High-Temperature Oxidation of NbTi-Bearing Refractory Medium- and High-Entropy Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Design
2.2. Experiment Procedure
3. Results
3.1. As-Cast Microstructure and Segregation Analysis
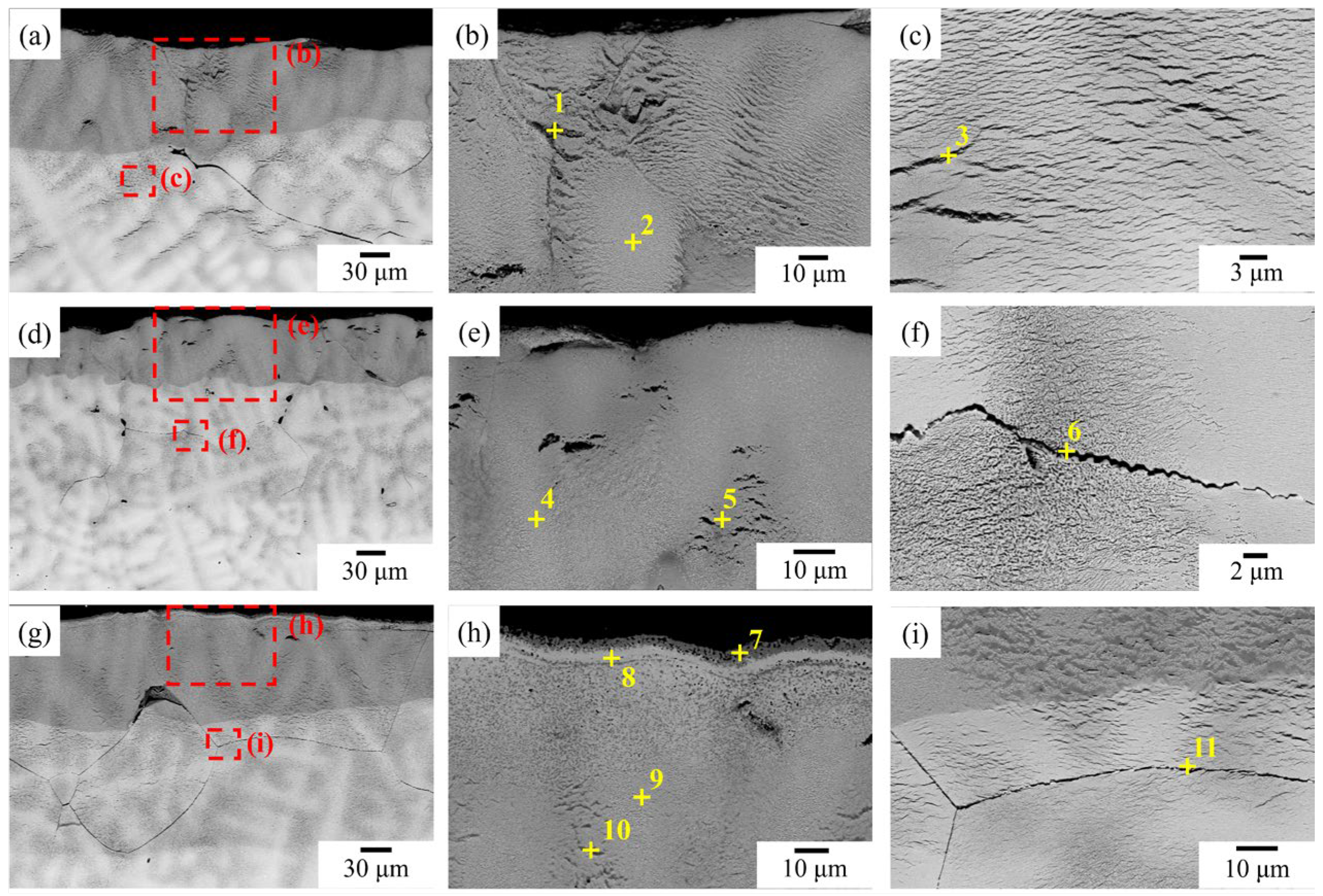
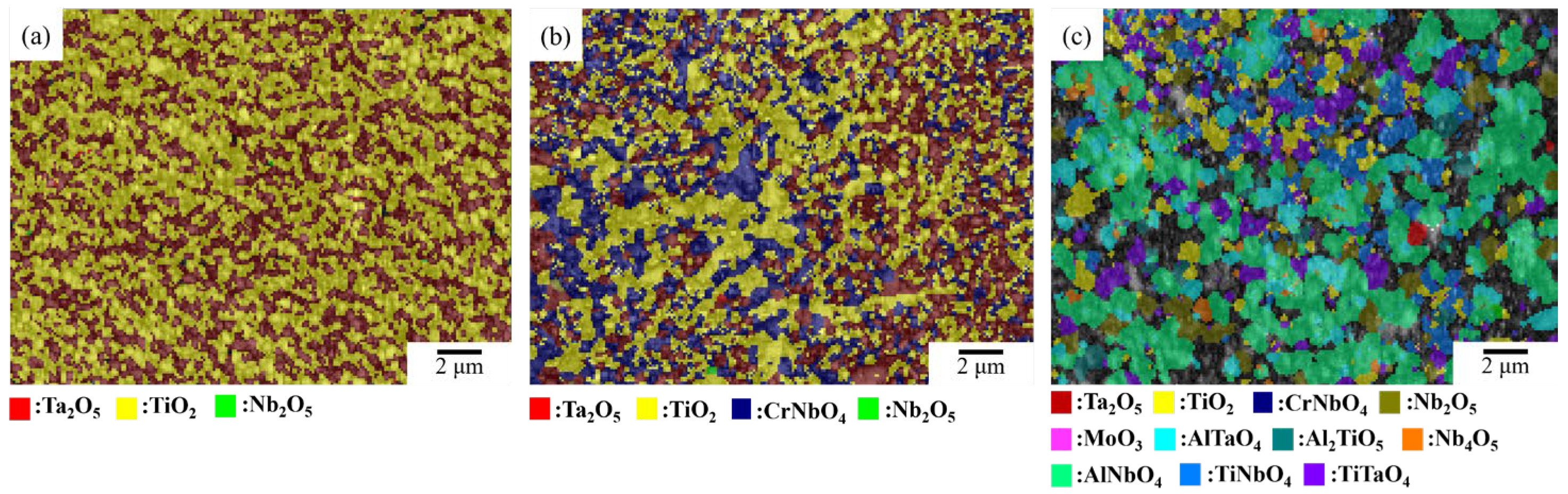
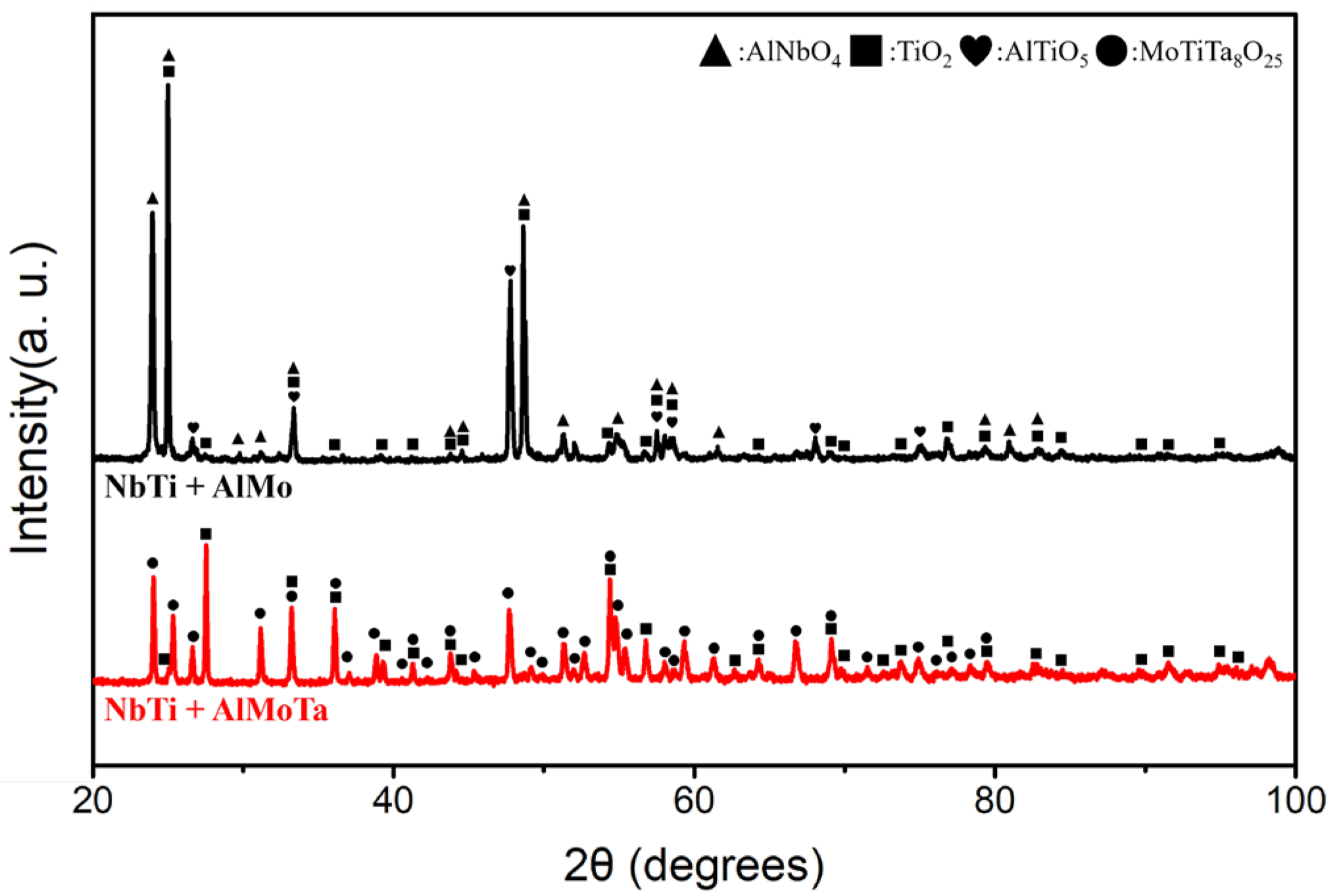
3.2. TGA and Oxidized Microstructure
4. Discussion
4.1. Elemental Segregation
4.2. The Oxidation Mechanism of NbTi-Bearing Refractory High Entropy
4.2.1. Al-Contained NbTi-Bearing RMEAs and RHEAs
4.2.2. The Oxidation Mechanism Difference between NbTi + Ta, NbTi + AlTa and NbTi + CrTa
4.2.3. The Elemental Effect of Mo and Ta during the Oxide Layer Formation
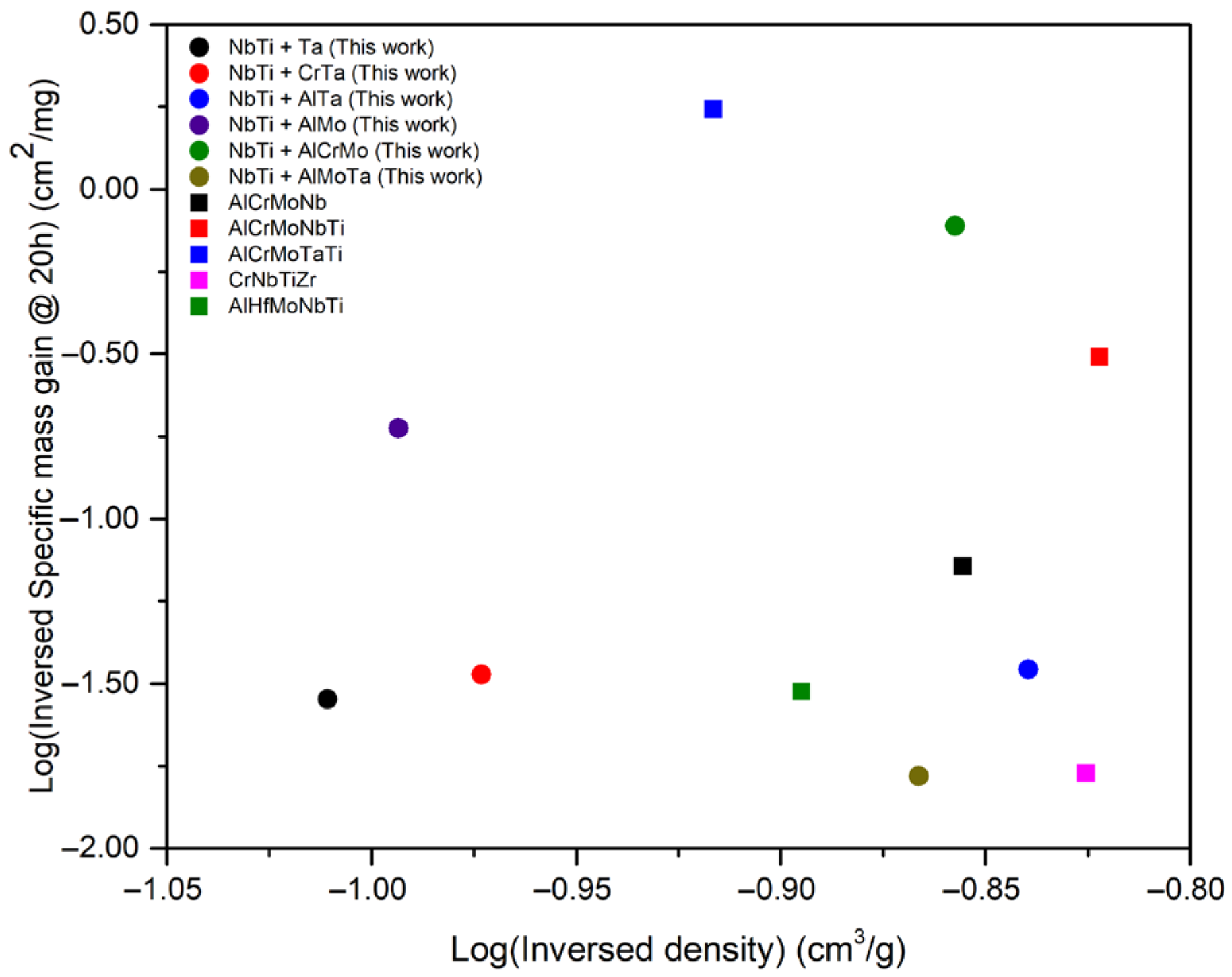
4.3. Comparison to the RHEAs Reported Recently
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhakal, S.; Minx, J.C.; Toth, F.L.; Abdel-Aziz, A.; Figueroa Meza, M.J.; Hubacek, K.; Jonckheere, I.G.C.; Kim, Y.-G.; Nemet, G.F.; Pachauri, S.; et al. Emissions Trends and Drivers. In IPCC, 2022: Climate Change 2022—Mitigation of Climate Change: Working Group III Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA; pp. 215–294. [CrossRef]
- Saravanamuttoo, H.I.; Rogers, G.F.C.; Cohen, H. Gas Turbine Theory; Pearson Education: London, UK, 2001. [Google Scholar]
- Chowdhury, T.S.; Mohsin, F.T.; Tonni, M.M.; Mita, M.N.H.; Ehsan, M.M. A critical review on gas turbine cooling performance and failure analysis of turbine blades. Int. J. Thermofluids 2023, 18, 100329. [Google Scholar] [CrossRef]
- Sivakumar, R.; Mordike, B.L. High temperature coatings for gas turbine blades: A review. Surf. Coat. Technol. 1989, 37, 139–160. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Dimiduk, D.M.; Woodward, C.; Miracle, D.B. Oxidation behavior of a refractory NbCrMo0.5Ta0.5TiZr alloy. J. Mater. Sci. 2012, 47, 6522–6534. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Wan, Y.; Mo, J.; Wang, X.; Zhang, Z.; Shen, B.; Liang, X. Mechanical Properties and Phase Stability of WTaMoNbTi Refractory High-Entropy Alloy at Elevated Temperatures. Acta Met. Sin. Engl. Lett. 2021, 34, 1585–1590. [Google Scholar] [CrossRef]
- Anne, B.R.; Shaik, S.; Tanaka, M.; Basu, A. A crucial review on recent updates of oxidation behavior in high entropy alloys. SN Appl. Sci. 2021, 3, 366. [Google Scholar] [CrossRef]
- Veselkov, S.; Samoilova, O.; Shaburova, N.; Trofimov, E. High-Temperature Oxidation of High-Entropic Alloys: A Review. Materials 2021, 14, 2595. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.-P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef]
- Lo, K.-C.; Chang, Y.-J.; Murakami, H.; Yeh, J.-W.; Yeh, A.-C. An oxidation resistant refractory high entropy alloy protected by CrTaO4-based oxide. Sci. Rep. 2019, 9, 7266. [Google Scholar] [CrossRef]
- Lo, K.-C.; Murakami, H.; Yeh, J.-W.; Yeh, A.-C. Oxidation behaviour of a novel refractory high entropy alloy at elevated temperatures. Intermetallics 2020, 119, 106711. [Google Scholar] [CrossRef]
- Müller, F.; Gorr, B.; Christ, H.-J.; Chen, H.; Kauffmann, A.; Heilmaier, M. Effect of microalloying with silicon on high temperature oxidation resistance of novel refractory high-entropy alloy Ta-Mo-Cr-Ti-Al. Mater. High. Temp. 2018, 35, 168–176. [Google Scholar] [CrossRef]
- Yurchenko, N.; Panina, E.; Zherebtsov, S.; Stepanov, N. Oxidation behaviour of eutectic refractory high-entropy alloys at 800–1000 °C. Corros. Sci. 2022, 205, 110464. [Google Scholar] [CrossRef]
- Gorr, B.; Müller, F.; Azim, M.; Christ, H.-J.; Müller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High-Temperature Oxidation Behavior of Refractory High-Entropy Alloys: Effect of Alloy Composition. Oxid. Met. 2017, 88, 339–349. [Google Scholar] [CrossRef]
- Sheikh, S.; Gan, L.; Ikeda, A.; Murakami, H.; Guo, S. Alloying effect on the oxidation behavior of a ductile Al0.5Cr0.25Nb0.5Ta0.5Ti1.5 refractory high-entropy alloy. Mater. Today Adv. 2020, 7, 100104. [Google Scholar] [CrossRef]
- Waseem, O.A.; Ryu, H.J. Combinatorial synthesis and analysis of AlxTayVz-Cr20Mo20Nb20Ti20Zr10 and Al10CrMoxNbTiZr10 refractory high-entropy alloys: Oxidation behavior. J. Alloys Compd. 2020, 828, 154427. [Google Scholar] [CrossRef]
- Liu, C.M.; Wang, H.M.; Zhang, S.Q.; Tang, H.B.; Zhang, A.L. Microstructure and oxidation behavior of new refractory high entropy alloys. J. Alloys Compd. 2014, 583, 162–169. [Google Scholar] [CrossRef]
- Cao, Y.-K.; Liu, Y.; Liu, B.; Zhang, W.-D.; Wang, J.-W.; Du, M. Effects of Al and Mo on high temperature oxidation behavior of refractory high entropy alloys. Trans. Nonferrous Met. Soc. China 2019, 29, 1476–1483. [Google Scholar] [CrossRef]
- Butler, T.; Senkov, O.; Daboiku, T.; Velez, M.; Schroader, H.; Ware, L.; Titus, M. Oxidation behaviors of CrNb, CrNbTi, and CrNbTaTi concentrated refractory alloys. Intermetallics 2022, 140, 107374. [Google Scholar] [CrossRef]
- Lo, K.-C.; Murakami, H.; Glatzel, U.; Yeh, J.-W.; Gorsse, S.; Yeh, A.-C. Elemental effects on the oxidation of refractory compositionally complex alloys. Int. J. Refract. Met. Hard Mater. 2022, 108, 105918. [Google Scholar] [CrossRef]
- Gorr, B.; Schellert, S.; Müller, F.; Christ, H.-J.; Kauffmann, A.; Heilmaier, M. Current Status of Research on the Oxidation Behavior of Refractory High Entropy Alloys. Adv. Eng. Mater. 2021, 23, 2001047. [Google Scholar] [CrossRef]
- Müller, F.; Gorr, B.; Christ, H.-J.; Müller, J.; Butz, B.; Chen, H.; Kauffmann, A.; Heilmaier, M. On the oxidation mechanism of refractory high entropy alloys. Corros. Sci. 2019, 159, 108161. [Google Scholar] [CrossRef]
- Cui, D.; Liu, X.; Yang, Z.; Guo, B.; Wang, Z.; Li, J.; Wang, J.; He, F. Uniting superior mechanical properties with oxidation resistance in a refractory high-entropy alloy via Cr and Al alloying. Scr. Mater. 2024, 244, 116031. [Google Scholar] [CrossRef]
- Gorr, B.; Mueller, F.; Christ, H.-J.; Mueller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High temperature oxidation behavior of an equimolar refractory metal-based alloy 20Nb 20Mo 20Cr 20Ti 20Al with and without Si addition. J. Alloys Compd. 2016, 688, 468–477. [Google Scholar] [CrossRef]
- Butler, T.; Chaput, K.; Dietrich, J.; Senkov, O. High temperature oxidation behaviors of equimolar NbTiZrV and NbTiZrCr refractory complex concentrated alloys (RCCAs). J. Alloys Compd. 2017, 729, 1004–1019. [Google Scholar] [CrossRef]
- Butler, T.; Chaput, K. Native oxidation resistance of Al20Nb30Ta10Ti30Zr10 refractory complex concentrated alloy (RCCA). J. Alloys Compd. 2019, 787, 606–617. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Chen, Z.; Zhang, J.; Shen, B. Oxidation response of a vacuum arc melted NbZrTiCrAl refractory high entropy alloy at 800–1200 °C. Vacuum 2019, 162, 20–27. [Google Scholar] [CrossRef]
- Senkov, O.; Gild, J.; Butler, T. Microstructure, mechanical properties and oxidation behavior of NbTaTi and NbTaZr refractory alloys. J. Alloys Compd. 2021, 862, 158003. [Google Scholar] [CrossRef]
- Gawel, R.; Rogal, Ł.; Przybylski, K. Oxidation Resistance of Ti-Al-Cr-Nb-Based High-Entropy Alloys in Air at 1073 K. J. Mater. Eng. Perform. 2019, 28, 4163–4170. [Google Scholar] [CrossRef]
- Pilone, D.; Felli, F.; Brotzu, A. High temperature oxidation behaviour of TiAl–Cr–Nb–Mo alloys. Intermetallics 2013, 43, 131–137. [Google Scholar] [CrossRef]
- Schellert, S.; Gorr, B.; Christ, H.J.; Pritzel, C.; Laube, S.; Kauffmann, A.; Heilmaier, M. The Effect of Al on the Formation of a CrTaO4 Layer in Refractory High Entropy Alloys Ta-Mo-Cr-Ti-xAl. Oxid. Met. 2021, 96, 333–345. [Google Scholar] [CrossRef]
- Li, Y.; Gan, B.; Bi, Z.; Yu, H.; Zhou, C.; Sha, J. Improvement of “pest” oxidation resistance of Nb-Mo-W-Zr solid- solution alloy at 800 °C by gas nitridation. Corros. Sci. 2021, 187, 109513. [Google Scholar] [CrossRef]
- Chang, C.; Titus, M.S.; Yeh, J. Oxidation Behavior between 700 and 1300 °C of Refractory TiZrNbHfTa High-Entropy Alloys Containing Aluminum. Adv. Eng. Mater. 2018, 20, 1700948. [Google Scholar] [CrossRef]
- Yurchenko, N.; Panina, E.; Zherebtsov, S.; Salishchev, G.; Stepanov, N. Oxidation Behavior of Refractory AlNbTiVZr0.25 High-Entropy Alloy. Materials 2018, 11, 2526. [Google Scholar] [CrossRef]
- Yang, X.; An, Z.; Zhai, Y.; Wang, X.; Chen, Y.; Mao, S.; Han, X. Effect of Al content on the thermal oxidation behaviour of AlHfMoNbTi high-entropy alloys analysed by in situ environmental TEM. Corros. Sci. 2021, 191, 109711. [Google Scholar] [CrossRef]
- Qiao, D.; Liang, H.; Wu, S.; He, J.; Cao, Z.; Lu, Y.; Li, T. The mechanical and oxidation properties of novel B2-ordered Ti2ZrHf0.5VNb0.5Alx refractory high-entropy alloys. Mater. Charact. 2021, 178, 111287. [Google Scholar] [CrossRef]
- An, Z.; Mao, S.; Yang, T.; Liu, Y.; Chen, Y.; Yang, X.; Liu, S.; Wang, X.; Deng, Q.; Zhang, Z.; et al. Simultaneously enhanced oxidation resistance and mechanical properties in a novel lightweight Ti2VZrNb0.5Al0.5 high-entropy alloy. Sci. China Mater. 2022, 65, 2842–2849. [Google Scholar] [CrossRef]
- Lu, S.; Li, X.; Liang, X.; He, J.; Shao, W.; Li, K.; Chen, J. Effect of Y additions on the oxidation behavior of vacuum arc melted refractory high-entropy alloy AlMo0.5NbTa0.5TiZr at elevated temperatures. Vacuum 2022, 201, 111069. [Google Scholar] [CrossRef]
- Lin, Y.; Guo, Y.; Dong, Q.; Huang, R.; Tan, J. Effects of vanadium content on the high temperature oxidation behavior of NbTiZrAlV refractory complex concentrated alloys. J. Alloys Compd. 2022, 905, 164180. [Google Scholar] [CrossRef]
- Welch, N.J.; Quintana, M.J.; Butler, T.M.; Collins, P.C. High-temperature oxidation behavior of TaTiCr, Ta4Ti3Cr, Ta2TiCr, and Ta4TiCr3 concentrated refractory alloys. J. Alloys Compd. 2023, 941, 169000. [Google Scholar] [CrossRef]
- Zhang, S.; Li, R.; Xu, Y. Zr alloying effects on the microstructure, compression performance and oxidation resistance of refractory high entropy alloys. Mater. Res. Express 2022, 9, 096510. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Li, Q.; Jin, C.; Hua, K.; Wang, H. The determining role of Al addition on tribology properties and oxidation behavior at elevated temperatures of TiZrHfNb refractory high-entropy alloy. Mater. Charact. 2022, 189, 111921. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, Z.-J.; Yu, H.-B.; Chan, K.; Liu, L. Oxidation behavior of the Ti38V15Nb23Hf24 refractory high-entropy alloy at elevated temperatures. Corros. Sci. 2022, 198, 110153. [Google Scholar] [CrossRef]
- Zhang, H.; Du, Y.; Lai, L.; Guo, N.; Li, N.; Guo, S. The as-cast AlxCrTaTi refractory medium entropy alloys with good room-temperature mechanical properties and high-temperature oxidation resistance. J. Alloys Compd. 2023, 932, 167675. [Google Scholar] [CrossRef]
- Lu, S.; Li, X.; Liang, X.; Shao, W.; Yang, W.; Chen, J. Effect of Al content on the oxidation behavior of refractory high-entropy alloy AlMo0.5NbTa0.5TiZr at elevated temperatures. Int. J. Refract. Met. Hard Mater. 2022, 105, 105812. [Google Scholar] [CrossRef]
- Schellert, S.; Weber, M.; Christ, H.; Wiktor, C.; Butz, B.; Galetz, M.; Laube, S.; Kauffmann, A.; Heilmaier, M.; Gorr, B. Formation of rutile (Cr,Ta,Ti)O2 oxides during oxidation of refractory high entropy alloys in Ta-Mo-Cr-Ti-Al system. Corros. Sci. 2023, 211, 110885. [Google Scholar] [CrossRef]
- Yan, Y.; McGarrity, K.A.; Delia, D.J.; Fekety, C.; Wang, K. The oxidation-resistance mechanism of WTaNbTiAl refractory high entropy alloy. Corros. Sci. 2022, 204, 110377. [Google Scholar] [CrossRef]
- Du, Y.; Ding, D.; Lai, L.; Xiao, S.; Guo, N.; Song, B.; Guo, S. Effect of Y on the high-temperature oxidation behavior of CrMoTaTi refractory high entropy alloy. Int. J. Refract. Met. Hard Mater. 2022, 103, 105755. [Google Scholar] [CrossRef]
- Jayaraj, J.; Thirathipviwat, P.; Han, J.; Gebert, A. Microstructure, mechanical and thermal oxidation behavior of AlNbTiZr high entropy alloy. Intermetallics 2018, 100, 9–19. [Google Scholar] [CrossRef]
- Zhang, R.; Meng, J.; Han, J.; Tulugan, K.; Zhang, R. Oxidation resistance properties of refractory high-entropy alloys with varied AlxCrTiMo content. J. Mater. Sci. 2021, 56, 3551–3561. [Google Scholar] [CrossRef]
- Waseem, O.A.; Auyeskhan, U.; Lee, H.M.; Ryu, H.J. A combinatorial approach for the synthesis and analysis of AlxCryMozNbTiZr high-entropy alloys: Oxidation behavior. J. Mater. Res. 2018, 33, 3226–3234. [Google Scholar] [CrossRef]
- Li, L.-C.; Li, M.-X.; Liu, M.; Sun, B.-Y.; Wang, C.; Huo, J.-T.; Wang, W.-H.; Liu, Y.-H. Enhanced oxidation resistance of MoTaTiCrAl high entropy alloys by removal of Al. Sci. China Mater. 2021, 64, 223–231. [Google Scholar] [CrossRef]
- Gungor, M.N. A statistically significant experimental technique for investigating microsegregation in cast alloys. Met. Trans. A 1989, 20, 2529–2533. [Google Scholar] [CrossRef]
- Xiong, W.; Du, Y.; Liu, Y.; Huang, B.; Xu, H.; Chen, H.; Pan, Z. Thermodynamic assessment of the Mo–Nb–Ta system. Calphad 2004, 28, 133–140. [Google Scholar] [CrossRef]
- Okamoto, H. Al-Mo (Aluminum-Molybdenum). J. Phase Equilibria Diffus. 2010, 31, 492–493. [Google Scholar] [CrossRef]
- Elliott, R.P.; Shunk, F.A. The Al−Nb system (Aluminum-Niobium). Bull. Alloy. Phase Diagr. 1981, 2, 75–81. [Google Scholar] [CrossRef]
- Kaufman, L. Calculation of multicomponent tantalum based phase diagrams. Calphad 1991, 15, 261–282. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, H.; Guo, S.; Wang, T.; Cao, Z.; Li, T. A new strategy to design eutectic high-entropy alloys using mixing enthalpy. Intermetallics 2017, 91, 124–128. [Google Scholar] [CrossRef]
- Krapivka, N.A.; Firstov, S.A.; Karpets, M.V.; Myslivchenko, A.N.; Gorban’, V.F. Features of phase and structure formation in high-entropy alloys of the AlCrFeCoNiCu x system (x = 0, 0.5, 1.0, 2.0, 3.0). Phys. Met. Met. 2015, 116, 467–474. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Eckert, M.; Bencze, L.; Kath, D.; Nickel, H.; Hilpert, K. Thermodynamic Activities in the Alloys of the Ti-Al System. Berichte Der Bunsenges. Für Phys. Chem. 1996, 100, 418–424. [Google Scholar] [CrossRef]
- Luthra, K.L. Stability of protective oxide films on Ti-base alloys. Oxid. Met. 1991, 36, 475–490. [Google Scholar] [CrossRef]
- Schäfer, H.; Gruehn, R.; Schulte, F. The Modifications of Niobium Pentoxide. Angew. Chem. Int. Ed. Engl. 1966, 5, 40–52. [Google Scholar] [CrossRef]
- Spyridelis, J.; Delavignette, P.; Amelinckx, S. On the Superstructures of Ta2O5 and Nb2O5. Phys. Status Solidi (b) 1967, 19, 683–704. [Google Scholar] [CrossRef]
- Li, M.; Sun, X.; Jin, T.; Guan, H.; Hu, Z. Oxidation Behavior of a Single-Crystal Ni-Base Superalloy in Air—II: At 1000, 1100, and 1150 °C. Oxid. Met. 2003, 60, 195–210. [Google Scholar] [CrossRef]
- Gulbransen, E.A.; Andrew, K.F.; Brassart, F.A. Oxidation of Molybdenum 550° to 1700°C. J. Electrochem. Soc. 1963, 110, 952–959. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, M.; Zhang, Y.; Guo, S. Senary refractory high-entropy alloy Crx MoNbTaVW. Calphad 2015, 51, 193–201. [Google Scholar] [CrossRef]
- Jehn, H.; Olzi, E. High temperature solid-solubility limit and phase studies in the system tantalum-oxygen. J. Less Common. Met. 1972, 27, 297–309. [Google Scholar] [CrossRef]
- Gorr, B.; Azim, M.; Christ, H.-J.; Mueller, T.; Schliephake, D.; Heilmaier, M. Phase equilibria, microstructure, and high temperature oxidation resistance of novel refractory high-entropy alloys. J. Alloys Compd. 2015, 624, 270–278. [Google Scholar] [CrossRef]

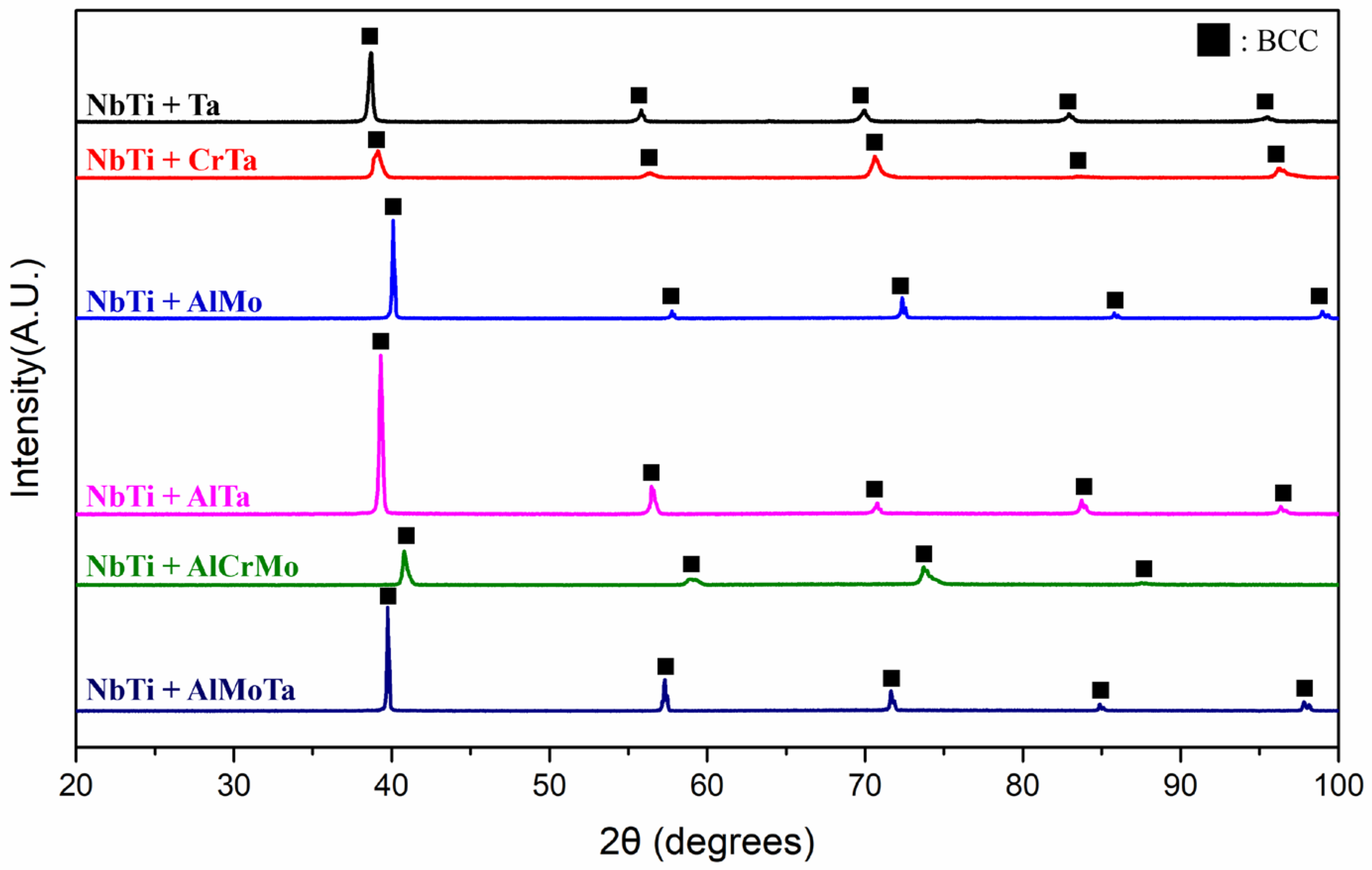
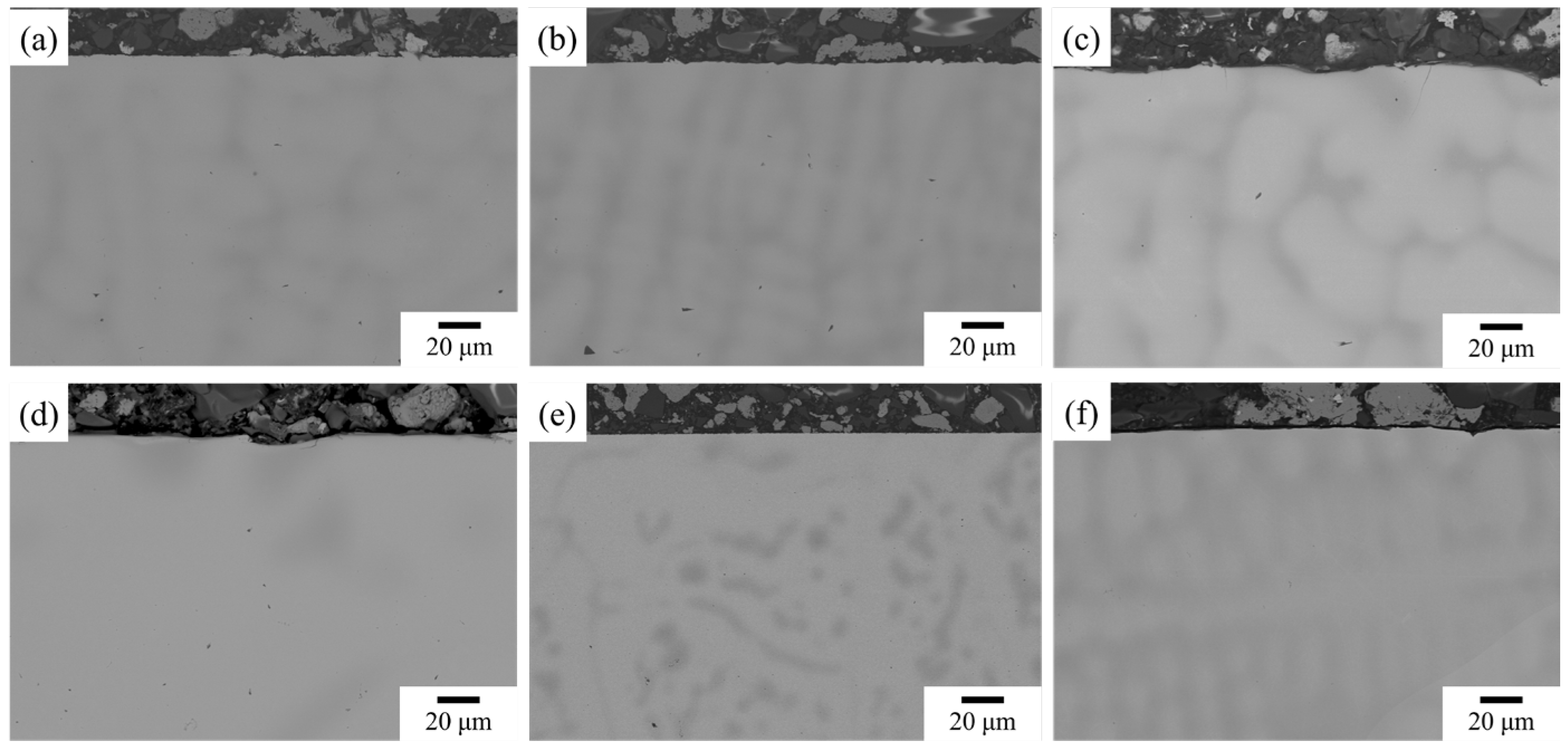
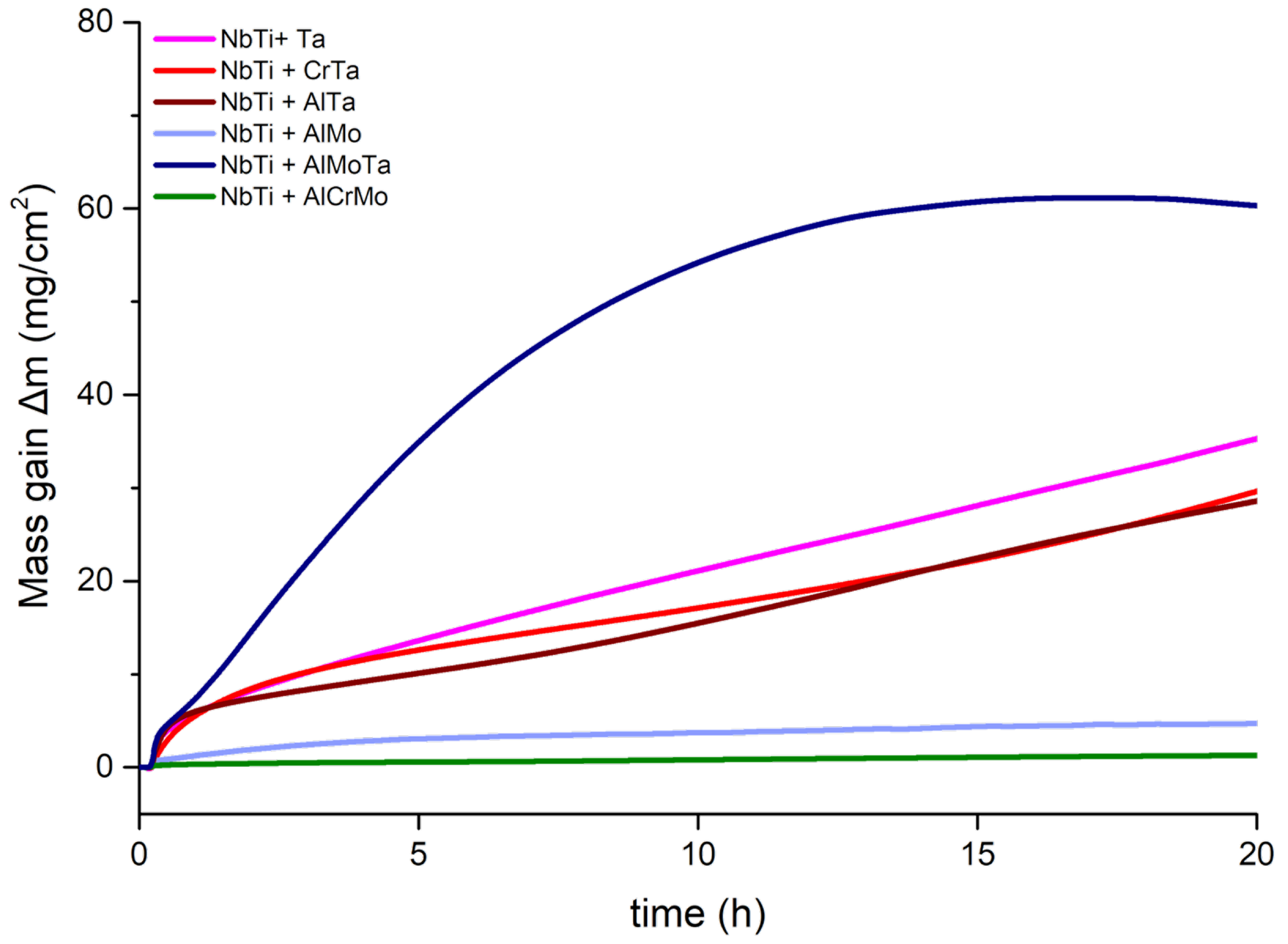

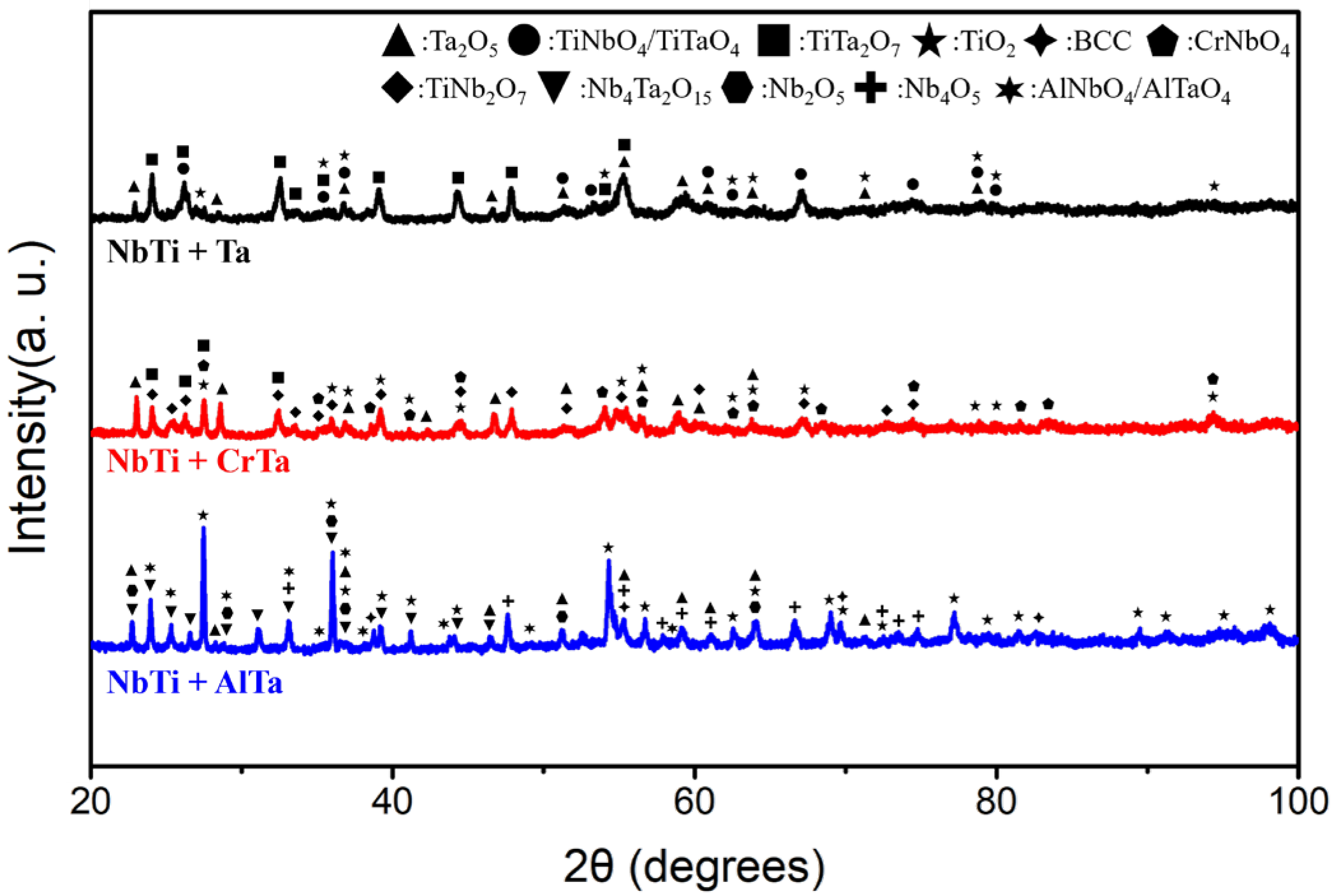
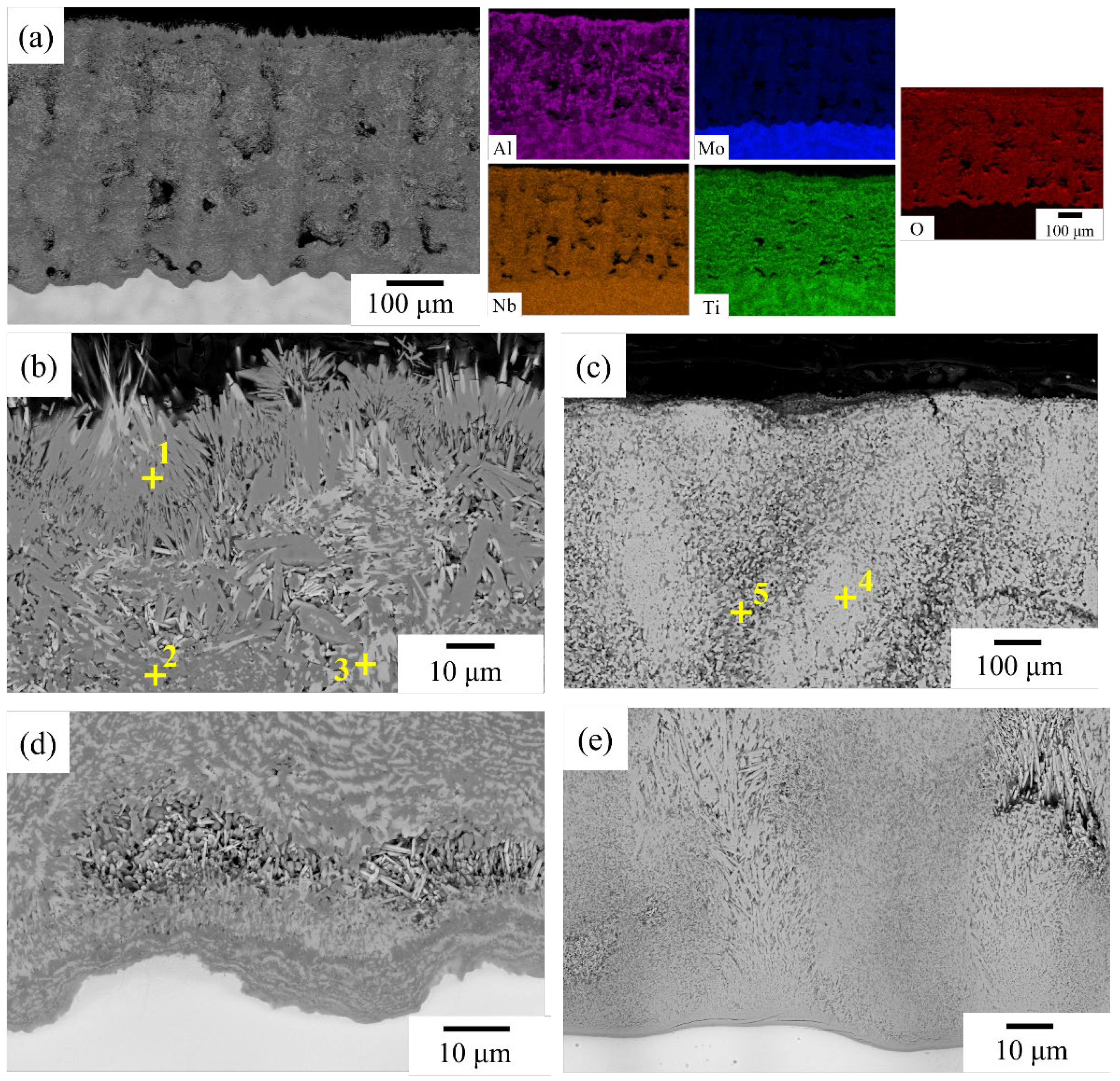
| Alloy | Al | Cr | Mo | Nb | Ta | Ti |
|---|---|---|---|---|---|---|
| NbTi + Ta | - | - | - | 33.1 ± 0.5 | 36.2 ± 0.3 | 30.7 ± 0.3 |
| NbTi + CrTa | - | 10.9 ± 0.5 | - | 27.1 ± 0.1 | 28.8 ± 0.2 | 33.2 ± 0.4 |
| NbTi + AlMo | 15.2 ± 0.4 | - | 26.9 ± 0.7 | 28.1 ± 0.2 | - | 29.8 ± 0.5 |
| NbTi + AlTa | 8.6 ± 0.4 | - | - | 30.1 ± 0.4 | 35.2 ± 1.2 | 26.1 ± 2.0 |
| NbTi + AlCrMo | 10.3 ± 0.3 | 21.0 ± 0.9 | 23.9 ± 1.0 | 23.4 ± 0.9 | - | 21.5 ± 0.7 |
| NbTi + AlMoTa | 5.1 ± 0.2 | - | 23.5 ± 0.9 | 25.0 ± 0.9 | 25.3 ± 0.9 | 21.1 ± 0.4 |
| Alloy | Region | Al | Cr | Mo | Nb | Ta | Ti |
|---|---|---|---|---|---|---|---|
| NbTi + Ta | D | - | - | - | 32.2 ± 0.4 | 37.8 ± 0.8 | 30.1 ± 0.4 |
| ID | - | - | - | 32.2 ± 0.7 | 28.0 ± 0.3 | 39.8 ± 2.0 | |
| NbTi + CrTa | D | - | 7.7 ± 1.4 | - | 29.8 ± 0.5 | 36.4 ± 1.2 | 26.2 ± 0.2 |
| ID | - | 18.9 ± 0.1 | - | 20.3 ± 0.5 | 13.3 ± 0.6 | 47.4 ± 0.9 | |
| NbTi + AlMo | D | 13.8 ± 0.7 | - | 31.0 ± 0.7 | 30.3 ± 0.3 | - | 24.8 ± 0.0 |
| ID | 18.3 ± 0.2 | - | 23.9 ± 0.4 | 27.0 ± 0.1 | - | 30.8 ± 0.3 | |
| NbTi + AlTa | D | 8.6 ± 0.0 | - | - | 31.3 ± 0.6 | 34.7 ± 0.3 | 25.5 ± 0.3 |
| ID | 14.9 ± 0.7 | - | - | 27.7 ± 0.1 | 21.9 ± 1.6 | 35.5 ± 1.0 | |
| NbTi + AlCrMo | D | 9.3 ± 0.1 | 16.7 ± 0.2 | 28.3 ± 0.2 | 24.6 ± 0.3 | - | 21.1 ± 0.4 |
| ID | 15.3 ± 0.8 | 31.3 ± 0.4 | 8.8 ± 1.4 | 17.7 ± 0.7 | - | 26.9 ± 1.0 | |
| NbTi + AlMoTa | D | 3.3 ± 0.1 | - | 25.0 ± 0.2 | 24.8 ± 0.1 | 29.2 ± 0.2 | 17.8 ± 0.1 |
| ID | 12.2 ± 1.5 | - | 17.2 ± 1.2 | 22.7 ± 0.4 | 12.3 ± 2.0 | 35.6 ± 2.0 |
| Alloy Name | k1 | n1 | Period | k2 | n2 | Period |
|---|---|---|---|---|---|---|
| NbTi + Ta | 5.62 | 0.55 | 1–9 h | 2.45 | 0.89 | 9–20 h |
| NbTi + CrTa | 5.88 | 0.46 | 1–14 h | 1.05 | 1.12 | 14–20 h |
| NbTi + AlTa | 5.79 | 0.34 | 1–7 h | 2.19 | 0.84 | 7–20 h |
| NbTi + AlMo | 1.27 | 0.57 | 1–4 h | 1.77 | 0.32 | 4–20 h |
| NbTi + AlCrMo | 0.33 | 0.34 | 1–7 h | 0.21 | 0.59 | 7–20 h |
| NbTi + AlMoTa | 9.12 | 0.76 | 1–15 h |
| Region | Al | Cr | Mo | Nb | Ti | O |
|---|---|---|---|---|---|---|
| 1 | 17.7 ± 1.0 | 9.8 ± 3.7 | 0.2 ± 0.1 | 2.8 ± 0.3 | 5.3 ± 1.0 | 64.2 ± 2.8 |
| 2 | 5.1 ± 0.3 | 25.0 ± 4.3 | 0.3 ± 0.2 | 2.4 ± 0.5 | 4.5 ± 1.7 | 62.7 ± 2.2 |
| 3 | 2.7 ± 0.2 | 3.3 ± 0.1 | 1.7 ± 0.1 | 17.8 ± 0.1 | 8.7 ± 0.3 | 66.0 ± 0.4 |
| 4 | 6.6 ± 0.4 | 17.0 ± 5.3 | 9.8 ± 1.7 | 12.3 ± 0.9 | 24.7 ± 3.3 | (N)29.7 ± 3.0 |
| 5 | 6.8 ± 0.3 | 22.7 ± 1.2 | 9.0 ± 0.1 | 13.9 ± 0.3 | 29.7 ± 1.8 | 18.1 ± 0.1 |
| Region | Al | Cr | Nb | Ta | Ti | O |
|---|---|---|---|---|---|---|
| 1 | - | - | 2.1 ± 0.6 | 2.4 ± 0.7 | 30.7 ± 3.1 | 64.8 ± 3.9 |
| 2 | - | - | 10.5 ± 0.6 | 14.4 ± 0.6 | 8.5 ± 2.3 | 66.6 ± 1.7 |
| 3 | - | - | 4.0 ± 0.3 | 4.1 ± 0.4 | 38.6 ± 0.1 | 53.4 ± 0.7 |
| 4 | - | 1.6 ± 0.3 | 10.4 ± 0.4 | 13.6 ± 0.7 | 7.2 ± 0.9 | 63.2 ± 6.5 |
| 5 | - | 3.3 ± 0.4 | 7.7 ± 0.7 | 6.4 ± 0.4 | 19.3 ± 0.5 | 63.3 ± 0.3 |
| 6 | - | 3.2 ± 1.0 | 3.2 ± 0.4 | 4.7 ± 1.3 | 38.3 ± 0.1 | 50.8 ± 0.6 |
| 7 | 7.3 ± 0.3 | - | 4.1 ± 0.2 | 4.9 ± 0.2 | 15.0 ± 0.7 | 68.7 ± 1.4 |
| 8 | 0.2 ± 0.1 | - | 14.2 ± 0.9 | 12.5 ± 0.7 | 4.0 ± 0.2 | 69.0 ± 0.2 |
| 9 | 2.8 ± 0.5 | - | 9.5 ± 0.6 | 11.9 ± 0.5 | 9.8 ± 1.8 | 66.1 ± 1.4 |
| 10 | 5.3 ± 0.2 | - | 4.7 ± 0.9 | 5.8 ± 1.1 | 20.1 ± 2.2 | 64.1 ± 0.1 |
| 11 | 8.9 ± 0.3 | - | 3.3 ± 0.9 | 5.0 ± 0.2 | 23.7 ± 1.2 | 59.2 ± 0.8 |
| Alloy | Ta2O5 | TiO2 | AlTaO4 | Al2TiO5 | Nb2O5 | Nb4O5 | AlNbO4 | TiNbO4 | TiTaO4 | CrNbO4 |
|---|---|---|---|---|---|---|---|---|---|---|
| NbTi + Ta | 41.8 | 57.6 | - | - | 0.6 | - | - | - | - | - |
| NbTi + CrTa | 29.52 | 41.02 | - | - | 0.04 | - | - | - | - | 29.42 |
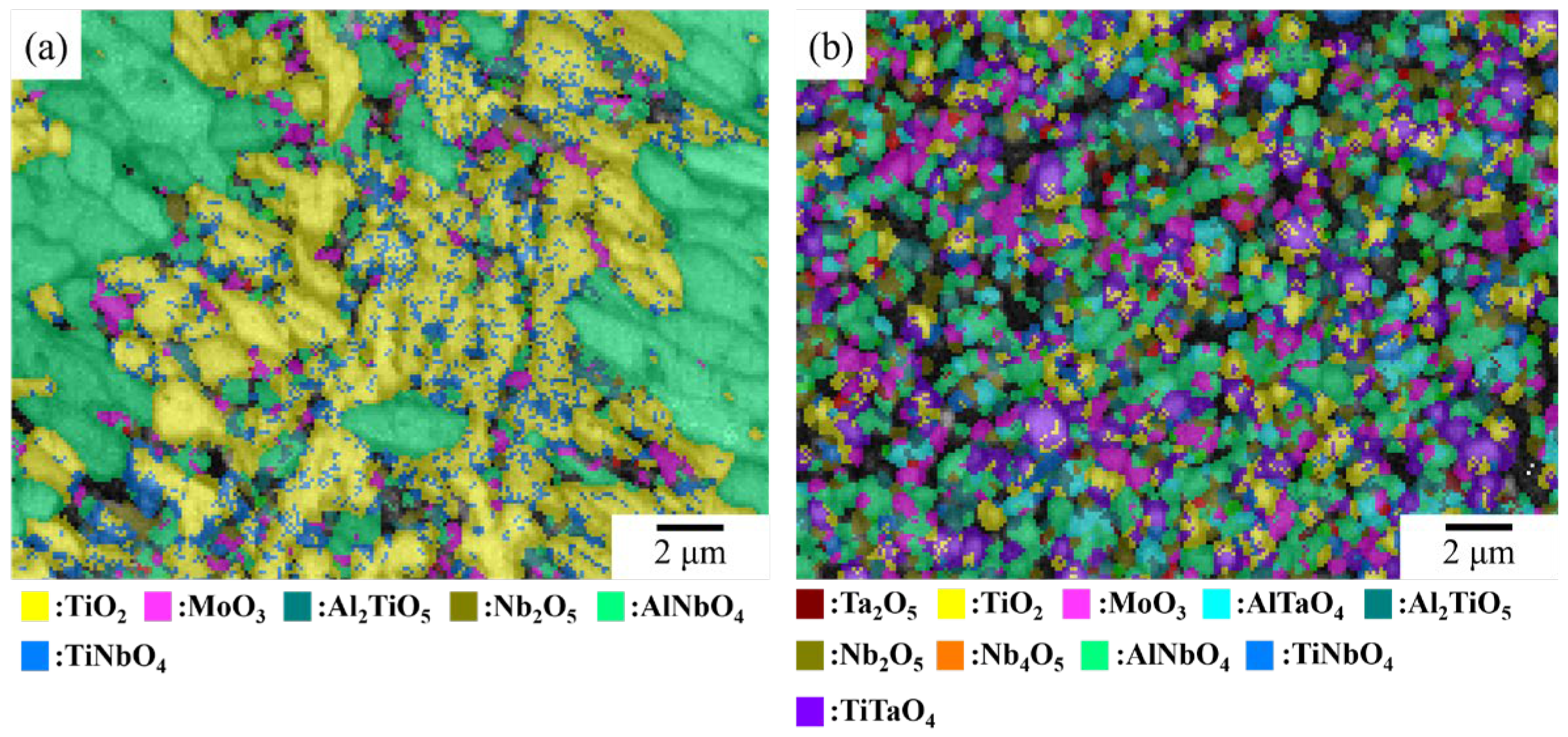
| NbTi + AlTa | 7.9 | 9.8 | 10.1 | 2.7 | 7.6 | 1.4 | 38.7 | 11.6 | 10.3 | - |
| Region | Al | Mo | Nb | Ta | Ti | O |
|---|---|---|---|---|---|---|
| 1 | 13.6 ± 0.3 | 0.0 ± 0.0 | 13.1 ± 0.3 | - | 9.7 ± 0.5 | 63.7 ± 0.1 |
| 2 | 7.0 ± 0.3 | 0.4 ± 0.2 | 8.6 ± 0.6 | - | 19.2 ± 0.3 | 64.8 ± 0.7 |
| 3 | 1.3 ± 0.4 | 2.7 ± 0.4 | 24.9 ± 4.2 | - | 5.7 ± 1.1 | 65.4 ± 3.8 |
| 4 | 0.4 ± 0.1 | 4.0 ± 0.2 | 9.2 ± 0.3 | 12.1 ± 0.1 | 4.9 ± 0.1 | 69.3 ± 0.1 |
| 5 | 2.7 ± 0.7 | 1.9 ± 0.5 | 6.8 ± 1.1 | 8.1 ± 0.8 | 12.5 ± 2.3 | 68.0 ± 0.6 |
| Alloy | Ta2O5 | TiO2 | MoO3 | AlTaO4 | Al2TiO5 | Nb2O5 | AlNbO4 | TiNbO4 | TiTaO4 |
|---|---|---|---|---|---|---|---|---|---|
| NbTi + AlMo | - | 43 | 4 | - | 2.5 | 2.2 | 37.2 | 11.1 | - |
| NbTi + AlMoTa | 2.1 | 14.1 | 12.3 | 5.9 | 9.1 | 10.7 | 24.9 | 5.5 | 15.3 |
| Alloy | Al | Cr | Mo | Nb | Ta | Ti |
|---|---|---|---|---|---|---|
| NbTi + Ta | - | - | - | 1.01 | 1.09 | 0.9 |
| NbTi + CrTa | - | 0.71 | - | 1.05 | 1.13 | 0.87 |
| NbTi + AlMo | 0.86 | - | 1.09 | 1.04 | - | 0.93 |
| NbTi + AlTa | 0.73 | - | - | 1.04 | 1.19 | 0.84 |
| NbTi + AlCrMo | 0.83 | 0.73 | 1.32 | 1.11 | - | 0.93 |
| NbTi + AlMoTa | 0.75 | - | 1.05 | 1.02 | 1.14 | 0.86 |
| Al | Cr | Mo | Nb | Ta | Ti | |
|---|---|---|---|---|---|---|
| Al | −10 | −5 | −18 | −19 | −30 | |
| Cr | −10 | 0 | −7 | −7 | −7 | |
| Mo | −5 | 0 | −6 | −5 | −4 | |
| Nb | −18 | −7 | −6 | 0 | 2 | |
| Ta | −19 | −7 | −5 | 0 | 1 | |
| Ti | −30 | −7 | −4 | 2 | 1 |
| Alloy | Region | Al | Cr | Mo | Nb | Ta | Ti |
|---|---|---|---|---|---|---|---|
| NbTi + Ta | D | - | - | - | 5.73 × 10−4 ± 8.53 × 10−6 | 3.06 × 10−4 ± 6.03 × 10−6 | 1.30 × 10−3 ± 1.01 × 10−5 |
| ID | - | - | - | 6.03 × 10−4 ± 2.08 × 10−6 | 2.61 × 10−4 ± 8.70 × 10−6 | 1.45 × 10−3 ± 3.33 × 10−5 | |
| NbTi + CrTa | D | - | 2.27 × 10−3 ± 5.86 × 10−6 | - | 5.31 × 10−4 ± 5.69 × 10−6 | 3.18 × 10−4 ± 5.46 × 10−6 | 1.14 × 10−3 ± 1.65 × 10−5 |
| ID | - | 2.49 × 10−3 ± 1.21 × 10−5 | - | 5.56 × 10−4 ± 6.18 × 10−6 | 1.83 × 10−4 ± 5.38 × 10−6 | 1.54 × 10−3 ± 1.15 × 10−5 | |
| NbTi + AlMo | D | 7.55 × 10−6 ± 1.01 × 10−6 | - | 6.06 × 10−4 ± 2.80 × 10−5 | 2.78 × 10−4 ± 6.79 × 10−6 | - | 2.24 × 10−4 ± 7.36 × 10−6 |
| ID | 1.49 × 10−5 ± 4.64 × 10−7 | - | 3.48 × 10−4 ± 1.13 × 10−5 | 2.80 × 10−4 ± 3.21 × 10−6 | - | 2.65 × 10−4 ± 6.74 × 10−6 | |
| NbTi + AlTa | D | 9.13 × 10−7 ± 4.14 × 10−9 | - | - | 6.28 × 10−4 ± 8.56 × 10−6 | 3.39 × 10−4 ± 3.67 × 10−6 | 6.85 × 10−4 ± 2.09 × 10−6 |
| ID | 1.36 × 10−6 ± 9.96 × 10−8 | - | - | 6.68 × 10−4 ± 4.18 × 10−6 | 2.30 × 10−4 ± 1.77 × 10−5 | 7.98 × 10−4 ± 1.37 × 10−5 | |
| NbTi + AlCrMo | D | 2.34 × 10−6 ± 6.77 × 10−8 | 4.14 × 10−3 ± 3.08 × 10−5 | 8.65 × 10−4 ± 1.44 × 10−5 | 1.91 × 10−4 ± 3.84 × 10−6 | - | 2.72 × 10−4 ± 7.10 × 10−6 |
| ID | 3.50 × 10−6 ± 3.08 × 10−7 | 3.23 × 10−3 ± 1.72 × 10−4 | 3.62 × 10−4 ± 6.96 × 10−5 | 1.81 × 10−4 ± 8.17 × 10−7 | - | 5.71 × 10−4 ± 4.94 × 10−5 | |
| NbTi + AlMoTa | D | 8.92 × 10−7 ± 1.11 × 10−8 | - | 2.28 × 10−4 ± 4.03 × 10−6 | 2.96 × 10−4 ± 2.99 × 10−6 | 1.97 × 10−4 ± 1.84 × 10−6 | 3.66 × 10−4 ± 2.41 × 10−6 |
| ID | 2.63 × 10−6 ± 6.24 × 10−7 | - | 1.47 × 10−4 ± 1.44 × 10−5 | 3.37 × 10−4 ± 1.24 × 10−7 | 1.11 × 10−4 ± 1.52 × 10−5 | 5.41 × 10−4 ± 1.85 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-C.; Lien, Y.-W.; Moreau, L.E.; Murakami, H.; Lo, K.-C.; Gorsse, S.; Yeh, A.-C. High-Temperature Oxidation of NbTi-Bearing Refractory Medium- and High-Entropy Alloys. Materials 2024, 17, 4579. https://doi.org/10.3390/ma17184579
Lin W-C, Lien Y-W, Moreau LE, Murakami H, Lo K-C, Gorsse S, Yeh A-C. High-Temperature Oxidation of NbTi-Bearing Refractory Medium- and High-Entropy Alloys. Materials. 2024; 17(18):4579. https://doi.org/10.3390/ma17184579
Chicago/Turabian StyleLin, Wei-Chih, Yi-Wen Lien, Louis Etienne Moreau, Hideyuki Murakami, Kai-Chi Lo, Stéphane Gorsse, and An-Chou Yeh. 2024. "High-Temperature Oxidation of NbTi-Bearing Refractory Medium- and High-Entropy Alloys" Materials 17, no. 18: 4579. https://doi.org/10.3390/ma17184579





