Analysis of Thermophysical Properties of Electro Slag Remelting and Evaluation of Metallographic Cleanliness of Steel
Abstract
:1. Introduction
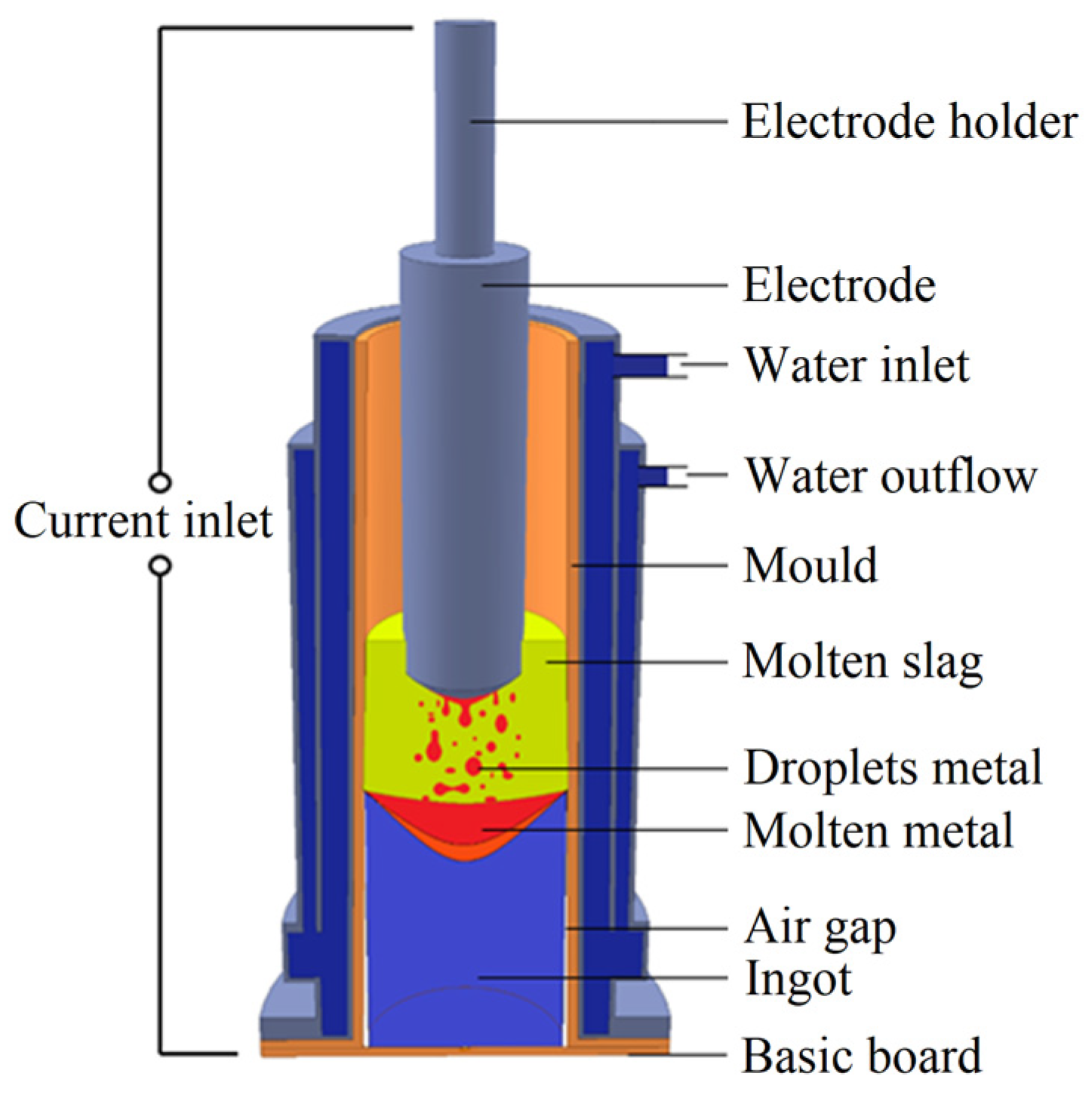
2. Materials and Methods
2.1. Defining the Input Data
2.2. Setting the Simulation and Calculations
2.3. Experimental Validation and Analyses
3. Results
3.1. ESR Slags Melting Intervals
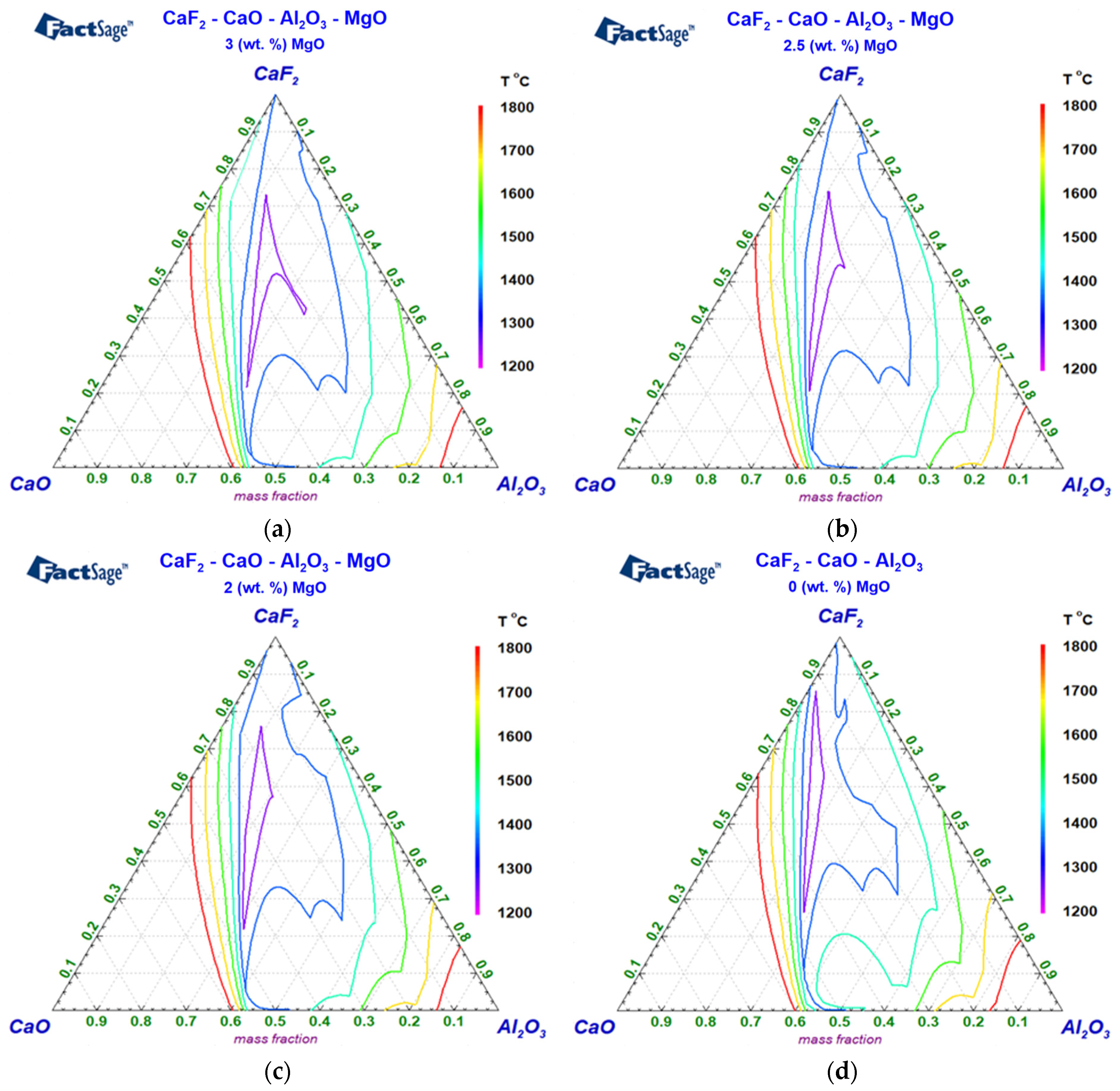
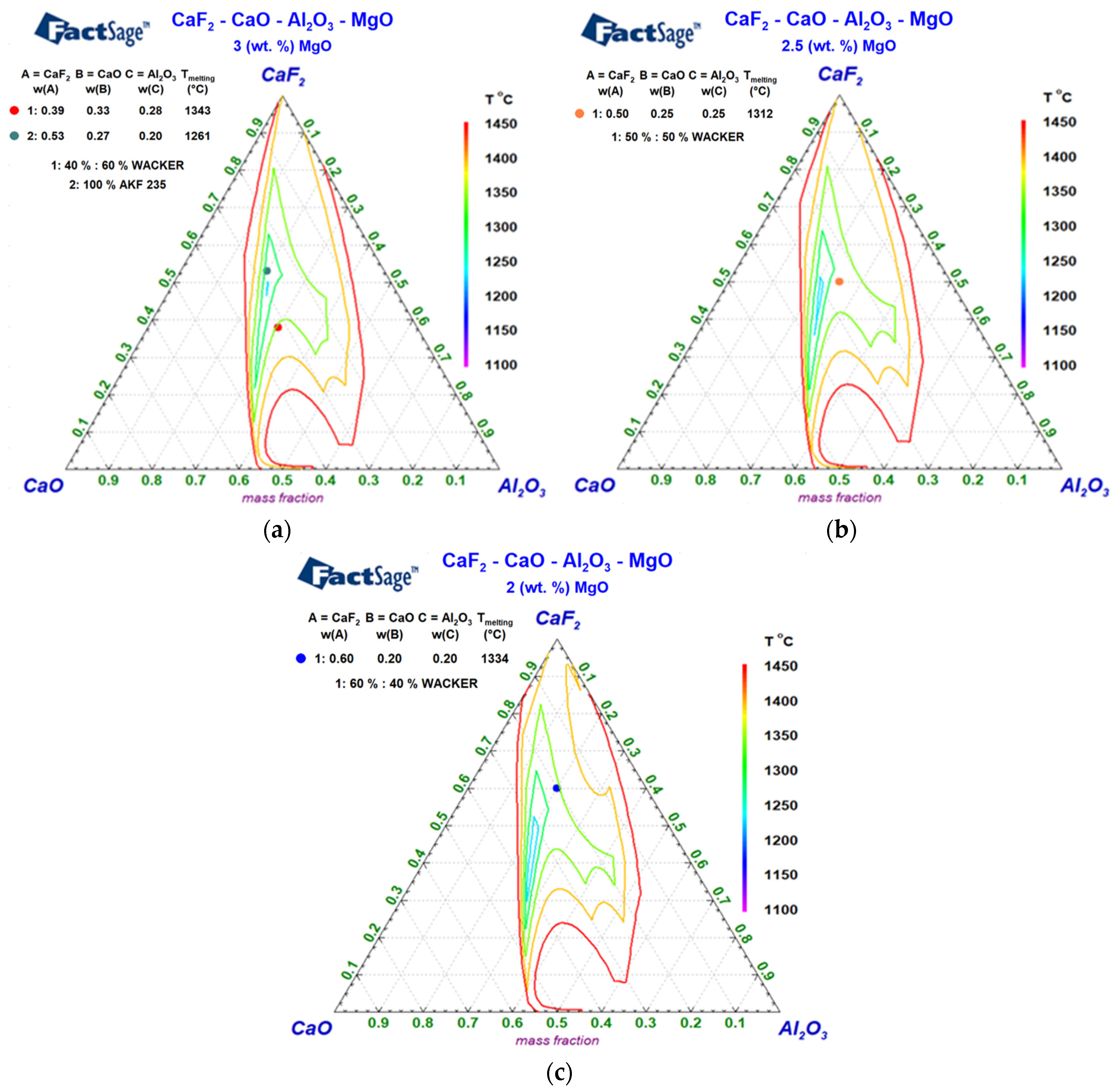
3.2. ESR Slags’ Solid and Liquid Temperatures and Quaternary Diagrams
3.3. Viscosity of Slags
3.4. Determination of Steel Melting Temperature
3.5. Metallographic Cleanliness of Steel
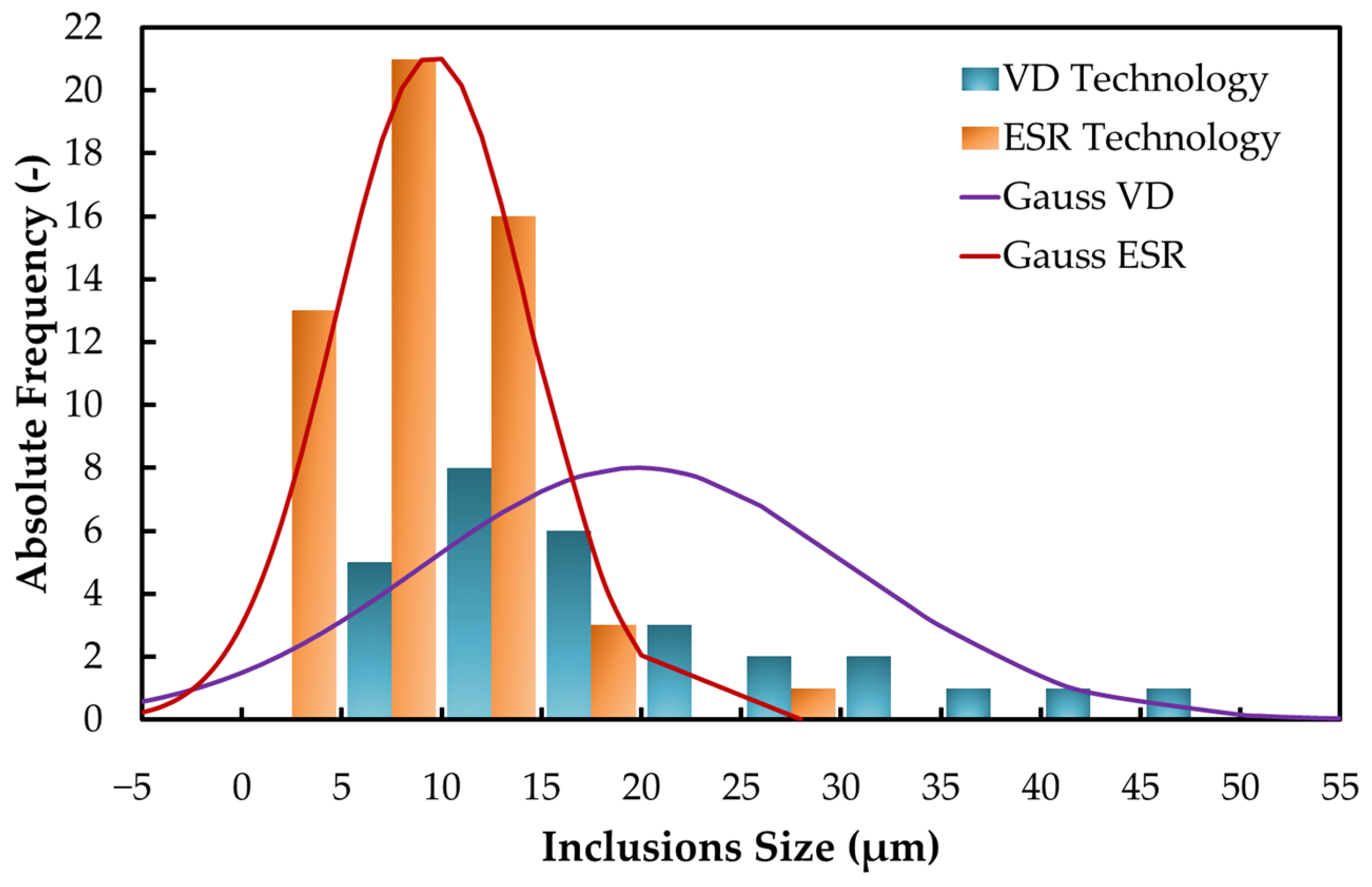
3.6. Evaluation of Non-Metallic Inclusions
3.7. Mechanical Properties
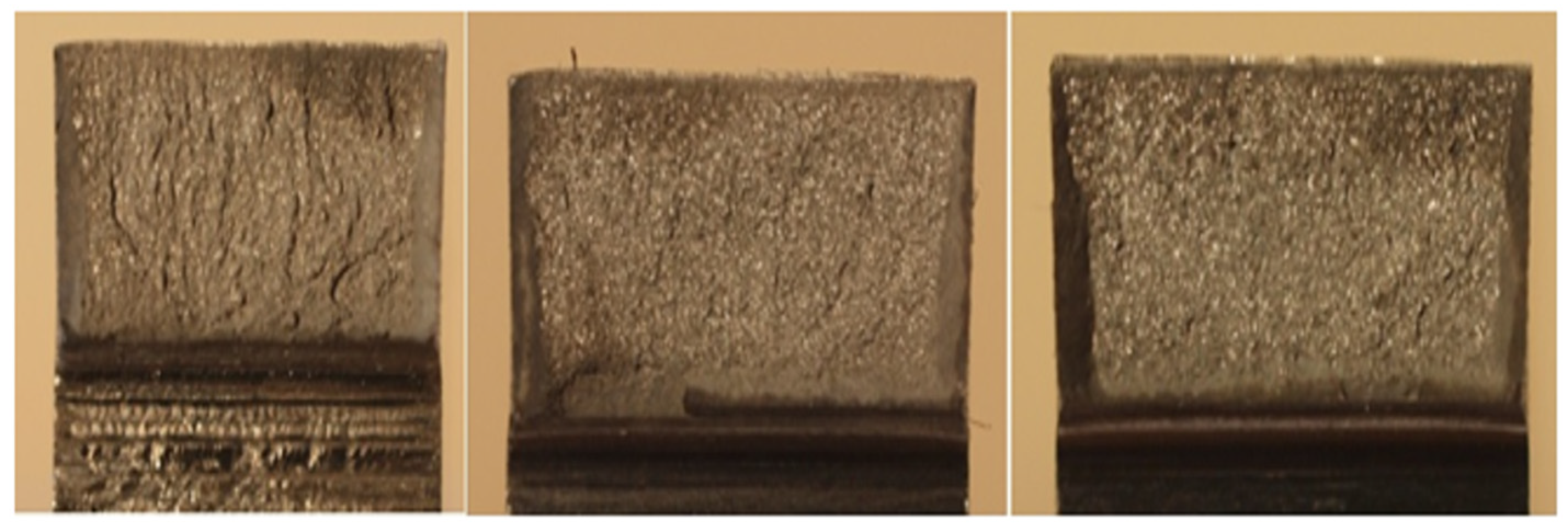
3.8. Fractography
3.9. Discussion of Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macháčková, A.; Kocich, R.; Bojko, M.; Kunčická, L.; Polko, K. Numerical and experimental investigation of flue gases heat recovery via condensing heat exchanger. Int. J. Heat Mass Trans. 2018, 124, 1321–1333. [Google Scholar] [CrossRef]
- Král, P.; Blum, W.; Dvořák, J.; Yurchenko, N.; Stepanov, N.; Zherebtsov, S.; Kunčická, L.; Kvapilová, M.; Sklenička, V. Creep behavior of an AlTiVNbZr0.25 high entropy alloy at 1073 K. Mater. Sci. Eng. A 2020, 783, 139291. [Google Scholar] [CrossRef]
- Kunčická, L.; Kocich, R. Comprehensive Characterisation of a Newly Developed Mg-Dy-Al-Zn-Zr Alloy Structure. Metals 2018, 8, 73. [Google Scholar] [CrossRef]
- Kocich, R.; Kursa, M.; Szurman, I.; Dlouhý, A. The influence of imposed strain on the development of microstructure and transformation characteristics of Ni-Ti shape memory alloys. J. Alloys Comp. 2011, 509, 2716–2722. [Google Scholar] [CrossRef]
- Kocich, R.; Kursa, M.; Macháčková, A. FEA of Plastic Flow in AZ63 Alloy during ECAP Process. Acta Phys. Pol. A 2012, 122, 581–587. [Google Scholar] [CrossRef]
- Weber, V.; Jardy, A.; Dussoubs, B.; Ablitzer, D.; Rybéron, S.; Schmitt, V.; Hans, S.; Poisson, H. A Comprehensive Model of the Electroslag Remelting Process: Description and Validation. Metall. Mater. Trans. B. 2009, 40, 271–280. [Google Scholar] [CrossRef]
- Shi, C.; Chen, X.; Guo, H.; Zhu, Z.; Ren, H. Assessment of Oxygen Control and Its Effect on Inclusion Characteristics during Electroslag Remelting of Die Steel. Steel Res. Int. 2012, 83, 472–486. [Google Scholar] [CrossRef]
- Li, S.; Cheng, G.; Miao, Z.; Chen, L.; Li, C.; Jiang, X. Kinetic Analysis of Aluminum and Oxygen Variation of G20CrNi2Mo Bearing Steel during Industrial Electroslag Remelting Process. ISIJ Int. 2017, 57, 2148–2156. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, Q.; Fu, T.; Wang, Z. Superior Through-Thickness Homogeneity of Microstructure and Mechanical Properties of Ultraheavy Steel Plate by Advanced Casting and Quenching Technologies. Steel Res. Int. 2021, 92, 2000698. [Google Scholar] [CrossRef]
- Ju, J.; Zhu, Z.; Gu, Y.; Yang, K.; Zhang, Q. Evolution of Inclusions in Incoloy825 during Electroslag Remelting. Metals 2021, 12, 208. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Michelic, S.; Wei, F.; Zhuang, C.; Han, Z.; Li, S. Characteristics of AIN inclusions in low carbon Fe-Mn-Si-Al TWIP steel produced by AOD-ESR method. Ironmak. Steelmak. 2016, 43, 171–179. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Jojo-Cunningham, Y.; Silaen, A.; Walla, N.; Zhou, C. Mixing Time Prediction in a Ladle Furnace. Metals 2024, 14, 518. [Google Scholar] [CrossRef]
- Artyushov, V.N.; Bochkarev, S.P.; Igolkin, S.V.; Makarevich, A.N.; Fomchenko, S.M. Mastering a Technology for Making Stainless Steel on a VOD Unit at the Chelyabinsk Metallurgical Combine. Metallurgist 2013, 57, 292–294. [Google Scholar] [CrossRef]
- Malayeri, K.M. Vacuum degassing impact on non-metallic inclusions during clean steelmaking. Ironmak. Steelmak. 2023, 50, 878–883. [Google Scholar] [CrossRef]
- Tridello, A.; Paolino, D.S.; Chiandussi, G.; Rossetto, M. Effect of electroslag remelting on the VHCF response of an AISI H13 steel. Fatique Fract. Eng. Mater. Struct. 2017, 40, 1783–1794. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Yang, S.; Ma, J.; Hao, Y.; Zhang, P. Microstructure and mechanical properties investigation of carbon/stainless steel composite rod prepared by a special device. Mater. Lett. 2023, 352, 135195. [Google Scholar] [CrossRef]
- Sun, C.; Li, J.; Zhang, J.; Yan, W.; Li, S. Formation and evolution of primary carbides in high-carbon martensitic stainless steel. J. Iron Steel Res. Int. 2023, 30, 2000–2009. [Google Scholar] [CrossRef]
- Kang, C.; Liu, F.; Geng, X.; Jiang, Z.; Chen, K.; Gao, J.; An, R. Desulfurization Behavior of Low-sulfur Plastic Die Steel during ESR Process under Different Atmospheres. ISIJ Int. 2021, 61, 219–228. [Google Scholar] [CrossRef]
- Huang, X.; Duan, Y.; Liu, Z.; Li, B.; Wang, F. Role of Electrode Rotation on Improvement of Metal Pool Profile in Electroslag Remelting Process. Metals 2021, 11, 1675. [Google Scholar] [CrossRef]
- Shi, C.; Huang, Y.; Zhang, J.; Li, J.; Zheng, X. Review on desulfurization in electroslag remelting. Int. J. Miner. Metall. Mater. 2021, 28, 18–29. [Google Scholar] [CrossRef]
- Boštajn, A.; Podgornik, B.; Burja, J. Electroslag Remelting: A Process Overview. Mater. Technol. 2016, 50, 971–979. [Google Scholar]
- Li, W.; Wang, W.; Hu, Y.; Chen, Y. The Estimation and Control of the Electroslag Remelting Melt Rate by Mechanism-Based Modeling. Metall. Mater. Trans. B 2012, 43, 276–289. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, Y.; Chen, W.; Li, J. Characteristics and Formation Tendency of Freckle Segregation in Electroslag Remelted Bearing Steel. Metals 2020, 10, 246. [Google Scholar] [CrossRef]
- Shi, C. Deoxidation of Electroslag Remelting (ESR)—A Review. ISIJ Int. 2020, 60, 1083–1096. [Google Scholar] [CrossRef]
- Hou, D.; Jiang, Z.; Dong, Y.; Li, Y.; Gong, W.; Liu, F. Mass Transfer Model of Desulfurization in the Electroslag Remelting Process. Metall. Mater. Trans. 2017, 48, 1885–1897. [Google Scholar] [CrossRef]
- Wang, Q.; He, Z.; Li, G.; Li, B.; Zhu, C.; Chen, P. Numerical investigation of desulfurization behavior in electroslag remelting process. Int. J. Heat Mass Transf. 2017, 104, 943–951. [Google Scholar] [CrossRef]
- Karimi-Sibaki, E.; Khricha, A.; Vakhrushev, A.; Wu, M.; Ludwig, A.; Bohacek, J. Investigation of effect of electrode polarity on electrochemistry and magnetohydrodynamics using tertiary current distribution in electroslag remelting process. J. Iron Steel Res. Int. 2021, 28, 1551–1561. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Lu, R.; Wang, F.; He, Z.; Li, G. Influence of Electro-Emulsification on Desulfurization of Rejected Electrolytic Manganese Metal in Electroslag Remelting Process. Metall. Mater. Trans. B 2020, 52, 107–122. [Google Scholar] [CrossRef]
- Sun, D.; Wang, Y.; Jin, L.; Pang, Z.; Huang, J.; Zhang, J. Controlling oxygen content in electro-slag remelting steel by optimizing slag-steel reaction process. China Foundry 2023, 20, 503–510. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Wang, M.; Zhao, L. Effect of electrical parameters and slag system on macrostructure of electroslag ingot. China Foundry 2024, 21, 44–50. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, C.; Wan, X.; Liang, Y.; Li, J.; Liu, S. Viscosity and surface tension of CaF2–CaO–Al2O3-based slag with varying SiO2 and B2O3 contents for ESR of rotor steel. J. Iron Steel Res. Int. 2023, 30, 74–81. [Google Scholar] [CrossRef]
- Schneider, R.S.E.; Molnar, M.; Gelder, S.; Reiter, G.; Martinez, C. Effect of the Slag Composition and a Protective Atmosphere on Chemical Reactions and Non-Metallic Inclusions during Electro-Slag Remelting of a Hot-Work Tool Steel. Steel Res. Int. 2018, 89, 1800161. [Google Scholar] [CrossRef]
- Jiang, M.; Li, K.; Wang, R.; Yang, E.; Wang, X. Cleanliness and Control of Inclusions in Al-Deoxidized Bearing Steel Refined by Basic Slags during LF-VD-Ar Bubbling. ISIJ Int. 2022, 62, 124–132. [Google Scholar] [CrossRef]
- Duan, S.; Shi, X.; Zhang, M.; Li, B.; Yang, W.; Wang, F.; Guo, H.; Guo, J. Effect of Slag Composition on the Deoxidation and Desulfurization of Inconel 718 Superalloy by ESR Type Slag Without Deoxidizer Addition. Metall. Mater. Trans. B. 2020, 51, 353–364. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Li, X.; Cui, Y.; Lu, X. Mechanism of Slag Composition Change During Electroslag Remelting Process. J. Iron Steel Res. Int. 2011, 18, 24–53. [Google Scholar] [CrossRef]
- Du, Y.; Dong, Y.; Jiang, Z.; Stovpchenko, G.; Li, Y.; Huang, J.; Wang, X.; Liu, Y. Dissolution kinetics and reaction mechanism of Al2O3 in molten CaF2–CaO–Al2O3 slag. J. Iron Steel Res. Int. 2024, 31, 861–869. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Zhang, J.; Shi, C. Effect of nitrogen on microstructure and microsegregation of martensitic stainless steel 4Cr13 produced by electroslag remelting. J. Iron Steel Res. Int. 2023, 30, 1854–1861. [Google Scholar] [CrossRef]
- Persson, E.S.; Brorson, S.; Mitchell, A.; Jonsson, P.G. Impact of Solidification on Inclusion Morphology in ESR and PESR Remelted Martensitic Stainless Steel Ingots. Metals 2021, 11, 408. [Google Scholar] [CrossRef]
- Brooksbank, D.; Andrews, K.W. Stress fields around inclusions and their relation to mechanical properties. JISI 1972, 210, 246–255. [Google Scholar]
- Kiessling, R.; Lange, N. Non-Metallic Inclusions in Steel; Metals Society: London, UK, 1976; pp. 74–101. [Google Scholar]
- Gladman, T. Sulphide Inclusions in Steel; ASM: New York, NY, USA, 1974; pp. 273–285. [Google Scholar]
- Schneider, R.S.E.; Molnar, M.; Klosch, G.; Schuller, C. Effect of the Al2O3 Content in the Slag on the Chemical Reactions and Nonmetallic Inclusions during Electroslag Remelting. Metall. Mater. Trans. B 2020, 51, 1904–1911. [Google Scholar] [CrossRef]
- Shi, C.; Wang, H.; Li, J. Effects of Reoxidation of Liquid Steel and Slag Composition on the Chemistry Evolution of Inclusions During Electroslag Remelting. Metall. Mater. Trans. B 2018, 49, 1675–1689. [Google Scholar] [CrossRef]
- Schneider, R.; Wiesinger, V.; Gelder, S.; Reiter, G. Effect of the Slag Composition on the Process Behavior, Energy Consumption, and Nonmetallic Inclusions during Electroslag Remelting. Steel Res. Int. 2023, 94, 2200483. [Google Scholar] [CrossRef]
- Li, S.; Cheng, G.; Miao, Z.; Chen, L.; Jiang, X. Effect of slag on oxide inclusions in carburized bearing steel during industrial electroslag remelting. Int. J. Miner. Metall. Mater. 2019, 26, 291–300. [Google Scholar] [CrossRef]
- Xuan, C.; Persson, E.S.; Sevastopolev, R.; Nzotta, M. Motion and Detachment Behaviors of Liquid Inclusion at Molten Steel-Slag Interfaces. Metall. Mater. Trans. B 2019, 50, 1957–1973. [Google Scholar] [CrossRef]
- Franceschini, A.; Ruby-Meyer, F.; Midroit, F.; Diawara, B.; Hans, S.; Poulain, T.; Trempint, C.; Hénault, E.; Roufié, A. An assessment of cleanliness techniques for low alloyed steel grades. Metall. Res. Technol. 2019, 116, 509. [Google Scholar] [CrossRef]
- Persson, E.S.; Karasev, A.; Mitchell, A.; Jonsson, P.G. Origin of the Inclusions in Production-Scale Electrodes, ESR Ingots, and PESR Ingots in a Martensitic Stainless Steel. Metals 2020, 10, 1620. [Google Scholar] [CrossRef]
- Liu, M.; Bernhard, M.; Kawuloková, M.; Walek, J.; Kern, M.; Zlá, P.; Presoly, P.; Smetana, B.; Tkadlečková, M.; Xu, G.; et al. Decomposition of γ-Fe in 0.4C-1.8Si-2.8Mn-0.5Al steel during a continuous cooling process: A comparative study using in-situ HT-LSCM, DSC and dilatometry. J. Mater. Res. Technol. 2023, 24, 3534–3547. [Google Scholar] [CrossRef]
- Ahmadian, P.; Taghizadeh, M. The effect of non-metallic inclusion size and orientation on tensile properties of stainless steel (simulation and experiment). Metall. Mater. Eng. 2020, 26, 43–55. [Google Scholar] [CrossRef]
- Řeháčková, L.; Novák, V.; Tokarský, J.; Heger, M.; Zimný, O.; Matýsek, D.; Peikertová, P.; Ritz, M.; Walek, J.; Leinweberová, S. Rheological behaviour of CaO-MgO-SiO2-Al2O3-B2O3 system with varying B2O3 content up to 30 wt% at basicity of 0.4. Ceram. Int. 2024, 50, 1389–1397. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, Y.; Hou, D.; Yang, D.; Zhang, Q.; Hu, Y.; Liu, X. Assessment of Porosity Defects in Ingot Using Machine Learning Methods during Electro Slag Remelting Process. Metals 2022, 12, 958. [Google Scholar] [CrossRef]
- Wang, J.; Song, S.; Xue, Z. Transient evolution of non-metallic inclusions in molten high aluminum and high manganese steel contacting with slag and crucible: Experimental investigation and FactSage macros modeling. J. Mater. Res. Technol. 2023, 25, 2841–2853. [Google Scholar] [CrossRef]
- Singha, P.; Shukla, A. Contribution of Hot-Spot Zone in Decarburization of BOF Steel-Making: Fundamental Analysis Based upon the FactSage-Macro Program. Metals 2022, 12, 638. [Google Scholar] [CrossRef]
- Jung, I.; Van Ende, M. Computational Thermodynamic Calculations: FactSage from CALPHAD Thermodynamic Database to Virtual Process Simulation. Metall. Mater. Trans. B 2020, 51, 1851–1874. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, Y.; Zhang, L. A kinetic model for Ca treatment of Al-killed steels using FactSage macro processing. Ironmak. Steelmak. 2017, 44, 497–504. [Google Scholar] [CrossRef]
- Vidhyasagar, M.; Kumar, D.; Viswanathan, N. A Static Model for Energy-Optimizing Furnace. Steel Res. Int. 2022, 93, 2200185. [Google Scholar] [CrossRef]
- Wang, Z.; Song, T.; Zhao, L.; Bao, Y. Study on Efficient Dephosphorization in Converter Based on Thermodynamic Calculation. Crystals 2023, 13, 1132. [Google Scholar] [CrossRef]
- DIN 50 602; Metallographic Examination–Microcopic Examination of Special Steels Using Standard Diagrams to Assess the Content of Non-Metallic Inclusions; UDC 669.14:620.186.14. Deutsches Institut fur Normung (DIN): Berlin, Germany, 1985.
- Jiang, Z.; Hou, D.; Dong, Y.; Cao, Y.; Cao, H.; Gong, W. Effect of Slag on Titanium, Silicon, and Aluminium Contents in Superalloy During Electroslag Remelting. Metall. Mater. Trans. B 2016, 47, 1465–1474. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, Z.; Geng, X. Design of ESR slag for remelting 9CrMoCoB steel through experiments and thermodynamic calculations. Calphad 2020, 70, 101782. [Google Scholar] [CrossRef]
- Hou, D.; Wang, D.; Qu, T.; Tian, J.; Wang, H. Kinetic Study on Alloying Element Transfer During an Electroslag Remelting Process. Metall. Mater. Trans. B 2019, 50, 3088–3102. [Google Scholar] [CrossRef]
- Shi, C.; Wang, S.; Li, J.; Cho, J. Non-metallic inclusions in electroslag remelting: A review. J. Iron Steel Res. Int. 2021, 28, 1483–1503. [Google Scholar] [CrossRef]
- Kunčická, L.; Kocich, R. Optimizing electric conductivity of innovative Al-Cu laminated composites via thermomechanical treatment. Mater. Des. 2022, 215, 110441. [Google Scholar] [CrossRef]
- Kocich, R.; Macháčková, A.; Fojtík, F. Comparison of strain and stress conditions in conventional and ARB rolling process. Int. J. Mech. Sci. 2012, 64, 54–61. [Google Scholar] [CrossRef]
- Kocich, R.; Kunčická, L. Develpoment of structure and properties in bimetallic Al/Cu sandwich composite during cumulative severe plastic deformation. J. Sand. Struct. Mater. 2021, 23, 4252–4275. [Google Scholar] [CrossRef]
- Kunčická, L.; Kocich, R.; Németh, G.; Dvořák, K.; Pagáč, M. Effect of post process shear straining on structure and mechanical properties of 316L stainless steel manufactured via powder bed fusion. Add. Manuf. 2022, 59, 103128. [Google Scholar]
- Wang, Z.; Chen, J.; Kocich, R.; Tardif, S.; Dolbnya, I.P.; Kunčická, L.; Micha, J.-S.; Liogas, K.; Magdysyuk, O.V.; Szurman, I.; et al. Grain Structure Engineering of NiTi Shape Memory Alloys by Intensive Plastic Deformaiton. ACS Appl. Mater. Interfaces 2022, 14, 31396–31410. [Google Scholar] [CrossRef]
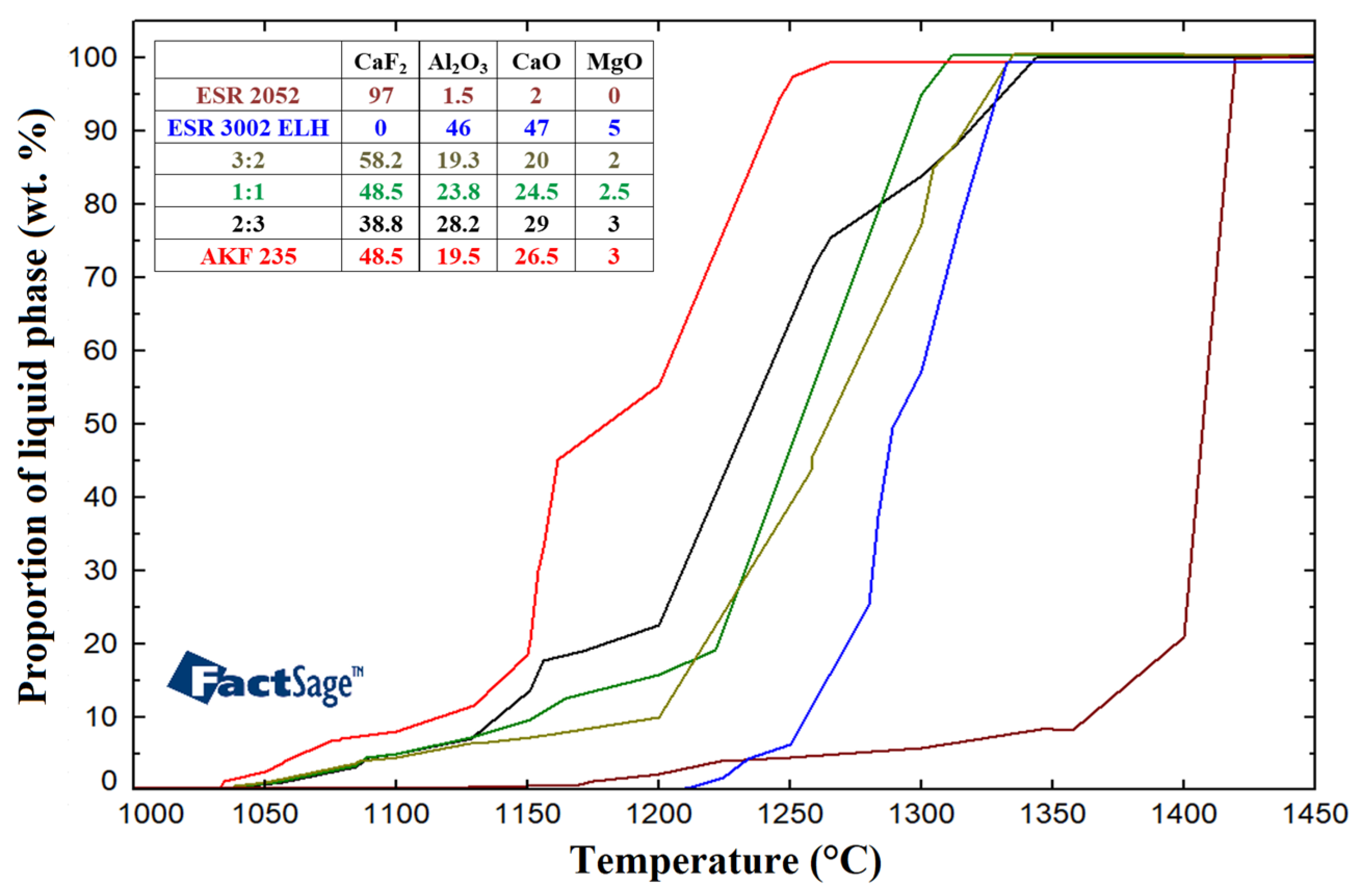
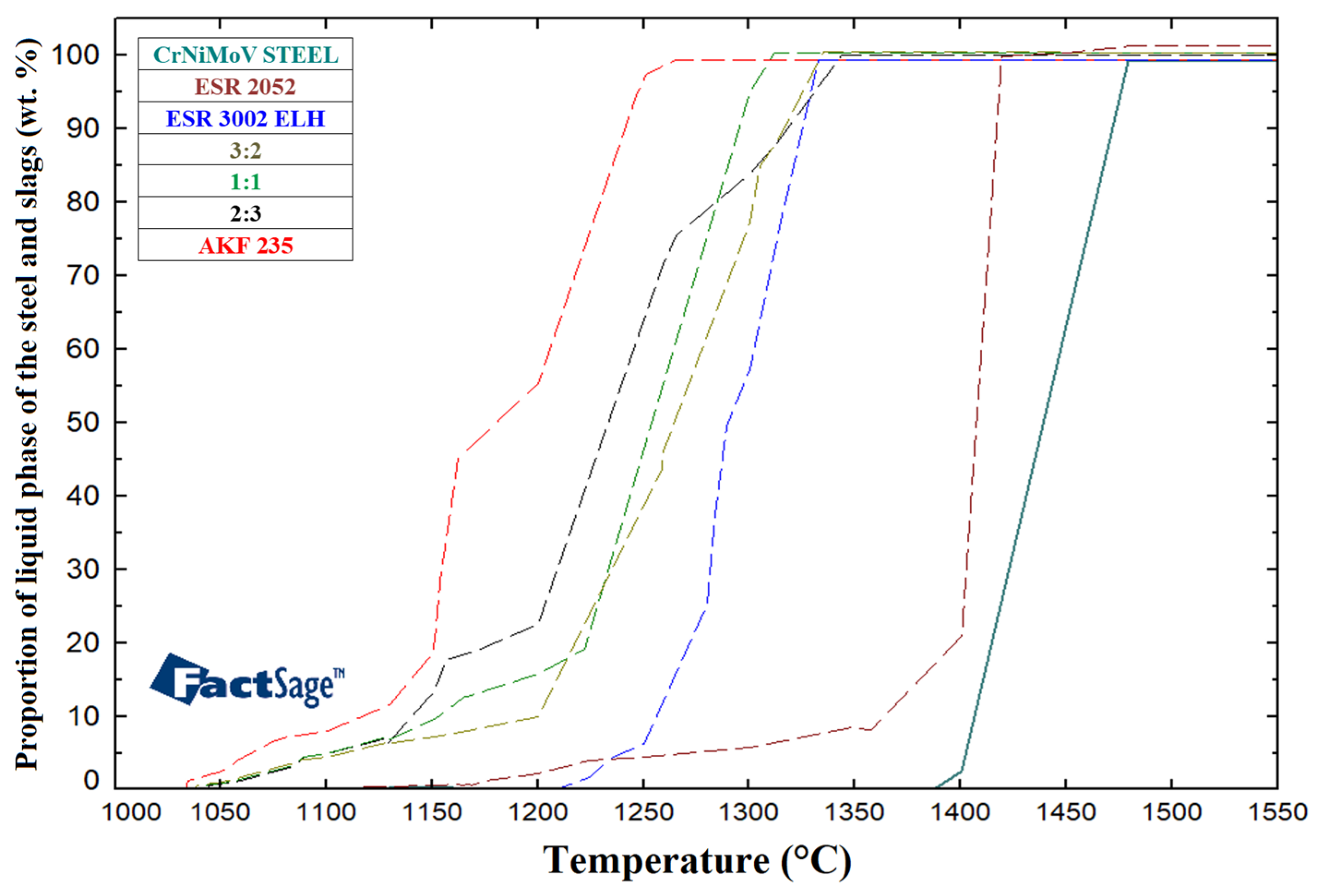
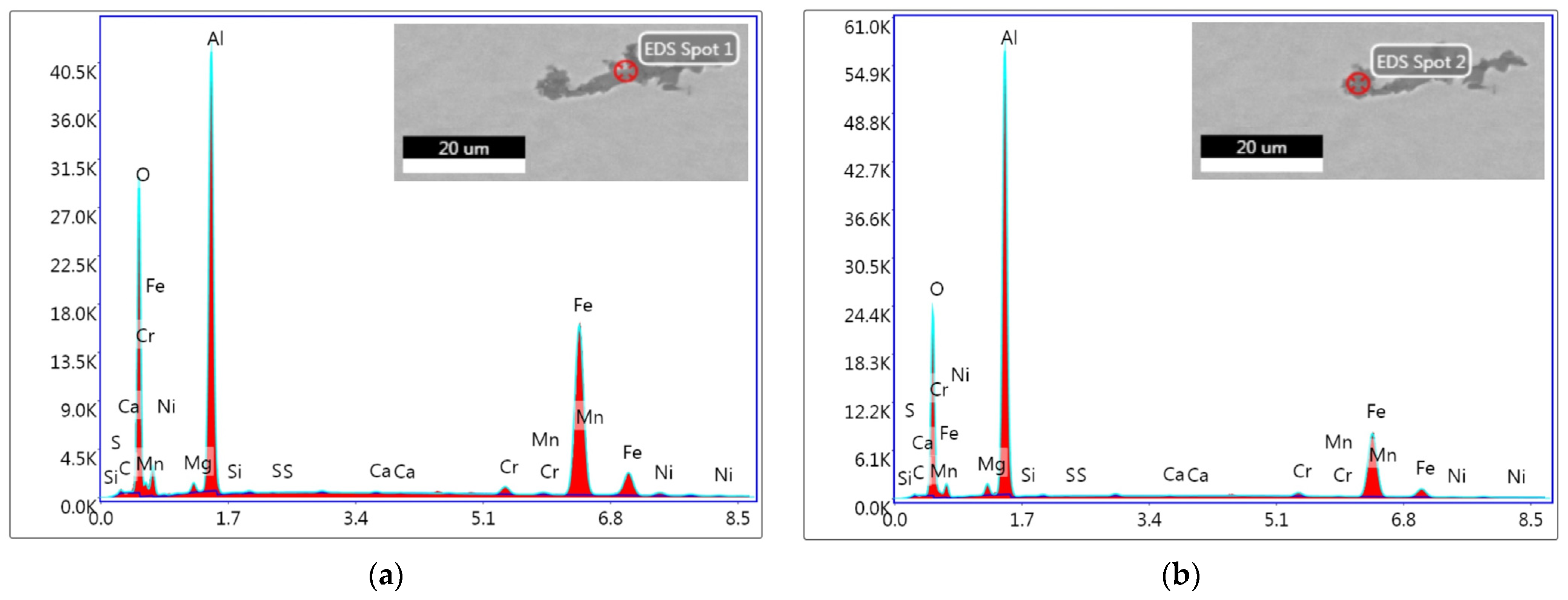
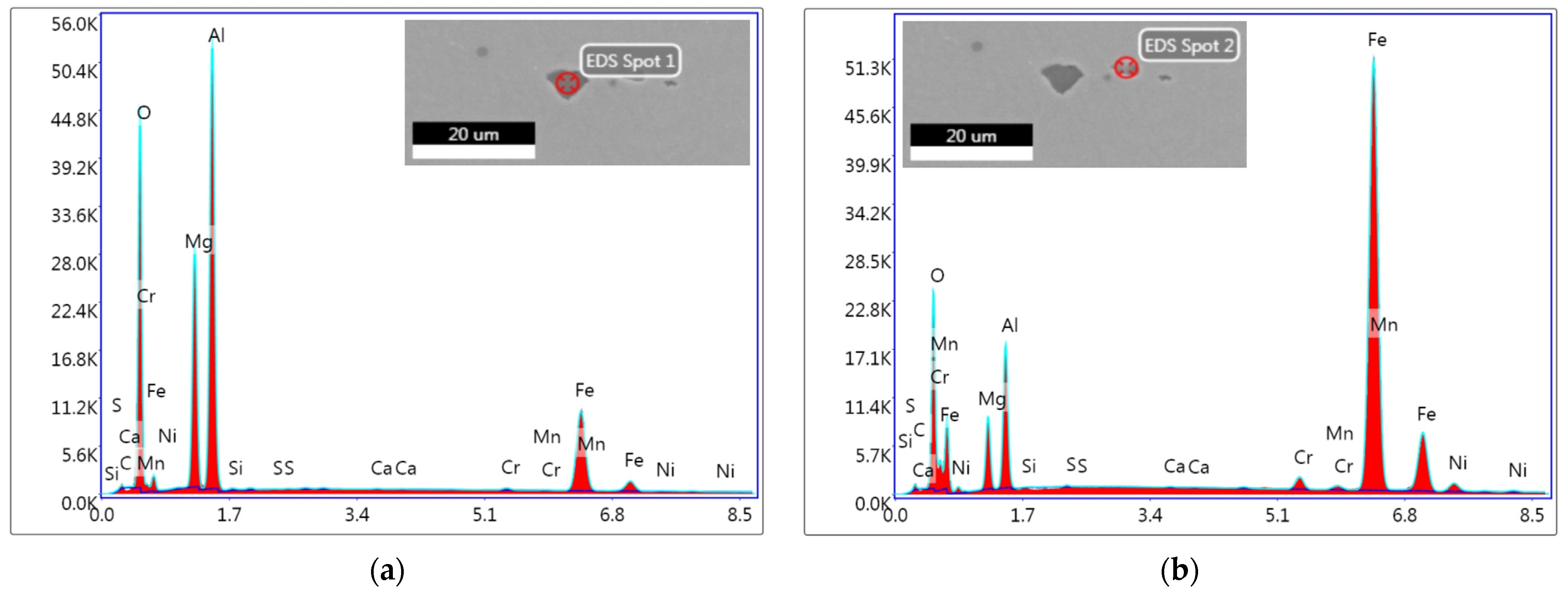
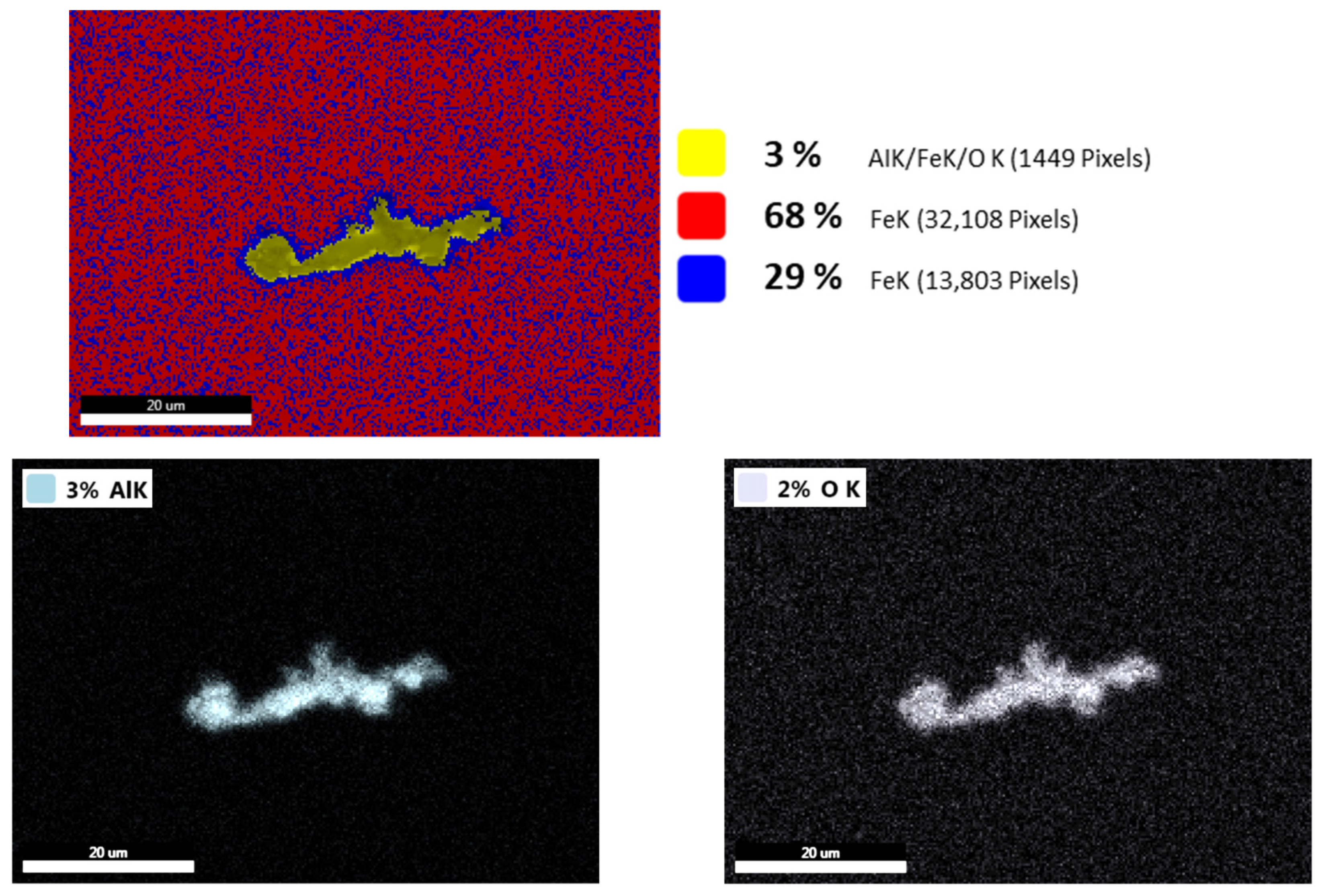


| ESR 2052 | |||||||||||
| CaF2 | Al2O3 | CaO | MgO | SiO2 | TiO2 | FeO | H2O | C | P | S | Pb |
| 97 | 1.5 | 2 | 0 | 0.5 | 0 | 0.2 | 0.005 | 0.03 | 0.005 | 0.03 | 0.0002 |
| ESR 3002 ELH | |||||||||||
| CaF2 | Al2O3 | CaO | MgO | SiO2 | TiO2 | FeO | H2O | C | P | S | Pb |
| 0 | 46 | 47 | 5 | 0.8 | 0.2 | 0.3 | 0.005 | 0.03 | 0.005 | 0.05 | 0 |
| AKF 235 | |||||||||||
| CaF2 | Al2O3 | CaO | MgO | SiO2 | TiO2 | FeO | H2O | C | P | S | Pb |
| 48.5 | 19.5 | 26.5 | 3 | 1 | 0.3 | 0.5 | 0.05 | 0.1 | 0.05 | 0.05 | 0.005 |
| C | Mn | Si | P | S | Cr | Ni | Cu | Mo | V |
|---|---|---|---|---|---|---|---|---|---|
| 0.37 | 0.40 | 0.27 | 0.007 | 0.004 | 1.37 | 3.30 | 0.14 | 0.40 | 0.14 |
| Slag | ESR 2052 and ESR 3002 ELH | AKF 235 | ||||||
|---|---|---|---|---|---|---|---|---|
| Ratio | 2:3 | 1:1 | 3:2 | 1 | ||||
| Temperature (°C) | 1750 | 1800 | 1750 | 1800 | 1750 | 1800 | 1750 | 1800 |
| Viscosity (Pa·s) | 0.017 | 0.015 | 0.014 | 0.012 | 0.011 | 0.009 | 0.013 | 0.011 |
| DIN 50602 | K0 | K1 | K2 | K3 | K4 | |
|---|---|---|---|---|---|---|
| VD technology | minimum | - | - | - | - | 0 |
| average | - | - | - | - | 6.1 | |
| maximum | - | - | - | - | 12.5 | |
| ESR technology | minimum | 0 | 0 | 0 | 0 | 0 |
| average | 4.5 | 1.6 | 0.5 | 0 | 0 | |
| maximum | 14.1 | 6.8 | 4.9 | 0 | 0 | |
| Size of the Non-Metallic Inclusions | ||
|---|---|---|
| (μm) | VD Technology | ESR Technology |
| minimum | 8 | 2 |
| average | 19.8 | 9.5 |
| maximum | 50 | 28 |
| Composition of the Inclusions | VD Technology | ESR Technology |
|---|---|---|
| Al2O3 | 21 | 21 |
| Al2O3-MgO | 7 | 49 |
| Al2O3-CaO | 45 | 13 |
| Al2O3-MgO-CaO | 10 | 8 |
| Al2O3-MgO-CaS-CaO | 3 | 2 |
| MgO | 0 | 2 |
| Al2O3-MgO-CaS | 3 | 0 |
| CaS | 0 | 2 |
| Al2O3-CaS-CaO | 10 | 0 |
| SiO2 | 0 | 2 |
| CaS-CaO | 0 | 2 |
| VD Technology | ||||||||||||||||
| Casting | Ru (MPa) | Rp0.2 (MPa) | Rm (MPa) | A5 (%) | Z (%) | KCU/20 °C (J·cm2) | KCU/−40 °C (J·cm2) | |||||||||
| 1 | 1157 | 1146 | 1309 | 1312 | 1436 | 1443 | 12.1 | 12.1 | 42.5 | 43.5 | 34 | 34 | 34 | 28 | 32 | 30 |
| 2 | 1143 | 1146 | 1330 | 1337 | 1457 | 1461 | 11.3 | 11.3 | 43.8 | 38.9 | 34 | 36 | 35 | 28 | 30 | 32 |
| 3 | 1178 | 1185 | 1348 | 1351 | 1468 | 1468 | 9.8 | 10.0 | 42.5 | 41.7 | 30 | 28 | 29 | 30 | 26 | 28 |
| 4 | 1185 | 1196 | 1348 | 1341 | 1479 | 1482 | 11.2 | 9.0 | 45.5 | 34.9 | 28 | 30 | 29 | 28 | 28 | 28 |
| 5 | 1196 | 1181 | 1362 | 1341 | 1482 | 1461 | 9.7 | 9.7 | 40.2 | 37.0 | 30 | 28 | 29 | 30 | 26 | 28 |
| 6 | 1150 | 1164 | 1326 | 1326 | 1447 | 1450 | 11.2 | 11.2 | 44.3 | 41.2 | 32 | 30 | 31 | 32 | 30 | 31 |
| Ø | 1169 | 1336 | 1461 | 10.7 | 41.3 | 31.2 | 29.2 | |||||||||
| ESR Technology | ||||||||||||||||
| Casting | Ru (MPa) | Rp0.2 (MPa) | Rm (MPa) | A5 (%) | Z (%) | KCU/20 °C (J·cm2) | KCU/−40 °C (J·cm2) | |||||||||
| 1 | 1249 | 1269 | 1359 | 1351 | 1488 | 1474 | 12.4 | 13.0 | 50.8 | 52.0 | 46 | 48 | 47 | 40 | 38 | 39 |
| 2 | 1280 | 1255 | 1416 | 1398 | 1548 | 1541 | 12.0 | 12.2 | 49.5 | 51.6 | 46 | 48 | 47 | 38 | 36 | 37 |
| 3 | 1266 | 1273 | 1406 | 1409 | 1542 | 1542 | 11.4 | 10.3 | 49.6 | 50.9 | 38 | 38 | 38 | 36 | 32 | 34 |
| 4 | 1259 | 1218 | 1404 | 1415 | 1504 | 1518 | 9.7 | 14.0 | 41.9 | 53.3 | 54 | 54 | 54 | 46 | 50 | 48 |
| 5 | 1278 | 1292 | 1360 | 1380 | 1490 | 1502 | 11.4 | 11.2 | 46.7 | 46.3 | 44 | 44 | 44 | 36 | 34 | 35 |
| 6 | 1268 | 1275 | 1446 | 1421 | 1571 | 1555 | 11.9 | 11.7 | 44.3 | 46.7 | 42 | 42 | 42 | 34 | 34 | 34 |
| Ø | 1265 | 1397 | 1523 | 11.8 | 48.6 | 45.3 | 37.8 | |||||||||
| Difference (%) | +8.2 | +4.6 | +4.2 | +9.8 | +17.7 | +45.5 | +29.7 | |||||||||
| p-value | 0 | 3 × 10−13 | 5 × 10−12 | 5 × 10−4 | 1 × 10−12 | 0 | 6 × 10−13 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walek, J.; Odehnalová, A.; Kocich, R. Analysis of Thermophysical Properties of Electro Slag Remelting and Evaluation of Metallographic Cleanliness of Steel. Materials 2024, 17, 4613. https://doi.org/10.3390/ma17184613
Walek J, Odehnalová A, Kocich R. Analysis of Thermophysical Properties of Electro Slag Remelting and Evaluation of Metallographic Cleanliness of Steel. Materials. 2024; 17(18):4613. https://doi.org/10.3390/ma17184613
Chicago/Turabian StyleWalek, Josef, Adéla Odehnalová, and Radim Kocich. 2024. "Analysis of Thermophysical Properties of Electro Slag Remelting and Evaluation of Metallographic Cleanliness of Steel" Materials 17, no. 18: 4613. https://doi.org/10.3390/ma17184613







