fabrics.
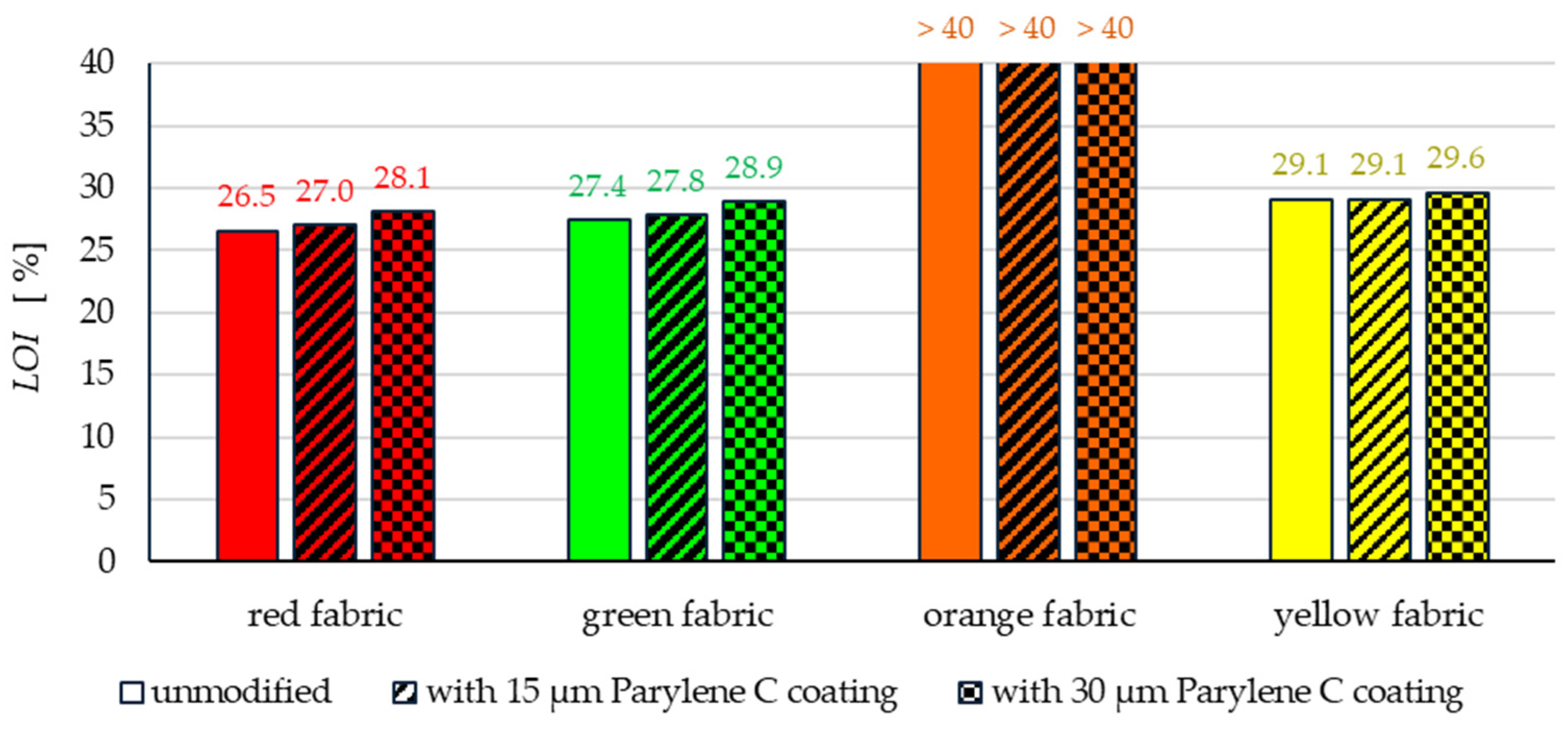
- The flammability test results showed that in the case of all aramids fibre fabrics, the Parylene C deposition method increases the LOI value of these fabrics, which means improving their flame retardancy properties. Samples of unmodified basalt fabric characterized by exceptionally high flame resistance retained this feature also after coating with Parylene C and can be classified as non-combustible materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorham, W.F. A new general synthetic method for the preparation of linear poly-p-xylylenes. J. Polym. Sci. Part A-1 Polym. Chem. 1966, 4, 3027–3039. [Google Scholar] [CrossRef]
- Ramachandran, A.; Junk, M.; Koch, K.P.; Hoffmann, K.P. A study of parylene C polymer deposition inside microscale gaps. IEEE Trans. Adv. Packag. 2007, 30, 712–724. [Google Scholar] [CrossRef]
- Gazicki-Lipman, M. Vapor deposition polymerization of para-xylylene derivatives—Mechanism and applications. J. Vac. Soc. Jpn. 2007, 50, 601–608. [Google Scholar] [CrossRef]
- Tokarska, M.; Miśkiewicz, P.; Puszkarz, A.K.; Nosal, A. Evaluation of Insulation against Contact Heat, Radiant Heat and Sensory Comfort of Basalt Fabric-Based Composites with Parylene C Coating. Fibers Text. East. Eur. 2023, 31, 99–108. [Google Scholar] [CrossRef]
- Cieślik, M.; Engvall, K.; Pan, J.; Kotarba, A. Silane–parylene coating for improving corrosion resistance of stainless steel 316L implant material. Corros. Sci. 2011, 53, 296–301. [Google Scholar] [CrossRef]
- Kamińska, M.; Okrój, W.; Szymański, W.; Jakubowski, W.; Komorowski, P.; Nosal, A.; Szymanowski, H.; Gazicki-Lipman, M.; Jerczyńska, H.; Pawłowska, Z.; et al. Interaction of Parylene C with biological objects. Acta Bioeng. Biomech. 2009, 11, 19–25. [Google Scholar]
- Frac, I.; Kucinska, M.; Gawrys, P.; Zagorska, M.; Maniukiewicz, W.; Nosal, A.; Gazicki-Lipman, M. Ambipolar organic thin film transistors prepared with a one step solution technique. Synth. Met. 2016, 220, 194–201. [Google Scholar] [CrossRef]
- Marszalek, T.; Gazicki-Lipman, M.J.; Ulański, J. Parylene C as versatile dielectric material for organic field-effect transistors. Beilstein J. Nanotechnol. 2017, 8, 1532–1545. [Google Scholar] [CrossRef]
- Spellman, G.P.; Carley, J.F.; Lopez, L.A. Vacuum deposition of parylene films: Influence of process factors and baffling on film-thickness distribution. J. Plast. Film Sheeting 1999, 15, 308–328. [Google Scholar] [CrossRef]
- Buchwalder, S.; Borzi, A.; Diaz Leon, J.J.; Bourgeois, F.; Nicolier, C.; Nicolay, S.; Neels, A.; Zywitzki, O.; Hogg, A.; Burger, J. Thermal analysis of parylene thin films. Polymers 2022, 14, 3677. [Google Scholar] [CrossRef]
- Kim, B.J.; Meng, E. Micromachining of Parylene C for bioMEMS. Polym. Adv. Technol. 2016, 27, 564–576. [Google Scholar] [CrossRef]
- Xu, C.; Lemon, W.; Liu, C. Design and fabrication of high-density metal microelectrode array for neural recording. Sens. Actuators A Phys. 2002, 96, 78–85. [Google Scholar] [CrossRef]
- Golda-Cepa, M.; Engvall, K.; Hakkarainen, M.; Kotarba, A. Recent progress on parylene C polymer for biomedical applications: A review. Prog. Org. Coat. 2020, 140, 105493. [Google Scholar] [CrossRef]
- Hsu, J.-M.; Rieth, L.; Kammer, S.; Orthner, M.; Solzbacher, F. Effect of thermal and deposition processes on the surface morphology. Crystallinity, and adhesion of parylene-C. Sens. Mater. 2008, 20, 87–102. [Google Scholar]
- Nosal, A.; Zydorczyk, A.; Sobczyk-Guzenda, A.; Głuchowski, L.; Szymanowski, H.; Gazicki-Lipman, M. Parylene coatings on biological specimens. J. Achiev. Mater. Manuf. Eng. 2009, 37, 442–447. [Google Scholar]
- Miśkiewicz, P.; Puszkarz, A.K. Assessment of Insulation against Contact Heat and Radiant Heat of Composites with TiO2-ZrO2-Al and Parylene C Coatings Intended for Protective Gloves Supported by Computational Fluid Dynamics. Appl. Sci. 2023, 13, 12420. [Google Scholar] [CrossRef]
- EN ISO 5084; Textiles—Determination of Thickness of Textiles and Textile Products. ISO: Geneva, Switzerland, 1996.
- EN 12127; Textiles—Fabrics—Determination of Mass per Unit Area Using Small Samples. CEN: Brussels, Belgium, 1997.
- Gorham, W.F. Para-Xylene Polymers (to Union Carbide Corporation). U.S. Patent 3,342,754, 18 February 1966. [Google Scholar]
- Gazicki, M.; James, W.; Yasuda, H. The EPR spectra of poly(chloro-para-xylylene) in vacuum and in air. J. Polym. Sci. Polym. Lett. Ed. 1985, 23, 639–645. [Google Scholar] [CrossRef]
- Broer, D.J.; Luijks, W. Penetration of p-xylylene vapor into small channels prior to polymerization. J. Appl. Polym. Sci. 1981, 26, 2415–2422. [Google Scholar] [CrossRef]
- EN ISO 4920; Textile Fabrics—Determination of Resistance to Surface Wetting (Spray Test). ISO: Geneva, Switzerland, 2012.
- EN ISO 6530; Protective Clothing—Protection Against Liquid Chemicals—Test Method for Resistance of Materials to Penetration by Liquids. ISO: Geneva, Switzerland, 2005.
- EN ISO 9237; Textiles, Determination of the Permeability of Fabrics to Air. ISO: Geneva, Switzerland, 1995.
- EN 1149-3; Protective Clothing—Electrostatic Properties—Part 3: Test Methods for Measurement of Charge Decay. CEN: Brussels, Belgium, 2004.
- EN 1149-1; Protective Clothing—Electrostatic Properties—Part 1: Test Method for Measurement of Surface Resistivity. CEN: Brussels, Belgium, 2006.
- EN 1149-2; Protective Clothing—Electrostatic Properties—Part 2: Test Method for Measurement of the Electrical Resistance through a Material (Vertical Resistance). CEN: Brussels, Belgium, 1997.
- EN ISO 4589-2; Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient Temperature Test. ISO: Geneva, Switzerland, 2017.
- Pabjańczyk-Wlazło, E.K.; Puszkarz, A.K.; Bednarowicz, A.; Tarzyńska, N.; Sztajnowski, S. The Influence of Surface Modification with Biopolymers on the Structure of Melt-Blown and Spun-Bonded Poly(lactic acid) Nonwovens. Materials 2022, 15, 7097. [Google Scholar] [CrossRef]
- Pabjanczyk-Wlazlo, E.; Tarzynska, N.; Bednarowicz, A.; Puszkarz, A.K.; Szparaga, G. Polymer-Based Electrophoretic Deposition of Nonwovens for Medical Applications: The Effect of Carrier Structure, Solution, and Process Parameters. Mar. Drugs 2021, 19, 533. [Google Scholar] [CrossRef] [PubMed]
- PubChem. Sulfuric Acid (Compound). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Sulfuric-Acid#section=pH (accessed on 15 June 2024).
- PubChem. Sodium Hydroxide (Compound). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/sodium%20hydroxide#section=pH (accessed on 16 June 2024).
- Choundhury, A.K.R. Principles of Textile Finishing; The Textile Institute Book Series; Woodhead Publishing: Duxford, UK, 2017; ISBN 978-0-08-100646-7. [Google Scholar]
- Regar, M.; Amjad, A.I. Basalt Fibre—Ancient Mineral Fibre for Green and Sustainable Development. Tekstilec 2016, 59, 321–334. [Google Scholar] [CrossRef]
- Textile Flame Retardancy Evaluation. Available online: https://www.polyestermfg.com/textile-flame-retardancy-evaluation/ (accessed on 1 July 2024).
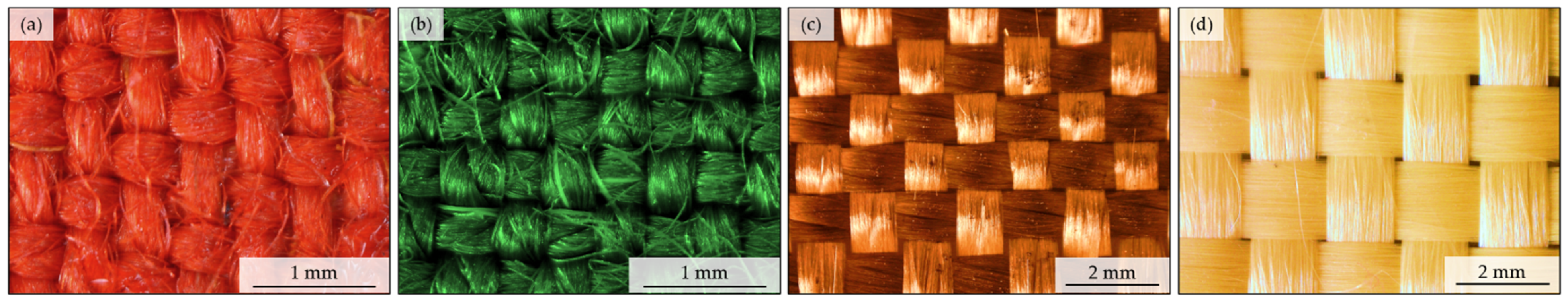


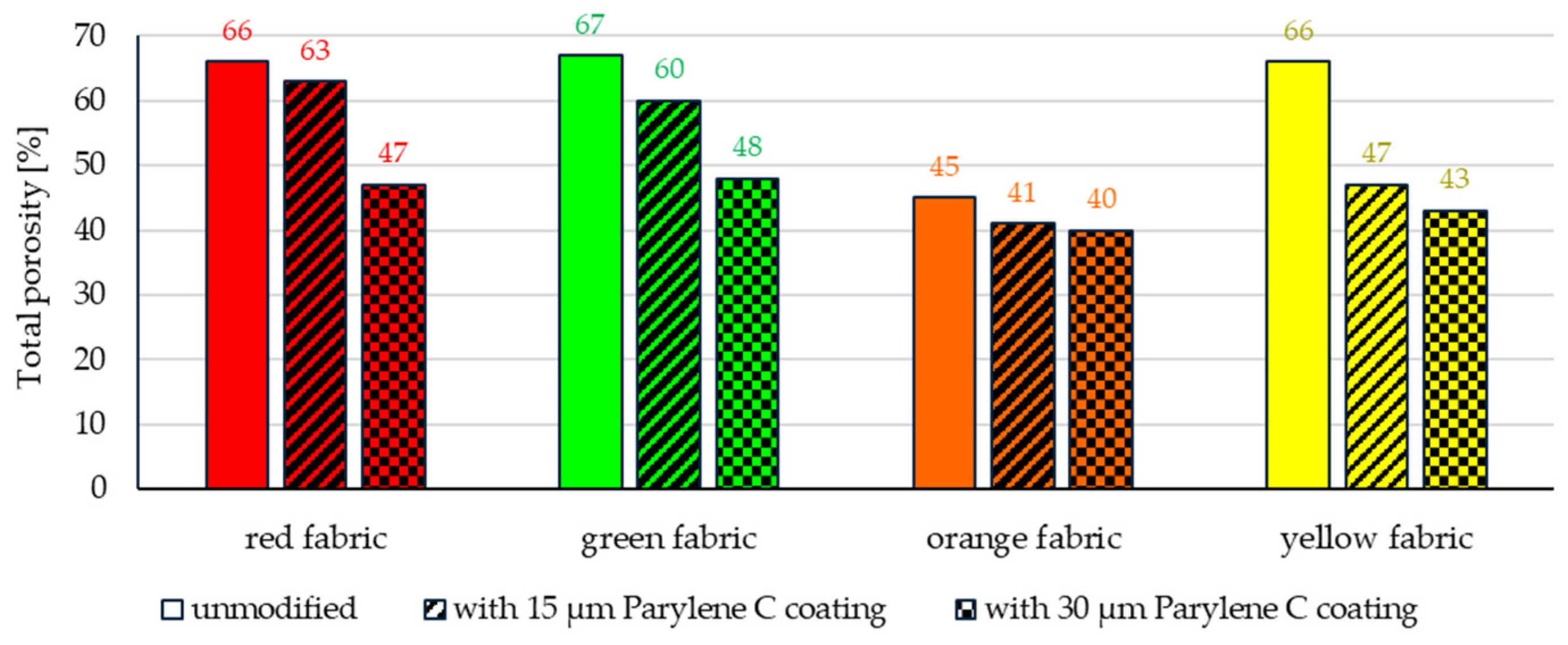



| Fabric Name | Composition | Thickness (a) [mm] | Mass per Unit Area (b) [g·m−2] | Weave | Warp Density [mm−1] | Weft Density [mm−1] | Total Porosity (c) [%] | Yarn Porosity (c) [%] |
|---|---|---|---|---|---|---|---|---|
| red | hydrophobic-finished meta-aramid | 0.37 | 233 | Twill | 3.01 | 2.25 | 66 | 47 |
| green | meta-aramid | 0.36 | 175 | Plain | 2.57 | 2.74 | 67 | 45 |
| orange | basalt | 0.50 | 380 | Plain | 0.82 | 0.89 | 45 | 11 |
| yellow | para-aramid | 0.45 | 214 | Plain | 0.62 | 0.64 | 66 | 58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miśkiewicz, P.; Puszkarz, A.K.; Machnowski, W.; Nosal, A. Evaluation of the Impact of Parylene C Deposition Method on the Functional Properties of Fabrics. Materials 2024, 17, 4073. https://doi.org/10.3390/ma17164073
Miśkiewicz P, Puszkarz AK, Machnowski W, Nosal A. Evaluation of the Impact of Parylene C Deposition Method on the Functional Properties of Fabrics. Materials. 2024; 17(16):4073. https://doi.org/10.3390/ma17164073
Chicago/Turabian StyleMiśkiewicz, Pamela, Adam K. Puszkarz, Waldemar Machnowski, and Andrzej Nosal. 2024. "Evaluation of the Impact of Parylene C Deposition Method on the Functional Properties of Fabrics" Materials 17, no. 16: 4073. https://doi.org/10.3390/ma17164073







