Analysis of Ni-Cu Interaction in Aluminum-Based Alloys: Hardness, Tensile and Precipitation Behavior
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
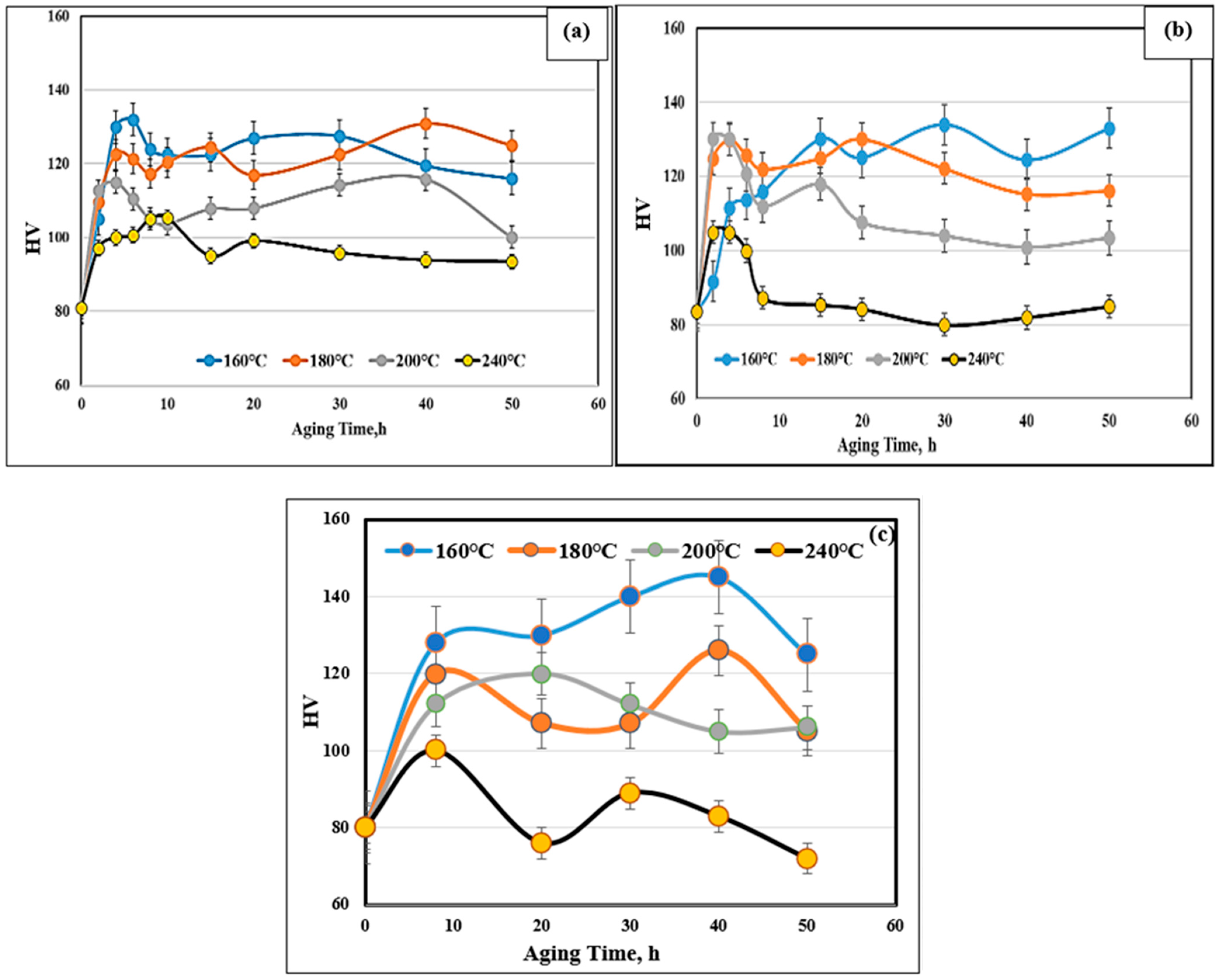
3.1. Microhardness and Tensile Testing
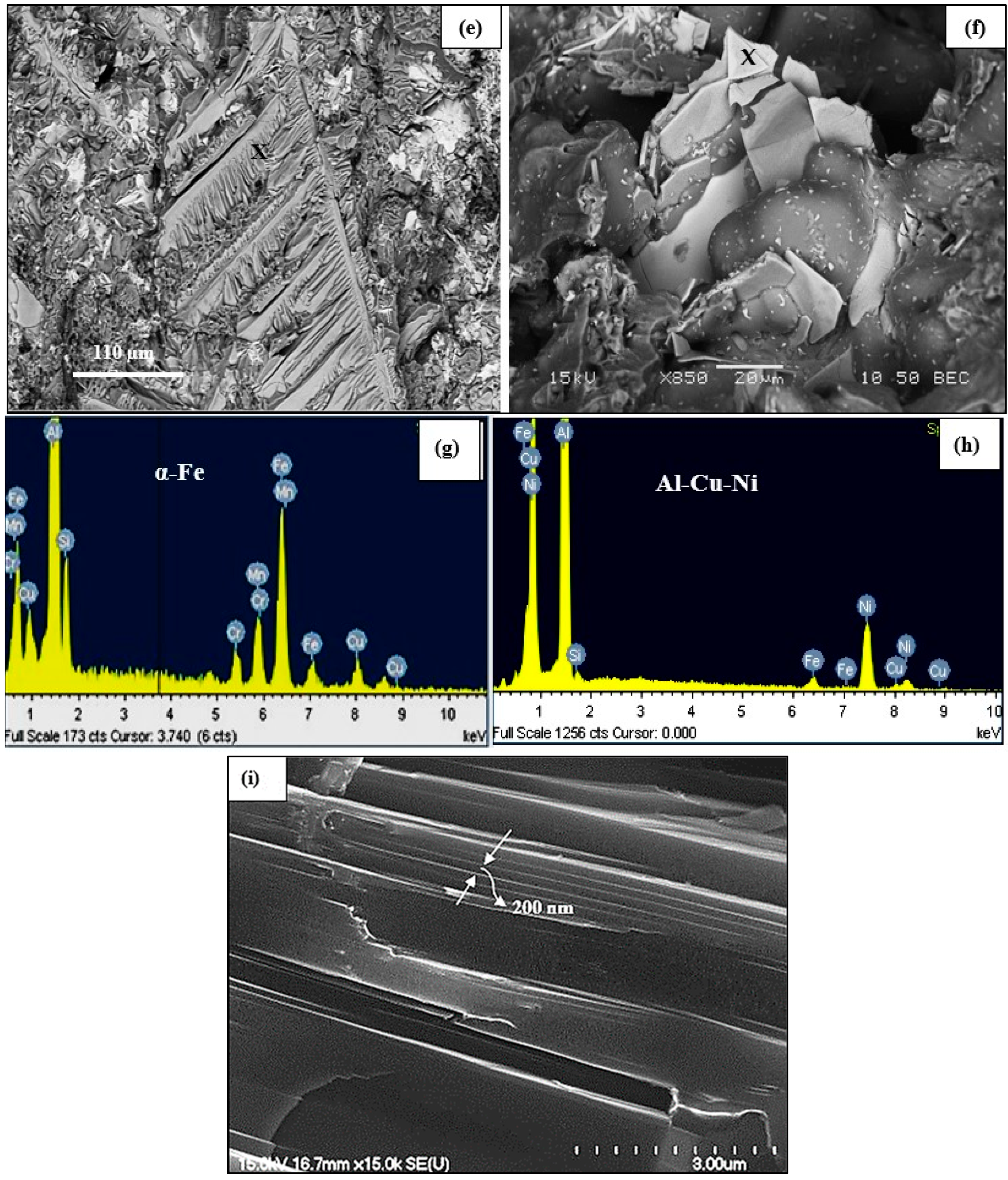
3.2. Microstructural Characterization of As-Cast Alloys
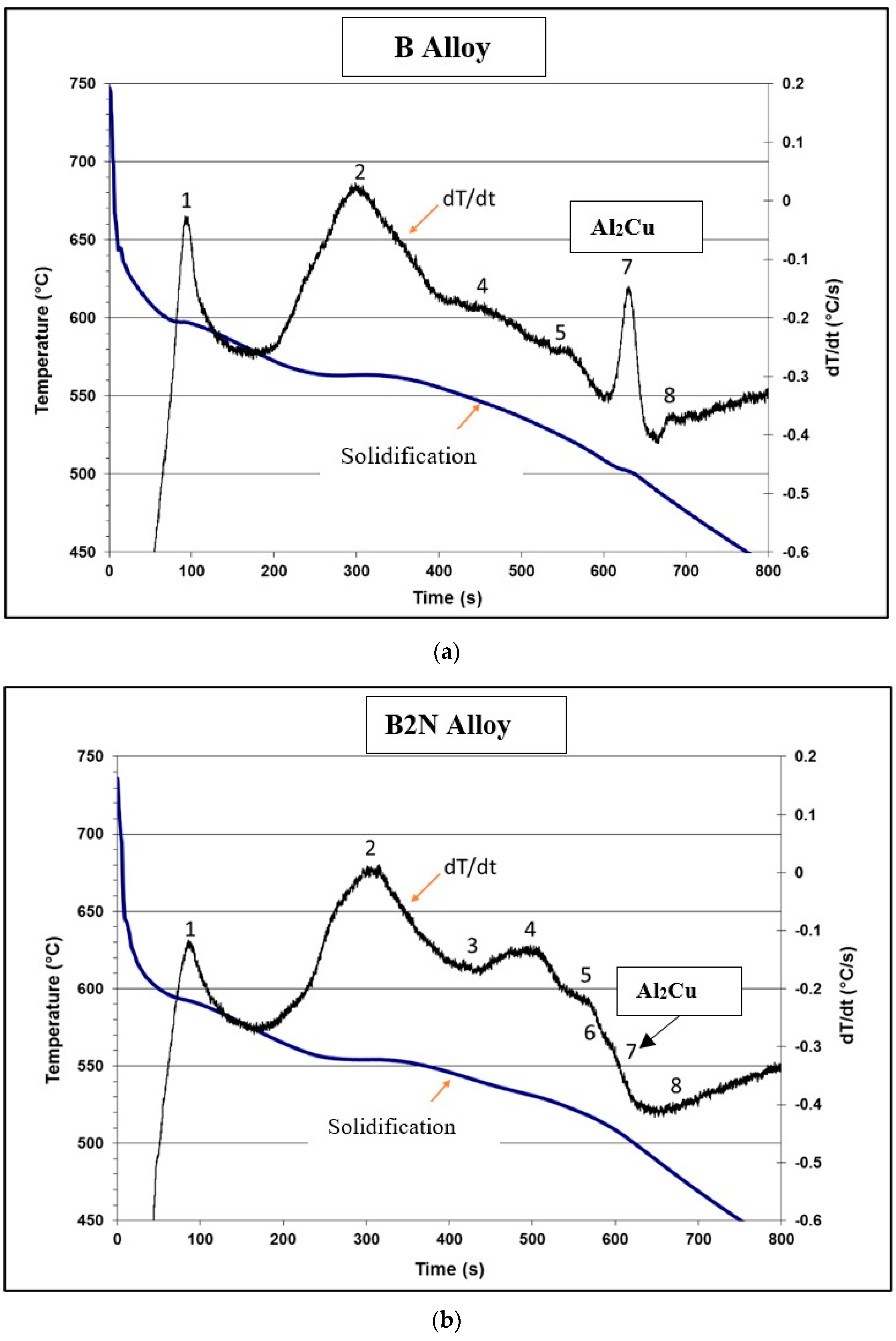
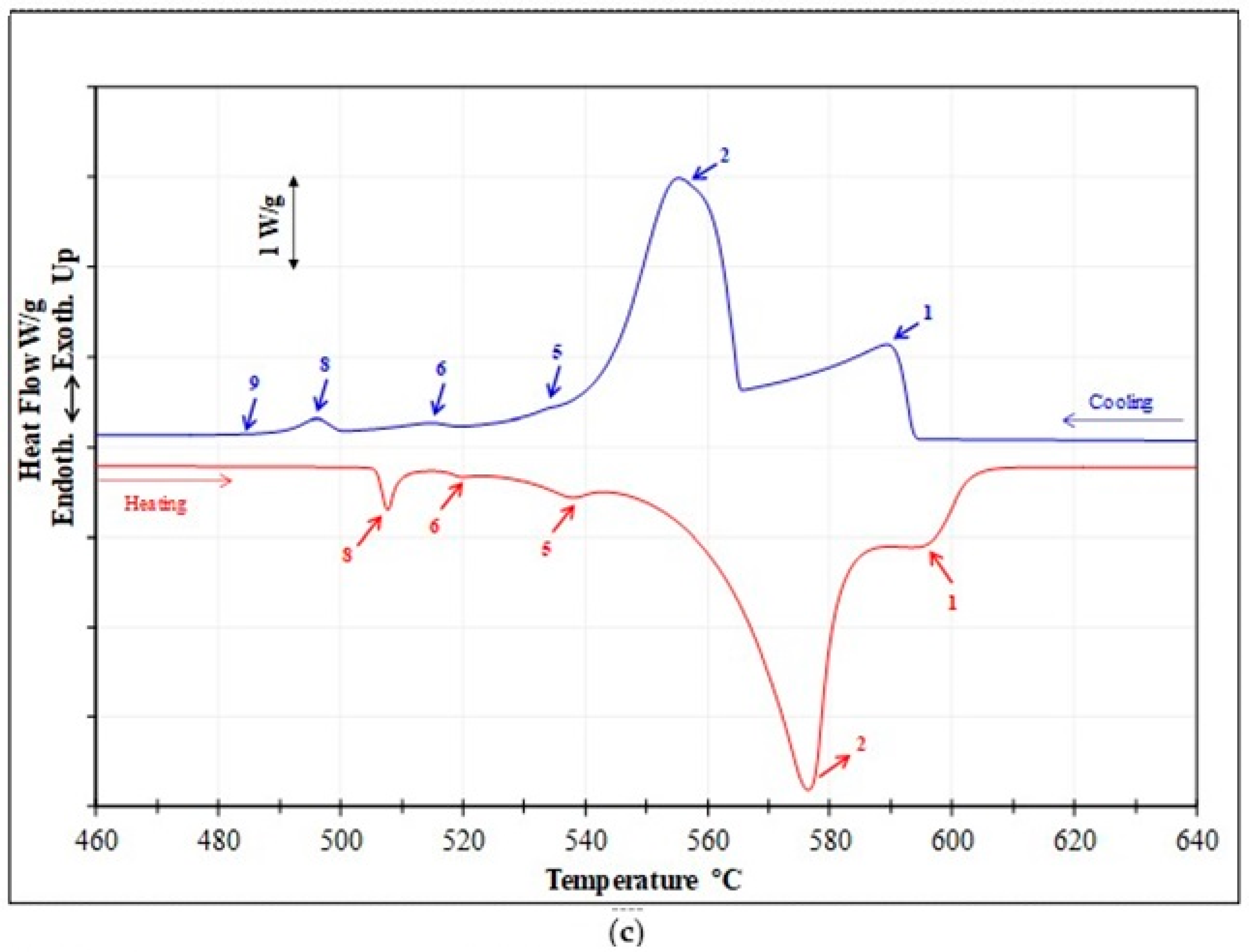
3.2.1. Thermal Analysis
| Reaction # | Transformation Temperature (°C) [24,54,57] | Reactions Occurring during Solidification and Melting | Temperatures Reported during Solidification (°C) | Temperatures Reported during Melting (°C) |
|---|---|---|---|---|
| BS | BS | |||
| 1 | 600–597 | α-Al dendritic network | 590 | 595 |
| 2 | 560–558 | Al-Si eutectic Post-eutectic β-Al5FeSi phase α-Al15(Fe,Mn)3Si2 phase for Mn-containing alloys | 556 | 576 |
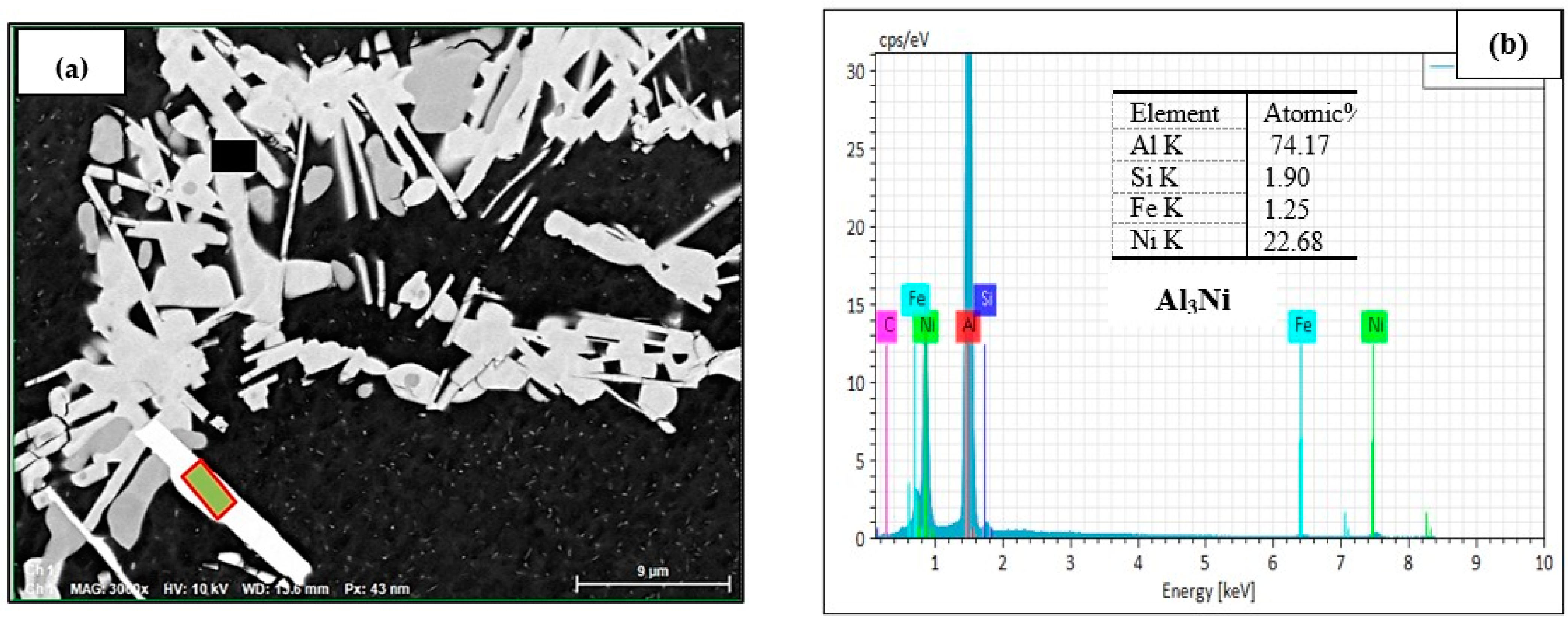
| 3 | 555–556 | Al9FeNi phase | - | - |
| 4 | 546–553 | Al3Ni phase | - | - |
| 5 | 540–538 | Mg2Si phase | 534 | 538 |
| 6 | 525–523 | Transformation of β to π-Al8Mg3FeSi6 phase | 516 | 520 |
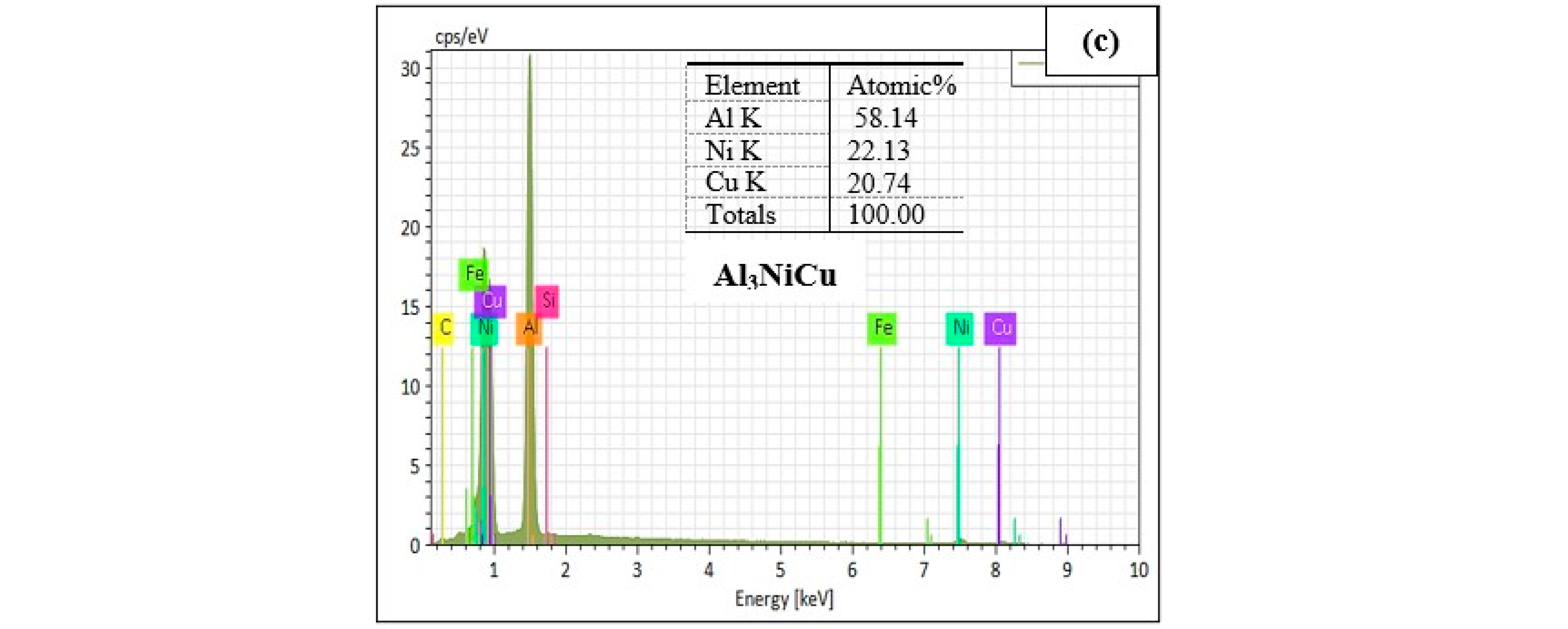
| 7 | 523–520 | Al3CuNi phase | - | - |
| 8 | 500–496 | eutectic Al-Al2Cu phase | 496 | 507 |
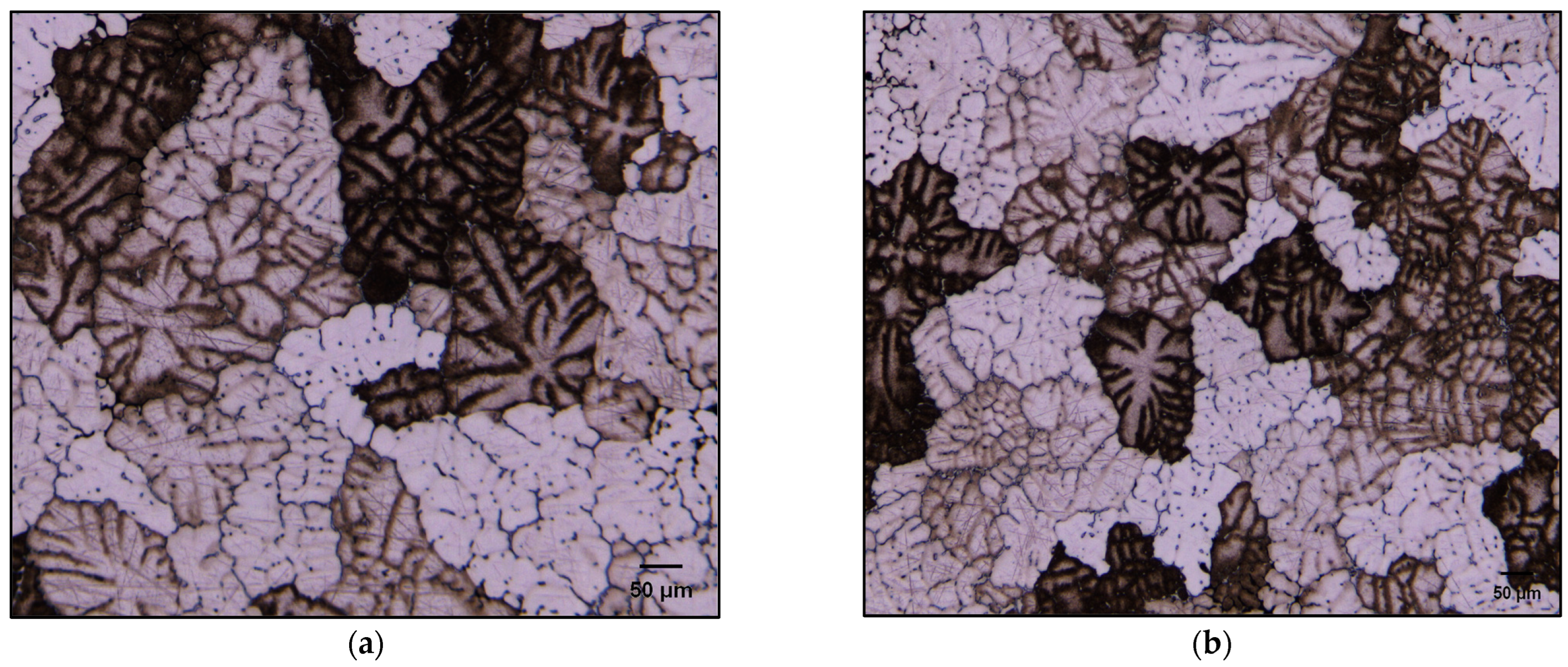
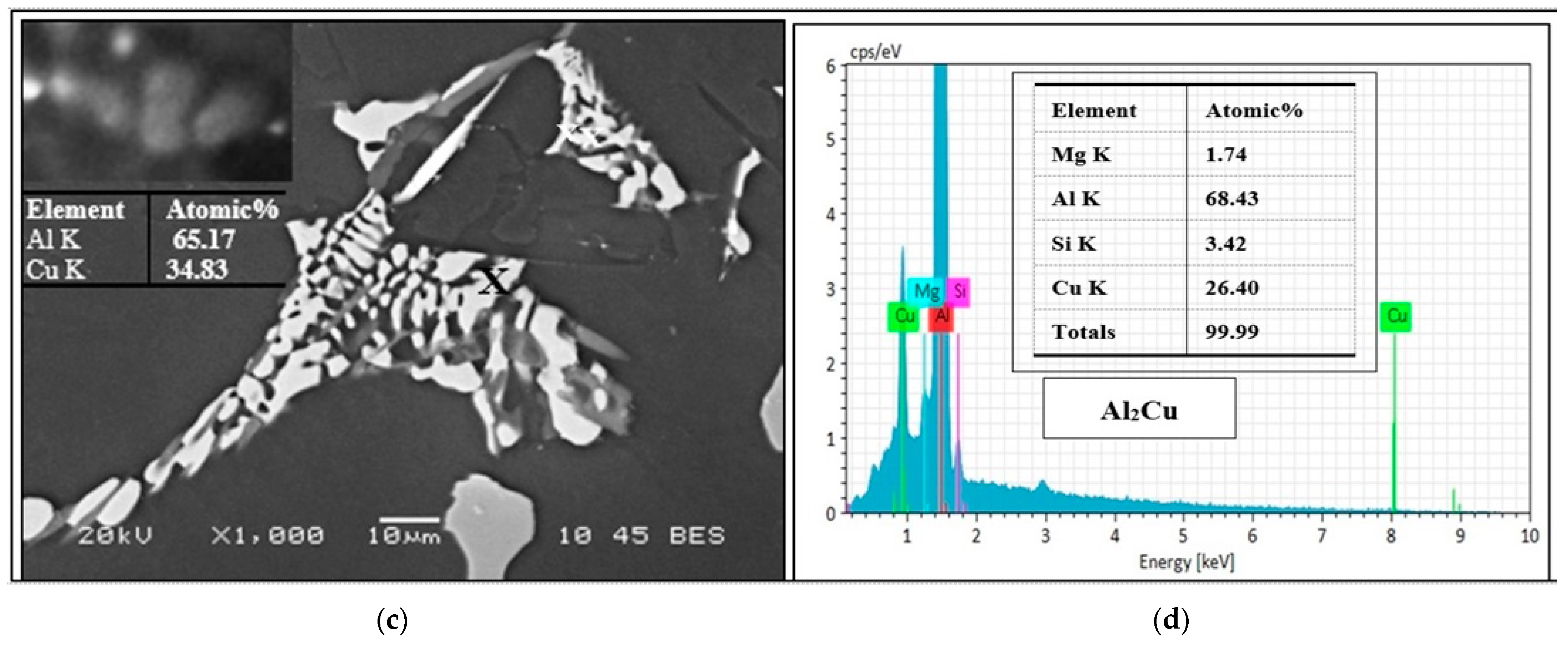
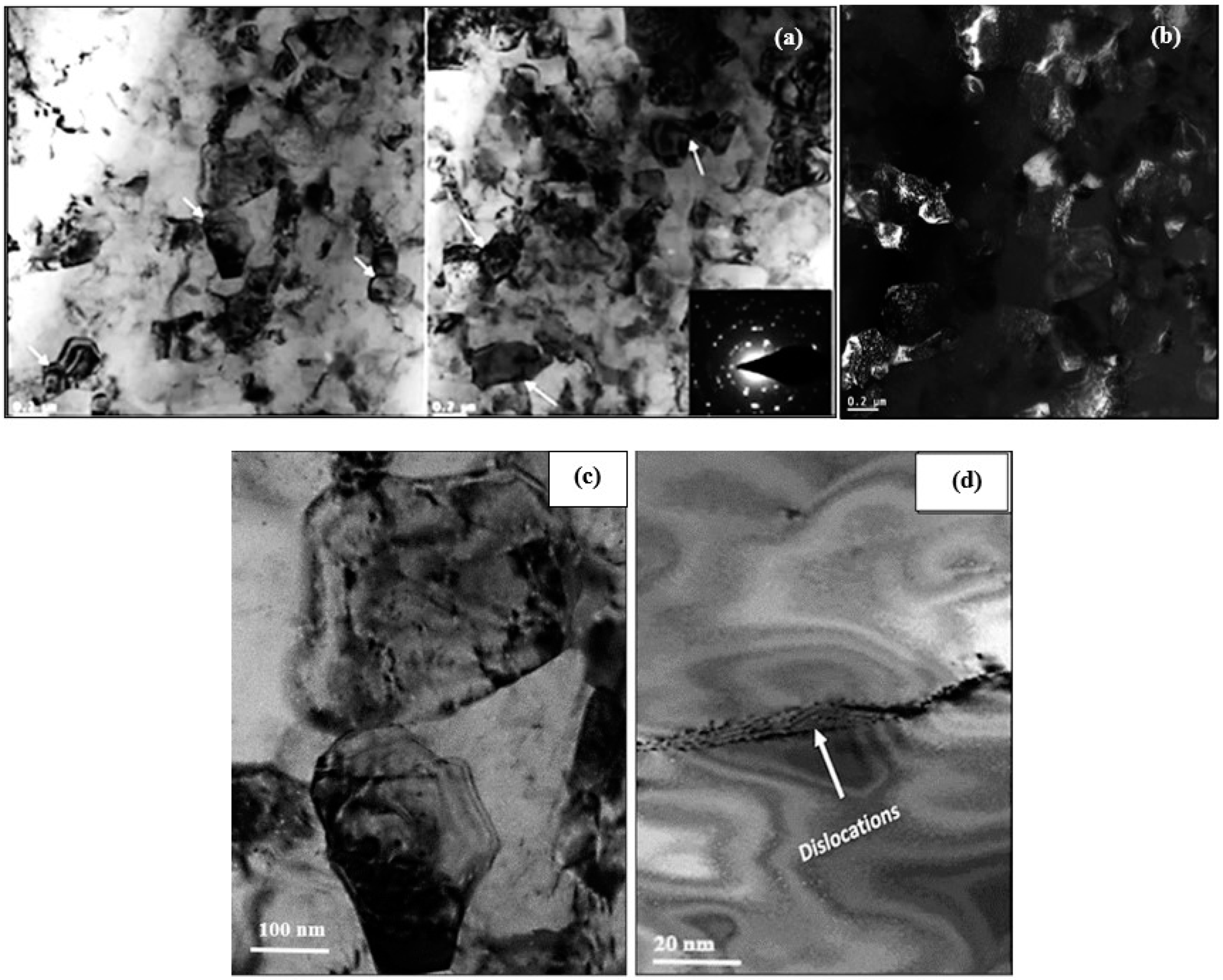
3.2.2. Optical and SEM Investigations of as Cast Alloys
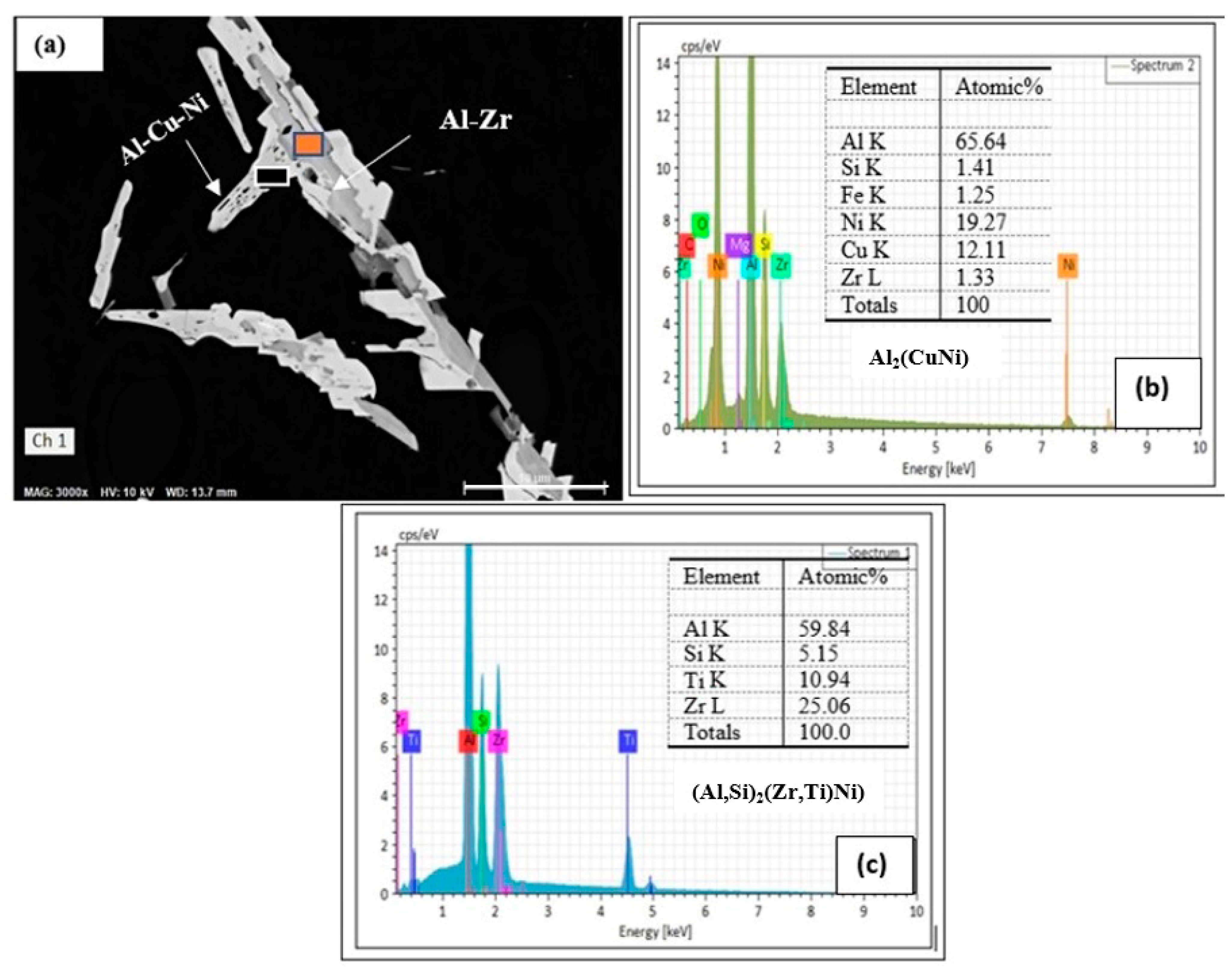
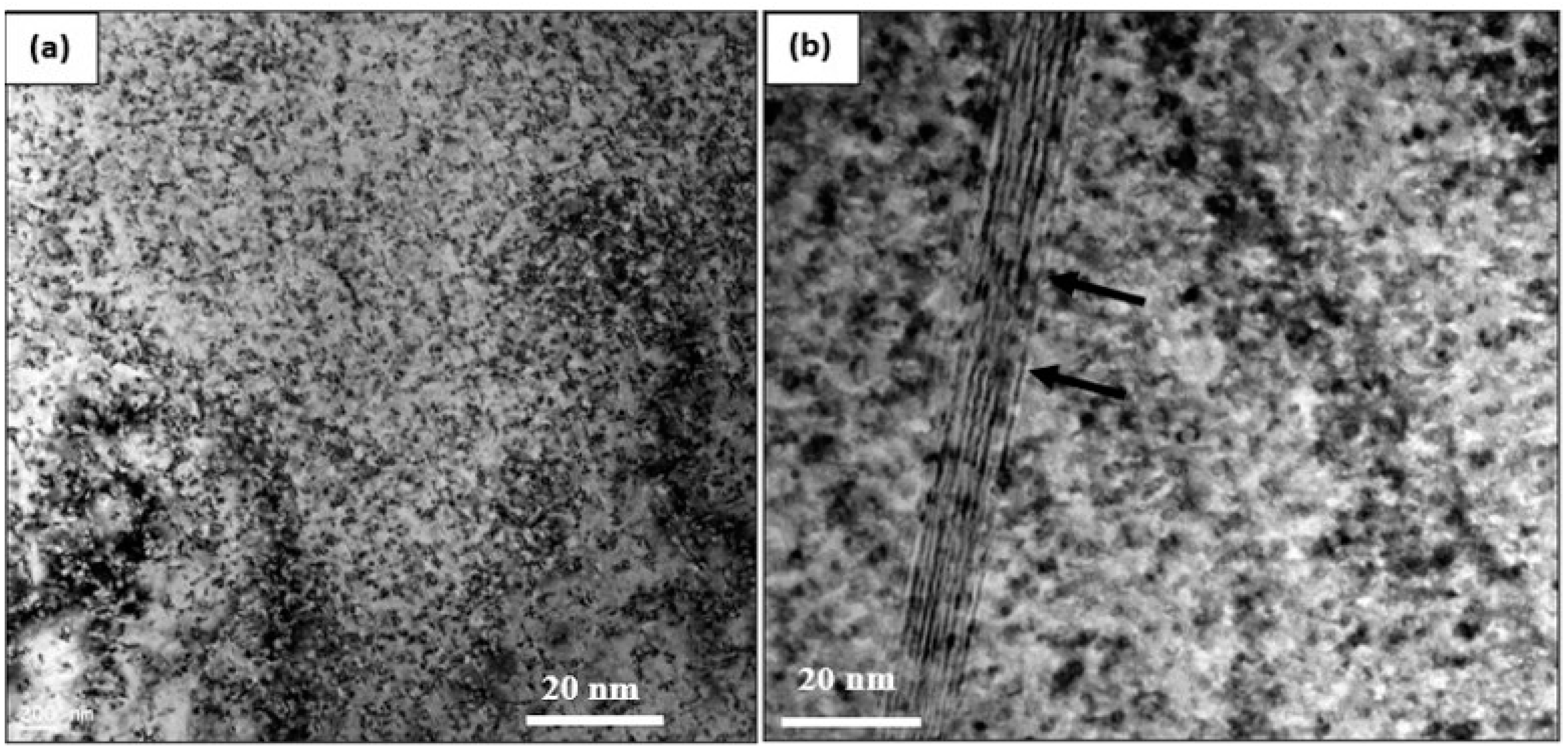
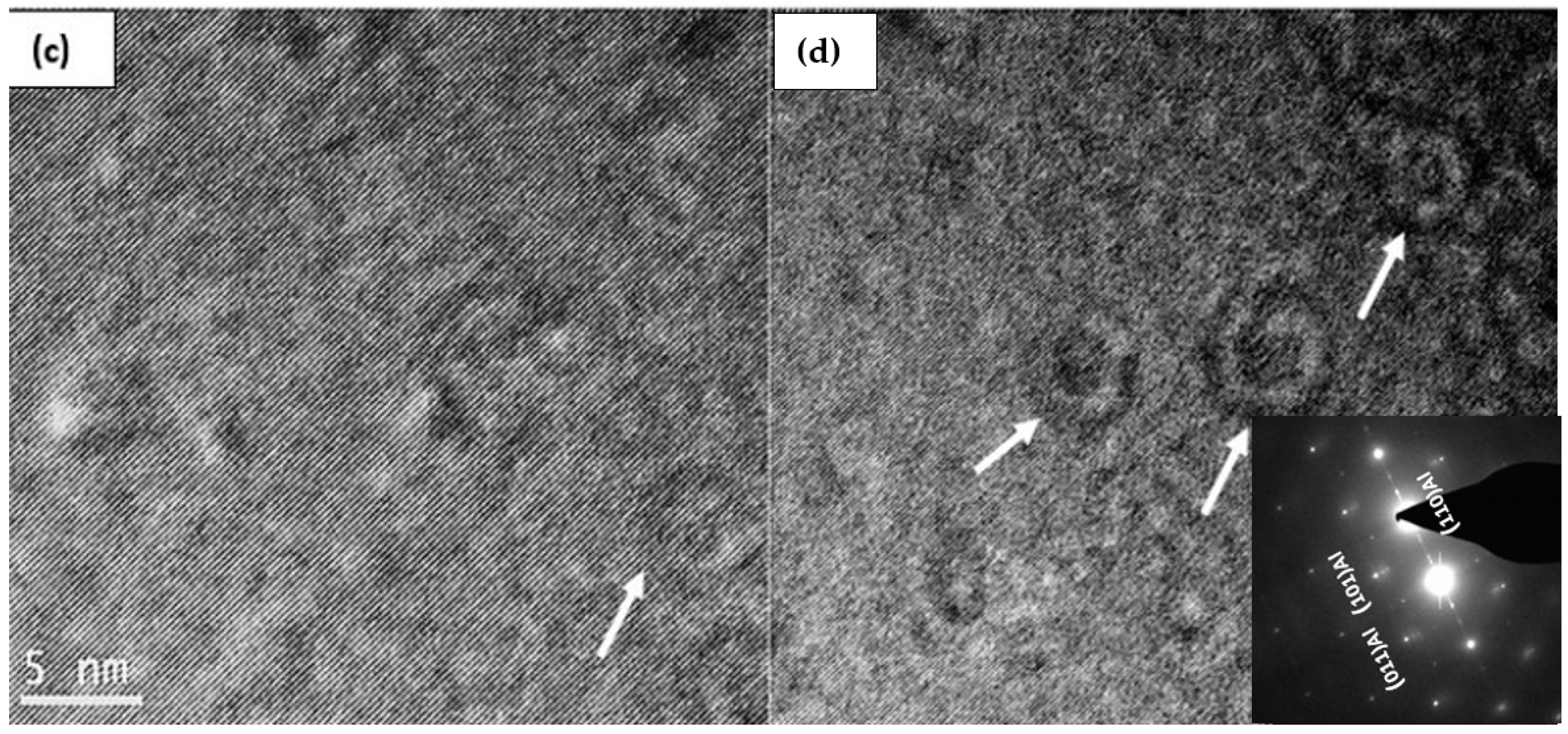
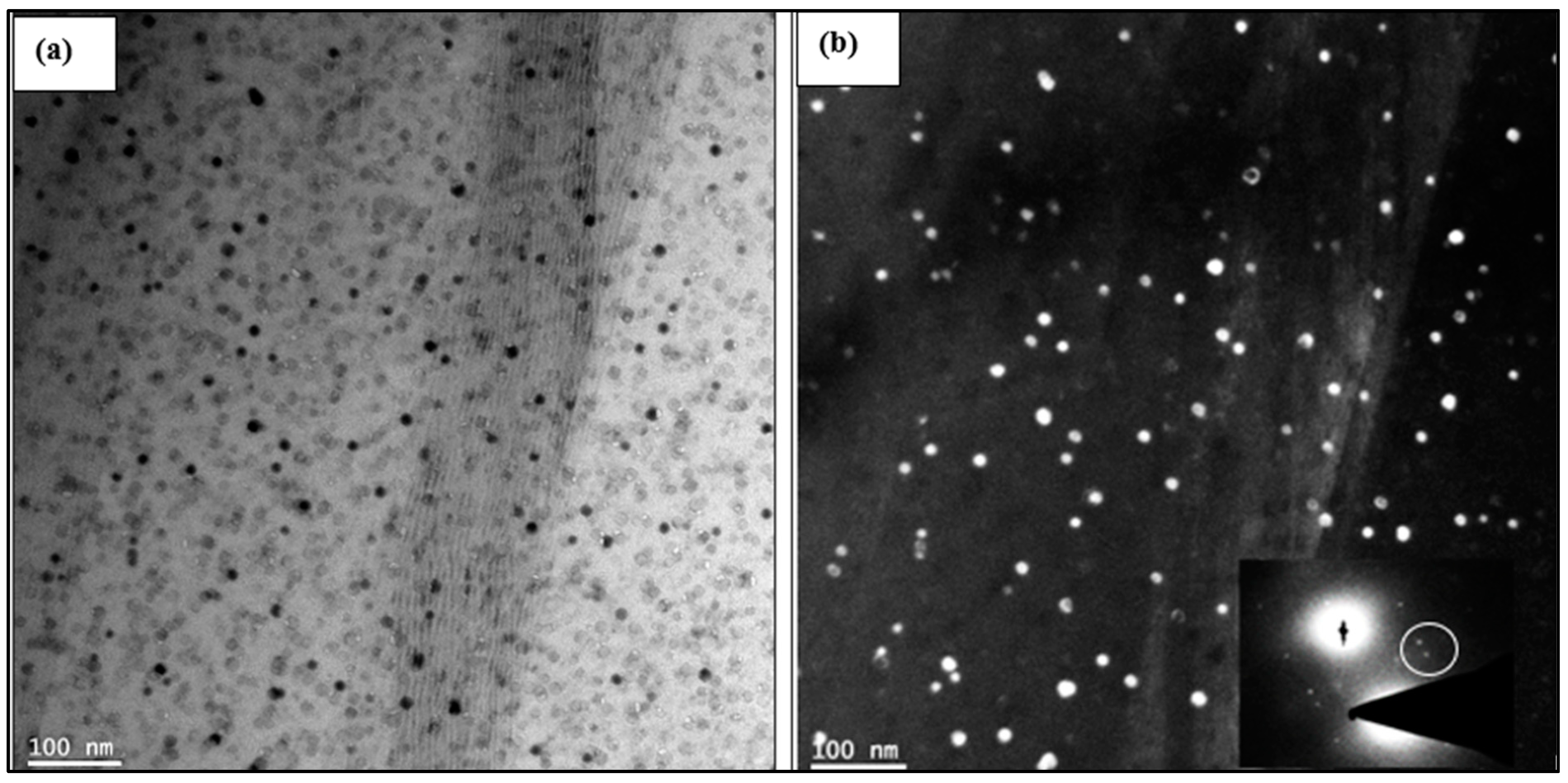
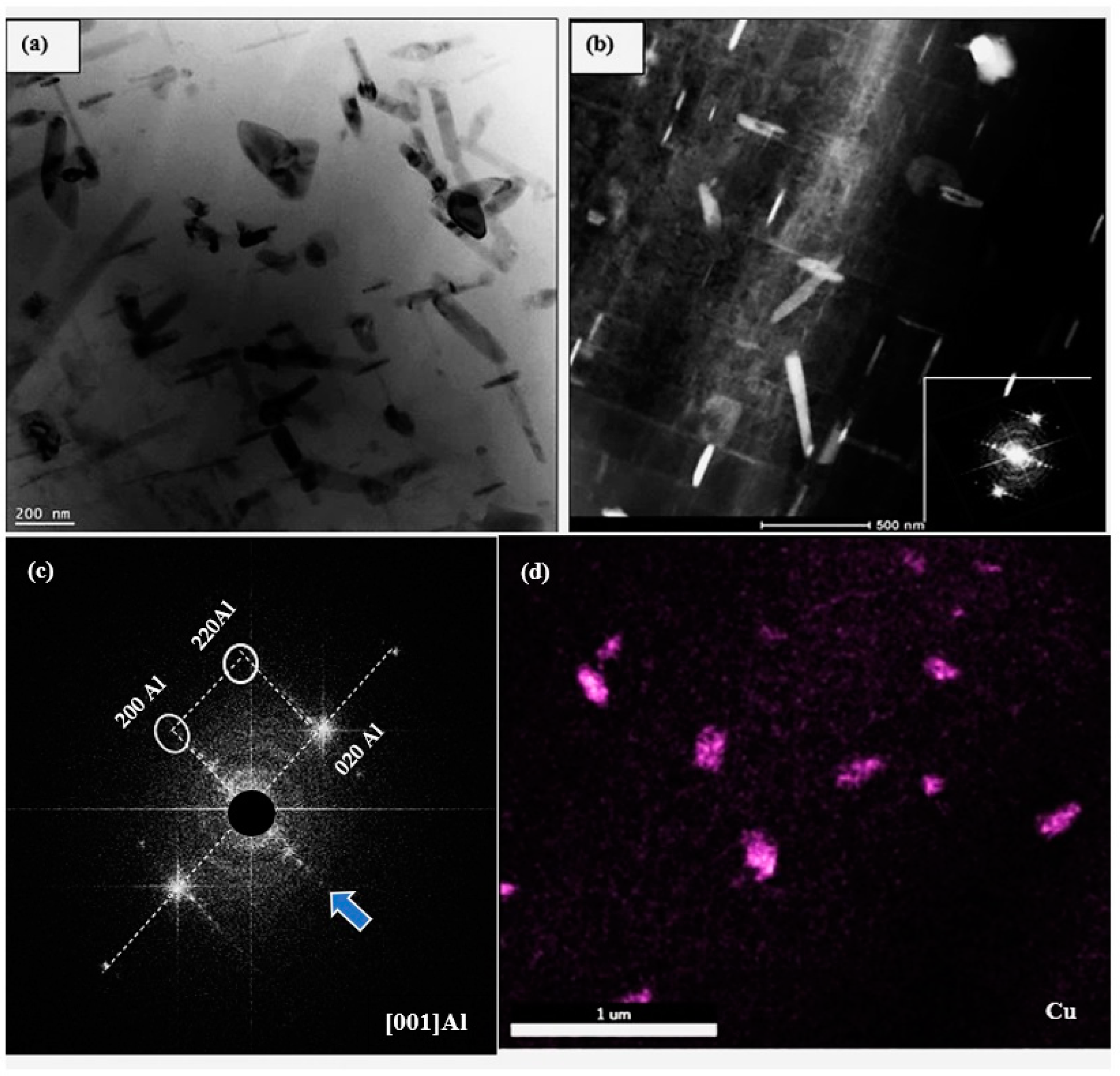
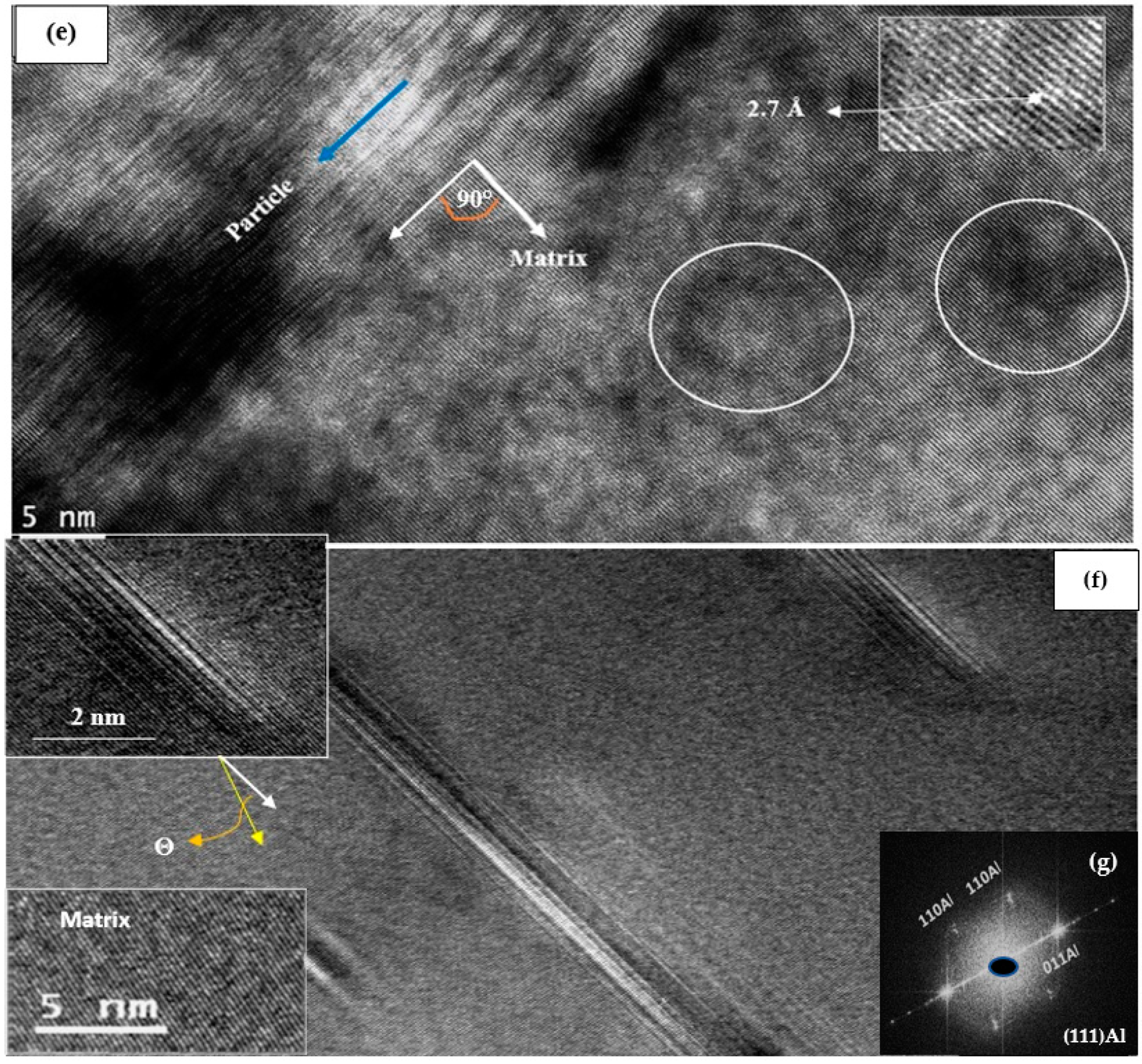
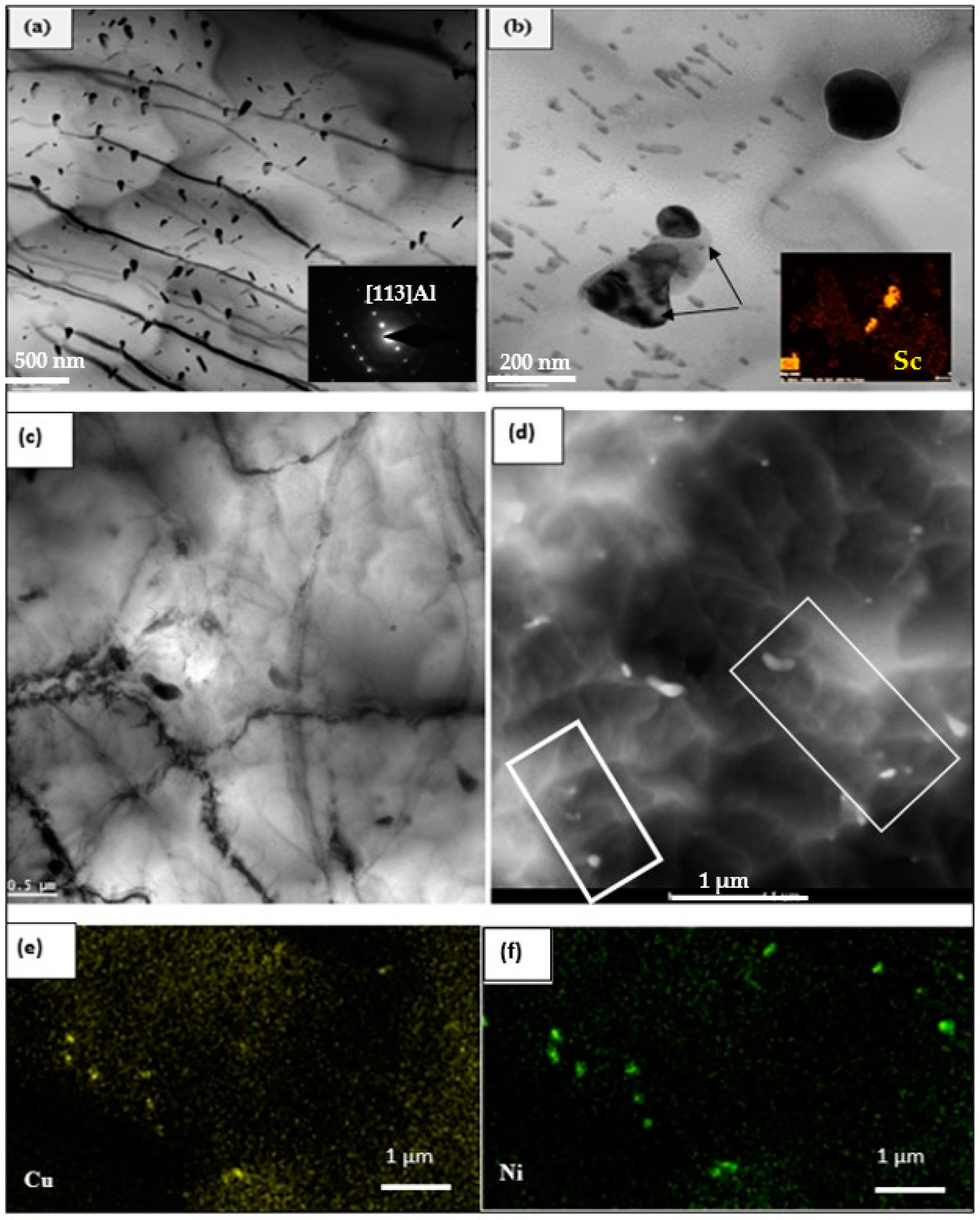
3.3. HR-TEM Analysis
4. Conclusions
- Aging of Al–Si–Cu–Mg alloys is characterized by the formation of multiple hardness peaks at the aging temperature with increasing aging time caused by simultaneous precipitation and coarsening of multiple phases.
- Increasing the Ni content to 4 wt% improves the alloy resistance for softening during aging at 240 °C.
- There are strong Cu–Ni, Zr–Ni, and Cu–Zr interactions between Cu, Ni, and Zr, causing a depletion in the matrix of Cu atoms needed for precipitation strengthening. This leads to a major reduction in the density of Al2Cu phase particles, and hence a lowering of the alloy strength.
- The Ni-rich compounds are observed to precipitate on the surface of precipitated primary Zr-phase particles.
- Aging the base 354 alloy at 240 °C for a long period of 200 h enhances the coarsening of the precipitated Al2Cu particles, leading to the commencement of incoherency.
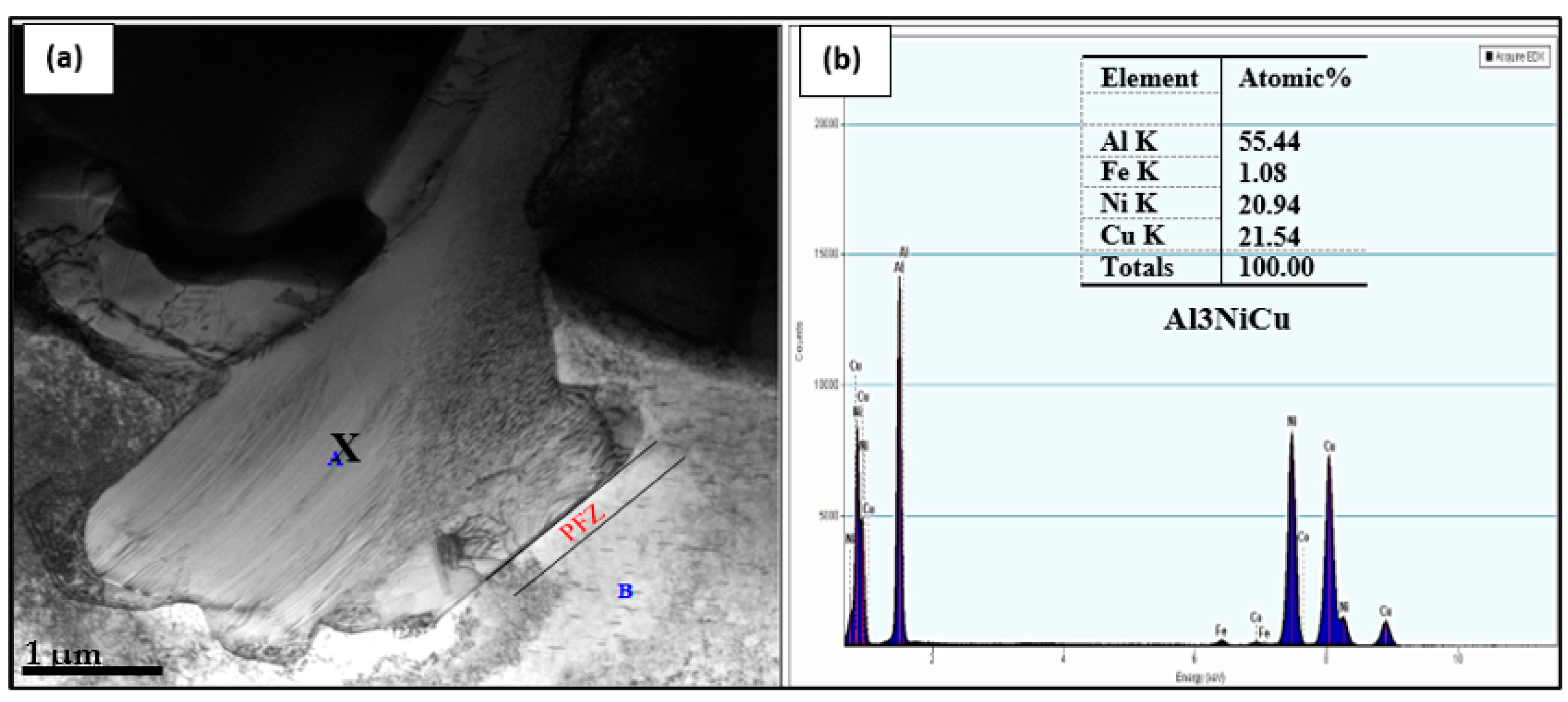
- In a 4 wt% Ni-containing alloy, a new phase was reported, namely Al3Ni2Cu2, representing almost 50% (about 50 at%) of the compound composition.
- Silicon particles were characterized by the presence of a large number of twins.
- In the precipitation of primary Cu–Ni-rich particles, the particles are surrounded by precipitate-free zones due to depletion of Cu atoms in their vicinity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Fu, Y.; Yang, B.; Gao, M.; Guan, R. Deformation behavior and microstructure evolution induced by nano-sized Al6Mn phase particles in a homogenized Al–6Mg–0.8Mn alloy during hot compression. Mater. Sci. Eng. A 2023, 882, 145461. [Google Scholar] [CrossRef]
- Yang, B.; Gao, M.; Liu, Y.; Pan, S.; Meng, S.; Fu, Y.; Guan, R. Formation mechanism of refined Al6 (Mn, Fe) phase particles during continuous rheo-extrusion and its contribution to tensile properties in Al–Mg–Mn–Fe alloys. Mater. Sci. Eng. A 2023, 872, 144952. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.; Gao, M.; Wang, C.; Guan, R. Microstructural evolution and strengthening mechanism of Al–Mg alloys with fine grains processed by accumulative continuous extrusion forming. J. Mater. Sci. Technol. 2022, 128, 195–204. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, B.; Gao, M.; Zhao, E.; Guan, R. Microstructure evolution, mechanical property response and strengthening mechanism induced by compositional effects in Al–6 Mg alloys. Mater. Des. 2022, 220, 111849. [Google Scholar] [CrossRef]
- Moustafa, M. Effect of iron content on the formation of β-Al5FeSi and porosity in Al–Si eutectic alloys. J. Mech. Work. Technol. 2009, 209, 605–610. [Google Scholar] [CrossRef]
- Yi, J.Z.; Gao, Y.X.; Lee, P.D.; Lindley, T.C. Effect of Fe-content on fatigue crack initiation and propagation in a cast aluminum–silicon alloy (A356–T6). Mater. Sci. Eng. 2004, 386, 396–407. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Ma, Z.; Tao, T.; Gui, J.; Song, W.; Yang, B.; Zhang, H. Interactions between Fe-rich intermetallics and Mg-Si phase in Al-7Si-xMg alloys. J. Alloys Compd. 2019, 786, 205–214. [Google Scholar] [CrossRef]
- Timelli, G.; Caliari, D.; Rakhmonov, J. Influence of Process Parameters and Sr Addition on the Microstructure and Casting Defects of LPDC A356 Alloy for Engine Blocks. J. Mater. Sci. Technol. 2016, 32, 515–523. [Google Scholar] [CrossRef]
- Limodin, N.; Dahdah, N.; Wang, L.; El Bartali, A.; Witz, J.-F.; Buffiere, J.-Y.; Charkaluk, E. Characterisation of 3D strain heterogeneity at the microstructure scale during low cycle fatigue of an AlSi7Cu3Mg alloy at 250 °C. Int. J. Fatigue 2023, 172, 107476. [Google Scholar] [CrossRef]
- Khisheh, S.; Azadi, M.; Hendoabadi, V.Z.; Parast, M.A.; Winter, G.; Seisenbacher, B.; Gruen, F.; Khalili, K. Influence of T6 heat-treating and over-ageing on out-of-phase thermo-mechanical fatigue behaviors of Al-Si-Cu alloy. Mater. Today Commun. 2022, 33, 104803. [Google Scholar] [CrossRef]
- Siddiqui, R.; Abdul-Wahab, S.; Pervez, T. Effect of aging time and aging temperature on fatigue and fracture behavior of 6063 aluminum alloy under seawater influence. Mater. Des. 2008, 29, 70–79. [Google Scholar] [CrossRef]
- Sadeler, R.; Totik, Y.; Gavgalı, M.; Kaymaz, I. Improvements of fatigue behaviour in 2014 Al alloy by solution heat treating and age-hardening. Mater. Des. 2004, 25, 439–445. [Google Scholar] [CrossRef]
- Wang, G.; Bian, X.; Wang, W.; Zhang, J. Influence of Cu and minor elements on solution treatment of Al–Si–Cu–Mg cast alloys. Mater. Lett. 2003, 57, 4083–4087. [Google Scholar] [CrossRef]
- Chen, B.A.; Pan, L.; Wang, R.H.; Liu, G.; Cheng, P.M.; Xiao, L.; Sun, J. Effect of solution treatment on precipitation behaviors and age hardening response of Al–Cu alloys with Sc addition. Mater. Sci. Eng. A 2011, 530, 607–617. [Google Scholar] [CrossRef]
- Tavitas-Medrano, F.; Gruzleski, J.; Samuel, F.; Valtierra, S.; Doty, H. Effect of Mg and Sr-modification on the mechanical properties of 319-type aluminum cast alloys subjected to artificial aging. Mater. Sci. Eng. A 2007, 480, 356–364. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Elgallad, E.M.; Doty, H.W.; Samuel, F.H. Strengthening precipitates and mechanical performance of Al–Si–Cu–Mg cast alloys containing transition elements. Mater. Sci. Eng. A 2021, 820, 141497. [Google Scholar] [CrossRef]
- De Wilde, J.; Froyen, L.; Rex, S. Coupled two-phase [α(Al) + θ(Al2Cu)] planar growth and destabilisation along the univariant eutectic reaction in Al-Cu-Ag alloys. Scr. Mater. 2004, 51, 533–538. [Google Scholar] [CrossRef]
- Weng, Y.; Chen, J.; Cheng, X.; Ding, L.; Sun, Q.; Tao, S.; Liu, X.; Jia, Z. Interface structures and thickening mechanisms of the Q′ and QP2 phases for Al-Mg-Si-Cu alloys. Mater. Charact. 2022, 194, 11235. [Google Scholar] [CrossRef]
- Ding, L.; Jia, Z.; Nie, J.-F.; Weng, Y.; Cao, L.; Chen, H.; Wu, X.; Liu, Q. The structural and compositional evolution of precipitates in Al-Mg-Si-Cu alloy. Acta Mater. 2018, 145, 437–450. [Google Scholar] [CrossRef]
- Li, Z.; Samuel, A.M.; Samuel, F.H.; Ravindran, C.; Valtierra, S. Effect of alloying elements on the segregation and dissolution of CuAl2 phase in Al-Si-Cu 319 alloys. J. Mater. Sci. 2003, 38, 1203–1218. [Google Scholar] [CrossRef]
- Meyers, C.; Hinton, K.; Chou, J.-S. Towards the Optimization of Heat-Treatment in Aluminium Alloys. Mater. Sci. Forum 1992, 102, 75–84. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Samuel, F.H. A review on the heat treatment of Al-Si-Cu/Mg casting alloys. In Heat Treatment-Conventional and Novel Applications; In Tech: London, UK, 2012; p. 229. [Google Scholar]
- Apelian, D. Aluminum Cast Alloys: Enabling Tools for Improved Performance; North American Die Casting Association: New York, NY, USA, 2009. [Google Scholar]
- Wang, Q. Microstructural Effects on the Tensile and Fracture Behavior of Aluminum Casting Alloys A356/357. Metall. Mater. Trans. A 2003, 34, 2887–2899. [Google Scholar] [CrossRef]
- Nakashima, K.; Miyamoto, K.; Kunimine, T.; Monzen, R.; Muramatsu, N. Precipitation behavior of Cu–Zr compounds in a Cu-0.13 wt%Zr alloy. J. Alloys Compd. 2020, 816, 152650. [Google Scholar] [CrossRef]
- Fadayomi, O.; Sanders, P.G.; Odegard, G.M. Microstructure and properties of precipitation-hardened Zr and Zn-Zr based aluminum alloys. J. Alloys Compd. 2019, 788, 1218–1230. [Google Scholar] [CrossRef]
- Nakamura, J.; Matsuda, K.; Sato, T.; Marioara, C.D.; Andersen, S.J.; Holmestad, R.; Ikeno, S. The Crystal Structure of the β’-Phase Including Ag in Al-Mg-Si-Ag Alloy. Adv. Mater. Res. Trans. Tech. Publ. 2012, 409, 67–70. [Google Scholar] [CrossRef]
- Medrano-Prieto, H.M.; Garay-Reyes, C.G.; Gómez-Esparza, C.D.; Aguilar-Santillán, J.; Maldonado-Orozco, M.C.; Martínez-Sánchez, R. Evolution of Microstructure in Al-Si-Cu System Modified with a Transition Element Addition and its Effect on Hardness. Mater. Res. 2016, 19, 59–66. [Google Scholar] [CrossRef]
- Hamasha, M.M.; Mayyas, A.T.; Hassan, A.M.; Hayajneh, M.T. The effect of time, percent of copper and nickel on naturally aged Al-Cu–Ni cast alloys. J. Miner. Mater. Charact. Eng. 2012, 11, 117–131. [Google Scholar] [CrossRef]
- Bogdanoff, T.; Dahle, A.K.; Seifeddine, S. Effect of Co and Ni Addition on the Microstructure and Mechanical Properties at Room and Elevated Temperature of an Al–7%Si Alloy. Int. J. Met. 2017, 12, 434–440. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Samuel, A.M.; Doty, H.W.; Valtierra, S.; Samuel, F.H. Effect of additives on the microstructure and tensile properties of Al–Si alloys. J. Mater. Res. Technol. 2019, 8, 2255–2268. [Google Scholar] [CrossRef]
- Medrano-Prieto, H.; Garay-Reyes, C.; Estrada-Guel, I.; Mendoza-Duarte, J.; Guía-Tello, J.; Ochoa-Gamboa, R.; Ornelas-Gutiérrez, C.; Rodríguez-Cabriales, G.; Castro-Carmona, J.; Camacho-Montes, H.; et al. Effect of Ni additions and hot deformation on precipitation behavior and hardness in Al–Si–Mg aged alloys. J. Alloys Compd. 2020, 828, 154429. [Google Scholar] [CrossRef]
- Zuo, L.; Ye, B.; Feng, J.; Xu, X.; Kong, X.; Jiang, H. Effect of δ-Al3CuNi phase and thermal exposure on microstructure and mechanical properties of Al-Si-Cu–Ni alloys. J. Alloys Compd. 2019, 791, 1015–1024. [Google Scholar] [CrossRef]
- Cai, Q.; Fang, C.; Mendis, C.; Chang, I.T.; Cantor, B. Thermal behaviour and microstructure evolution of new ternary eutectic alloy in Al-Cu-Si-Ni system. J. Alloys Compd. 2023, 941, 168942. [Google Scholar] [CrossRef]
- Czerwinski, F.; Aniolek, M.; Li, J. Strengthening retention and structural stability of the Al-Al3Ni eutectic at high temperatures. Scr. Mater. 2022, 214, 114679. [Google Scholar] [CrossRef]
- Bayram, Ü.; Maraşlı, N. Influence of Growth Rate on Eutectic Spacing, Microhardness, and Ultimate Tensile Strength in the Directionally Solidified Al-Cu–Ni Eutectic Alloy. Met. Mater. Trans. B 2018, 49, 3293–3305. [Google Scholar] [CrossRef]
- Li, G.J.; Guo, M.X.; Wang, Y.; Zheng, C.H.; Zhang, J.S.; Zhuang, L.Z. Effect of Ni addition on microstructure and mechanical properties of Al–Mg–Si–Cu–Zn alloys with a high Mg/Si ratio. Int J Min. Met. Mater 2019, 26, 740–751. [Google Scholar] [CrossRef]
- Abdelaziz, M.; Doty, H.; Valtierra, S.; Samuel, F. Static versus dynamic thermal exposure of transition elements-containing Al-Si-Cu-Mg cast alloy. Mater. Sci. Eng. A 2018, 739, 499–512. [Google Scholar] [CrossRef]
- Darlapudi, A.; McDonald, S.D.; StJohn, D.H. The influence of Cu, Mg and Ni on the solidification and microstructure of Al-Si alloys. IOP Conf. Ser. Mater. Sci. Eng. 2016, 117, 012022. [Google Scholar] [CrossRef]
- Milligan, B.; Ma, D.; Allard, L.; Clarke, A.; Shyam, A. Dislocation-θ′ (Al2Cu) interactions during creep deformation of an Al-Cu alloy. Scr. Mater. 2022, 217, 114739. [Google Scholar] [CrossRef]
- Belov, N.A.; Eskin, D.G.; Avxentieva, N.N. Constituent phase diagrams of the Al–Cu–Fe–Mg–Ni–Si system and their application to the analysis of aluminium piston alloys. Acta Mater. 2005, 53, 4709–4722. [Google Scholar] [CrossRef]
- Hernandez-Sandoval, J.; Zedan, Y.; Garza-Elizondo, G.H.; Abdelaziz, M.H.; Songmene, V.; Samuel, F.H. Effect of Minor Addition of Ni and Zr on the High-Temperature Performance of Al–Si–Cu–Mg Cast Alloys. Int. J. Met. 2022, 16, 1235–1251. [Google Scholar] [CrossRef]
- Djurdjevic, M.B.; Manasijevic, S. Impact of major alloying elements on the solidification parameters of cast hypoeutectic AlSi6Cu (1–4 wt.%) and AlSi8Cu (1–4 wt.%) alloys. Metall. Mater. Eng. 2014, 20, 235–246. [Google Scholar] [CrossRef]
- Hernandez-Sandoval, J.; Garza-Elizondo, G.H.; Abdelaziz, M.H.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. The Effect of Ni and Zr Additions on the Tensile Properties of Isothermally Aged Ai–Si–Cu–Mg Cast Alloys. Int. J. Met. 2021, 16, 435–457. [Google Scholar] [CrossRef]
- Medrano-Prieto, H.; Garay-Reyes, C.; Gómez-Esparza, C.; Estrada-Guel, I.; Aguilar-Santillan, J.; Maldonado-Orozco, M.; Martínez-Sánchez, R. Effect of Nickel addition and solution treatment time on microstructure and hardness of Al-Si-Cu aged alloys. Mater. Charact. 2016, 120, 168–174. [Google Scholar] [CrossRef]
- Hernandez-Sandoval, J.; Samuel, A.M.; Valtierra, S.; Samuel, F.H. Ni- and Zr-Based Intermetallics in Al–Si–Cu–Mg Cast Alloys. Metallogr. Microstruct. Anal. 2014, 3, 408–420. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Chen, Z.; Tsalanidis, A.; Weyland, M.; Findlay, S.; Allen, L.J.; Li, J.; Medhekar, N.V.; Bourgeois, L. The enhanced theta-prime (θ′) precipitation in an Al-Cu alloy with trace Au additions. Acta Mater. 2017, 125, 340–350. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Samuel, A.M.; Doty, H.W.; Songmene, V.; Samuel, F.H. Mechanical Performance and Precipitation Behavior in Al-Si-Cu-Mg Cast Alloys: Effect of Prolonged Thermal Exposure. Materials 2022, 15, 2830. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, C.L.; Xie, P.; Niu, F.J.; Ming, W.Q.; Du, K.; Chen, J.H. A hidden recipitation scenario of the θ′-phase in Al-Cu alloys. J. Mater. Sci. Technol. 2021, 75, 126–138. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Ibrahim, M.F.; Samuel, E.; Samuel, A.M.; Samuel, F.H.; Doty, H.W. Assessment of the Effect of Mg Addition on the Solidification Behavior, Tensile and Impact Properties of Al–Si–Cu Cast Alloys. Int. J. Met. 2022, 17, 82–108. [Google Scholar] [CrossRef]
- Joenoes, A.; Gruzleski, J. Magnesium effects on the microstructure of unmodified and modified Al–Si alloys. Cast Metals 1991, 4, 62–71. [Google Scholar] [CrossRef]
- Drouzy, M.; Jacob, S.; Richard, M. Interpretation of 749 tensile results by means of quality index and probable yield strength-application to Al–Si7 Mg foundry alloys-France. Int. Cast Met. J. 1980, 5, 43–50. [Google Scholar]
- Jacob, S. Quality index in prediction of properties of 755 aluminum castings—A review. In Proceedings of the Transactions of the American Foundry Society and the One Hundred Fourth Annual Castings Congress, Pittsburgh, PA, USA, 8–11 April 2000; pp. 709–712. [Google Scholar]
- Shaha, S.K. Development and Characterization of Cast Modified Al-Si-Cu-Mg Alloys for Heat Resistant Power Train Applications. Ph.D. Thesis, Ryerson University, Toronto, ON, Canda, 2015. [Google Scholar]
- Sankanit, P.; Uthaisangsuk, V.; Pandee, P. Tensile properties of hypoeutectic Al-Ni alloys: Experiments and FE simulations. J. Alloys Compd. 2021, 889, 161664. [Google Scholar] [CrossRef]
- Engin, S.; Büyük, U.; Maraşl, N. The effects of microstructure and growth rate on microhardness, tensile strength, and electrical resistivity for directionally solidified Al–Ni–Fe alloys. J. Alloys Compd. 2016, 660, 23–31. [Google Scholar] [CrossRef]
- Bäckerud, L.; Chai, G.; Tamminen, J. Solidification Characteristics of Aluminium Alloys, Volume 2: Foundry Alloys; AFS/Skanaluminium: Des Plaines, IL, USA, 1990; pp. 71–84. [Google Scholar]
- Wangi, J.; Reif, W. Effect of Cu Content on Grain Refinement of an Al–Cu Alloy AITiSBl Refiners. Int. J. Mater. Res. 1993, 84, 445–450. [Google Scholar] [CrossRef]
- Medrano-Prieto, H.M.; Garay-Reyes, C.; Gómez-Esparza, C.; Martínez-Sánchez, R. Influence of Solute Addition in the Microstructure and Hardness of the Al-Si-Cu Alloys. MRS Proc. 2016, 1815, 92. [Google Scholar] [CrossRef]
- Chen, Z.; Kang, H.; Fan, G.; Li, J.; Lu, Y.; Jie, J.; Zhang, Y.; Li, T.; Jian, X.; Wang, T. Grain refinement of hypoeutectic Al-Si alloys with B. Acta Mater. 2016, 120, 168–178. [Google Scholar] [CrossRef]
- Nadendla, H.B.; Nowak, M.; Bolzoni, L. Grain Refiner for Al-Si Alloys. In Light Metals 2013; Sadler, B.A., Ed.; The Minerals, Metals & Materials Series; Springer International Publishers: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Wang, T.; Fu, H.; Chen, Z.; Xu, J.; Zhu, J.; Cao, F.; Li, T. A novel fading-resistant Al–3Ti–3B grain refiner for Al–Si alloys. J. Alloys Compd. 2012, 51, 45–49. [Google Scholar] [CrossRef]
- Shaha, S.K.; Czerwinski, F.; Kasprzak, W.; Friedman, J.; Chen, D.L. Effect of Zr, V and Ti on hot compression behavior of the Al–Si cast alloy for powertrain applications. J. Alloys Compd. 2014, 615, 1119–1131. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Elsharkawi, E.A.; Doty, H.W.; Samuel, F.H. Mechanical Performance of Zr-Containing 354-Type Al-Si-Cu-Mg Cast Alloy: Role of Geometrical Discontinuities. J. Mater. Eng. Perform. 2020, 29, 6640–6651. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Timelli, G.; Bonollo, F. Characterization of the solidification path and microstructure of secondary Al-7Si-3Cu-0.3Mg alloy with Zr, V and Ni additions. Mater. Charact. 2017, 128, 100–108. [Google Scholar] [CrossRef]
- Garza-Elizondo, G.H.; Elsharkawi, E.A.; Samuel, A.M.; Doty, H.W.; Samuel, F.H. Effects of Alloying Elements Additions on Ambient Temperature Performance of Al–Si–Cu–Mg Base Alloys. Int. J. Met. 2021, 15, 1385–1401. [Google Scholar] [CrossRef]
- Biswas, A.; Siegel, D.J.; Seidman, D.N. Compositional evolution of Q-phase precipitates in an aluminum alloy. Acta Mater. 2014, 75, 322–336. [Google Scholar] [CrossRef]
- Bobel, A.; Kim, K.; Wolverton, C.; Walker, M.; Olson, G.B. Equilibrium composition variation of Q-phase precipitates in aluminum alloys. Acta Mater. 2017, 138, 150–160. [Google Scholar] [CrossRef]
- Pan, X.; Morral, J.E.; Brody, H.D. Predicting the Q-Phase in Al-Cu-Mg-Si Alloys. J. Phase Equilib. Diffus. 2010, 31, 144–148. [Google Scholar] [CrossRef]
- Qian, H.; Zhu, D.; Hu, C.; Jiang, X. Effects of Zr Additive on Microstructure, Mechanical Properties, and Fractography of Al-Si Alloy. Metals 2018, 8, 124. [Google Scholar] [CrossRef]
- Gao, T.; Ceguerra, A.; Breen, A. Precipitation behaviors of cubic and tetragonal Zr–rich phase in Al–(Si–) Zr alloys. J. Alloys Compd. 2016, 674, 125–130. [Google Scholar] [CrossRef]
- Rakhmonov, J.; Timelli, G.; Bonollo, F. The Effect of Transition Elements on High-Temperature Mechanical Properties of Al–Si Foundry Alloys–A Review. Adv. Eng. Mater. 2016, 18, 1187–1298. [Google Scholar] [CrossRef]
- Wang, N.; Li, C.; Du, Z.; Wang, F. Experimental study and thermodynamic re-assessment of the Ni–Zr system. Calphad 2007, 31, 413–421. [Google Scholar] [CrossRef]
- Delogu, F.; Cocco, G. Numerical simulations of structural modifications at a Ni−Zr sliding interface. Phys. Rev. B 2005, 72, 014124. [Google Scholar] [CrossRef]
- Kulikova, T.V.; Majorova, A.V.; Yu, K.; Shunyaev, K.Y.; Ryltsev, R.E. Thermodynamic properties of Cu–Zr melts: The role of chemical interaction. Phys. B Condens. Matter 2015, 466–467, 90–95. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, B.J. A modified embedded-atom method interatomic potential for the Cu–Zr system. J. Mater. Res. 2008, 23, 1095–1104. [Google Scholar] [CrossRef]
- Dorin, T.; Ramajayam, M.; Justin Lamb, J.; Langan, T. Effect of Sc and Zr additions on the microstructure/strength of Al-Cu binary alloys. Mater. Sci. Eng. A 2017, 707, 58–64. [Google Scholar] [CrossRef]
- Król, J. The pecipitation strengthening of directionally solidified Al Si Cu alloys. Mater. Sci. Eng. A 1997, 234–236, 169–172. [Google Scholar] [CrossRef]
- Weakley-Bollin, S.C.; Donlon, W.; Donlon, W.; Wolverton, C.; Allison, J.E.; Jones, J.W. Modeling the age-hardening behavior of Al-Si-Cu alloys. Met. Mater Trans A 2004, 35, 2407–2418. [Google Scholar] [CrossRef]
- Dong, X.; Guo, Y.; Wang, J.; Xia, F.; Ma, K.; Duan, H.; Yang, Z.; Wang, P.; Liang, M.; Li, J. Novel AlCu solute cluster precipitates in the Al–Cu alloy by elevated aging and the effect on the tensile properties. Mater. Sci. Eng. A 2023, 862, 144454. [Google Scholar] [CrossRef]
- Casas, B.Y.; Figueroa, I.A.; González, G.; González-Ojeda, R.; Alfonso, I. Dissolution of the second phases and transformation from Mg2Si to Q-Al5Cu2Mg8Si6 in solution heat treated quaternary Al-alloys. J. Alloys Compd. 2023, 955, 170003. [Google Scholar] [CrossRef]
- Piao, Y.; Jia, H.; Zha, M.; Ma, P.-K.; Gao, D.; Yin, C.-R.; Yang, Z.-Z.; Wang, H.-Y. The effect of quenching rates on microstructure and mechanical properties of an Al-Si-Cu-Mg alloy. Mater. Charact. 2023, 196, 112609. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Samuel, A.M.; Doty, H.W.; Samuel, H. Effect of Aging Conditions on Precipitation Hardening in Al–Si–Mg and Al–Si–Cu–Mg Alloys. Inter Met. 2017, 11, 274–286. [Google Scholar] [CrossRef]
- Xiao, Q.; Liu, H.; Yi, D.; Yin, D.; Chen, Y.; Zhang, Y.; Wang, B. Effect of Cu content on precipitation and age-hardening behavior in Al-Mg-Si-xCu alloys. J. Alloys Compd. 2017, 695, 1105–1113. [Google Scholar] [CrossRef]
- Starke, E. Accelerated Aging of Materials and Structures: The Effects of Long-Term Elevated-Temperature Exposure; NMAB-479; National Materials Advisory Board, National Research Council: Washington, DC, USA, 1996. [Google Scholar]
- Weatherly, G.; Nicholson, R. An Electron Microscope Investigation of the Interfacial Structure of Semi-Coherent Precipitates. Philos. Mag. 1968, 17, 801–831. [Google Scholar] [CrossRef]
- Buha, J.; Lumley, R.N.; Crosky, A.G. Secondary ageing in an aluminium alloy 7050. Mater. Sci. Eng. A 2008, 492, 1–11. [Google Scholar] [CrossRef]
- Idrac, J.; Blanc, C.; Kihn, Y.; Lafont, M.C.; Mankowski, G.; Skeldon, P.; Thompson, G.E. Electrochemical Behavior of Magnetron-Sputtered Al–Cu Alloy Films in Sulfate Solutions. J. Electrochem. Soc. 2007, 154, C286. [Google Scholar] [CrossRef]
- Tolley, A.; Mitlin, D.; Radmilovic, V.; Dahmen, U. Transmission electron microscopy analysis of grain boundary precipitate-free-zones (PFZs) in an AlCuSiGe alloy. Mater. Sci. Eng. A 2005, 412, 204–213. [Google Scholar] [CrossRef]
- Showe, P.; Poplawsky, J.; Bahl, S.; Shyam, A. The role of Si in determining the stability of the θ′ precipitate in Al-Cu-Mn-Zr alloys. J. Alloys Compd. 2021, 862, 158152. [Google Scholar] [CrossRef]
- Liu, H.; Papadimitriou, I.; Lin, F.X.; LLorca, J. Precipitation during high temperature aging of Al−Cu alloys: A multiscale analysis based on first principles calculations. Acta Mater. 2019, 167, 121–135. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, G.; Ji, Y. Study of θ’ precipitation behavior in Al-Cu-Cd alloys by phase- field modeling. Mater. Sci. Eng. A 2019, 746, 105–114. [Google Scholar] [CrossRef]
- Hofmeister, H. Fivefold Twinned Nanoparticles. Encycl. Nanosci. Nanotechnol. 2004, 3, 431–452. [Google Scholar]





| Elements (wt%) *,** | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alloy Code | Si | Cu | Mg | Fe | Ti | Zr | Ni | Ag | Sr | Al |
| B | 8.5 | 2.0 | 0.59 | 0.15 | 0.15 | 0.35 | 0.1 | 0.68 | -- | Bal |
| BS | 8.6 | 1.8 | 0.48 | 0.15 | 0.18 | 0.23 | 0.1 | 0.67 | 0.017 | Bal |
| B2N | 8.5 | 1.8 | 0.58 | 0.13 | 0.18 | 0.23 | 2.0 | 0.68 | 0.015 | Bal |
| B4N | 8.7 | 1.9 | 0.68 | 0.2 | 0.20 | 0.30 | 4.0 | 0.72 | 0.017 | Bal |
| (a) | ||
| Treatment # | Description | |
| 1 | AC | As cast (AC) T5 |
| 2 | AC + 8 h/240 °C | |
| 3 | AC + 110 h/240 °C | |
| 4 | AC + 200 h/240 °C | |
| 5 | SHT + 8 h/240 °C | Solution heat treatment (SHT) 495 °C/5 h/quenching in warm water (60 °C) T7 |
| (b) | ||
| Treatment # | Description | |
| 1 | AC | As cast (AC) T5 |
| 2 | AC + 8 h/240 °C | |
| 3 | AC + 110 h/240 °C | |
| 4 | AC + 200 h/240 °C | |
| 5 | SHT + 8 h/240 °C | Solution heat treatment (SHT) 495 °C/5 h/quenching in warm water (60 °C) T7 |
| 6 | SHT + 50 h/240 °C | |
| 7 | SHT + 110 h/240 °C | |
| 8 | SHT + 200 h/240 °C | |
| Reaction # | Suggested Temperature Range (°C) | Suggested Precipitated Phase |
|---|---|---|
| 1 | 600–597 | Formation of α-aluminum dendritic network |
| 2 | 560–558 | Precipitation of Al-Si eutectic Precipitation of post-eutectic β-Al5FeSi phase |
| 3 | 555–556 | Precipitation of Al9FeNi phase |
| 4 | 540–538 | Precipitation of Mg2Si phase |
| 5 | 525–523 | Transforming of β-phase into π-Al8Mg3FeSi6 phase |
| 6 | 523–520 | Precipitation of Al3CuNi phase |
| 7 | 500–496 | Formation of eutectic Al-Al2Cu phase |
| 8 | 485–489 | Precipitation of Q-Al5Mg8Cu2Si6 phase |
| Alloy Code | Element | at% | Calculated Formula | Suggested Formula |
|---|---|---|---|---|
| B | Al | 51.51 | Al11.6Fe6Mg4.02Si4.7 | π-Al8Mg3FeSi6 |
| Fe | 4.85 | |||
| Mg | 19.53 | |||
| Si | 23.12 | |||
| Total | 99.01 | |||
| Al | 68.11 | Al2.25Cu | Θ-Al2Cu | |
| Cu | 30.21 | |||
| Total | 98.31 | |||
| Mg | 11.14 | Al4.08Cu2Mg9.2Si7 | Q-Al5Mg8Cu2Si6 | |
| Al | 47.41 | |||
| Si | 8.70 | |||
| Cu | 33.74 | |||
| Total | 100.00 | |||
| Mg | 24.82 | Mg2.1Si | Mg2Si | |
| Al | 45.23 | |||
| Si | 21.98 | |||
| Cu | 7.97 | |||
| Total | 100.1 |
| Alloy Code | Element | wt% | at% | Calculated Formula | Suggested Formula |
|---|---|---|---|---|---|
| B2N | Al | 37.67 | 58.41 | Al5.88Si1.34Ti0.25Zr2.14 | (Al,Si)3(Ti,Zr) |
| Si | 11.94 | 16.29 | |||
| Ti | 3.79 | 3.31 | |||
| Zr | 47.14 | 21.62 | |||
| Total | 99.54 | 99.63 | |||
| Al | 66.69 | 80.44 | Al8.94Fe0.25Ni1.7 | Al9(FeNi) | |
| Fe | 3.86 | 2.25 | |||
| Ni | 27.62 | 15.31 | |||
| Total | 99.85 | 98.86 | |||
| Al | 40.77 | 61.03 | Al3.30Ni1.13Cu | Al3CuNi | |
| Ni | 29.77 | 20.48 | |||
| Cu | 28.44 | 18.07 | |||
| Total | 98.98 | 99.58 |
| Condition | Volume Fraction (%) | ||
|---|---|---|---|
| Alloy | B | B2N | B4N |
| As cast | 2.5 ± 0.4 | 12.2 ± 0.8 | 15.3 ± 0.8 |
| T4 | 1.2 ± 0.3 | 9.5 ± 0.7 | 11.5 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuel, E.; Samuel, A.M.; Songmene, V.; Doty, H.W.; Samuel, F.H. Analysis of Ni-Cu Interaction in Aluminum-Based Alloys: Hardness, Tensile and Precipitation Behavior. Materials 2024, 17, 4676. https://doi.org/10.3390/ma17184676
Samuel E, Samuel AM, Songmene V, Doty HW, Samuel FH. Analysis of Ni-Cu Interaction in Aluminum-Based Alloys: Hardness, Tensile and Precipitation Behavior. Materials. 2024; 17(18):4676. https://doi.org/10.3390/ma17184676
Chicago/Turabian StyleSamuel, Ehab, Agnes M. Samuel, Victor Songmene, Herbert W. Doty, and Fawzy H. Samuel. 2024. "Analysis of Ni-Cu Interaction in Aluminum-Based Alloys: Hardness, Tensile and Precipitation Behavior" Materials 17, no. 18: 4676. https://doi.org/10.3390/ma17184676








