Synergistically Boosting Li Storage Performance of MnWO4 Nanorods Anode via Carbon Coating and Additives
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Preparation
2.1.1. Materials
2.1.2. Synthesis of MnWO4
2.1.3. Synthesis of MnWO4@C
2.1.4. Material Characterization
2.1.5. Electrochemical Measurements
3. Result and Discussion
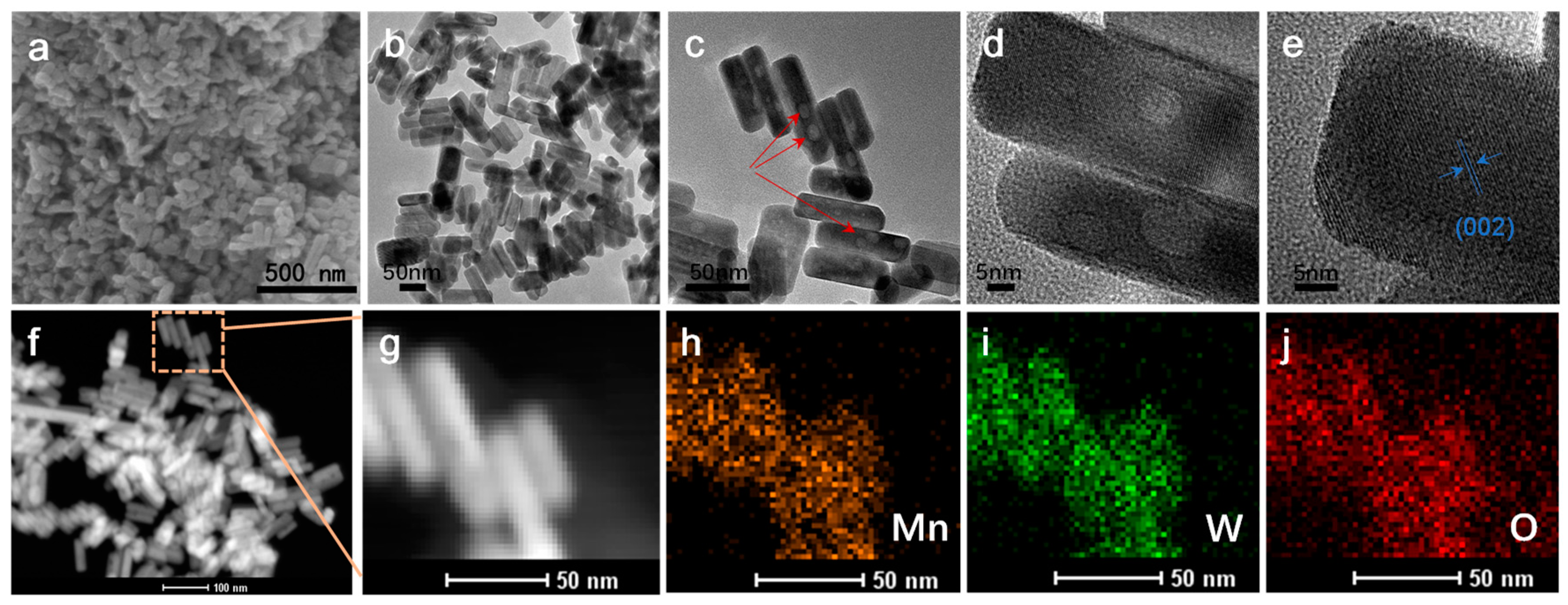
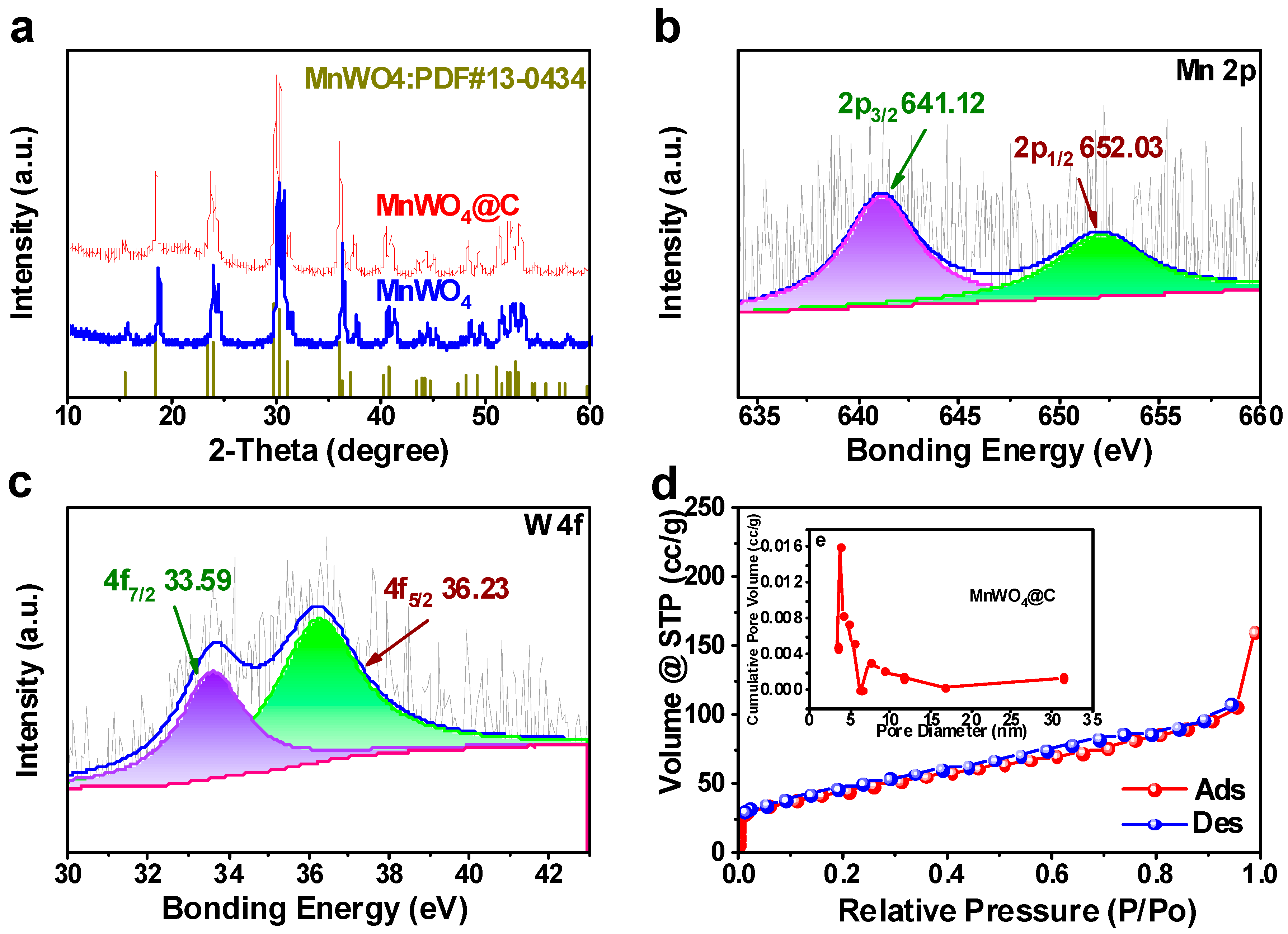
3.1. The Characterization of MnWO4 and MnWO4@C
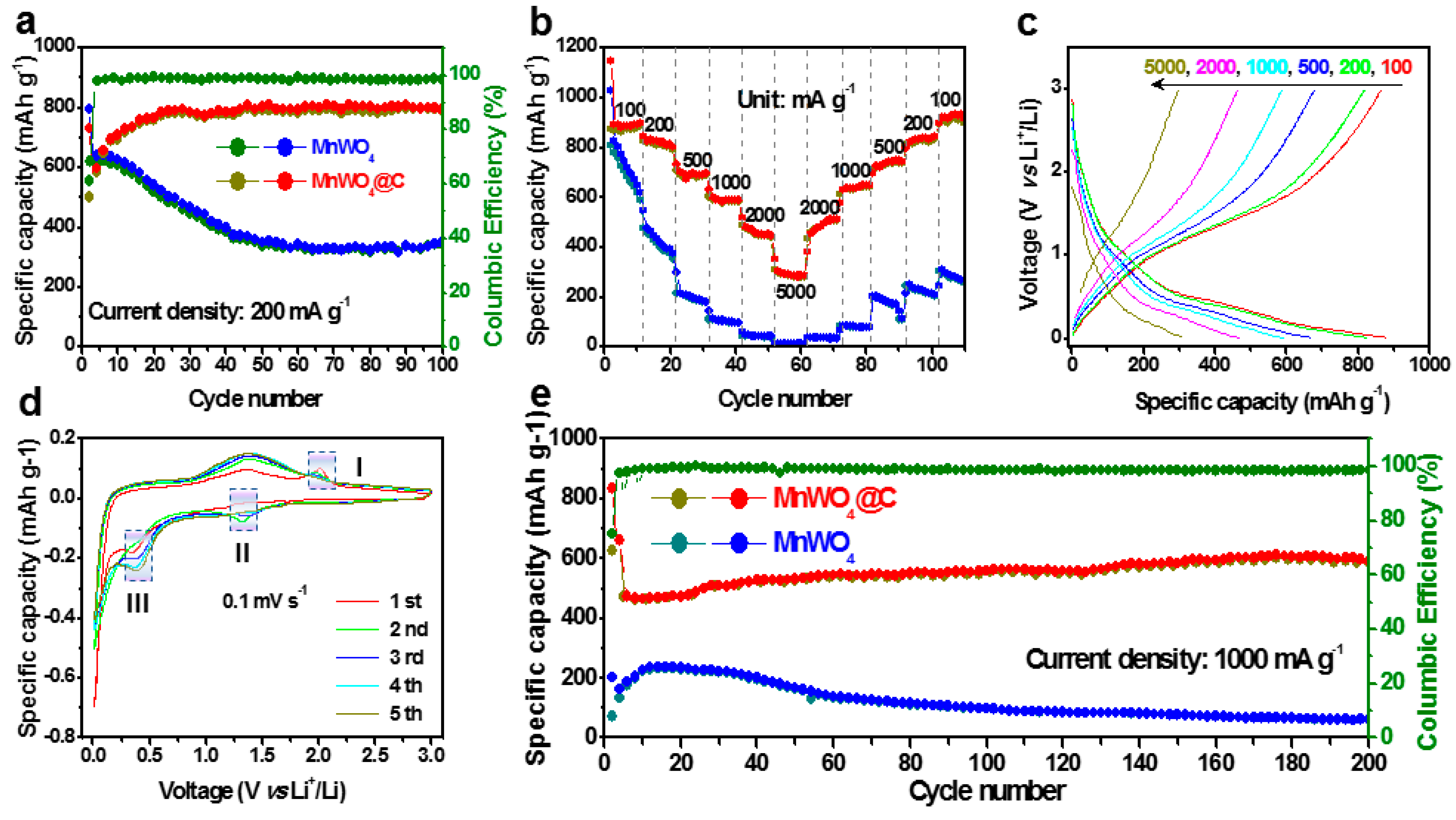
3.2. The Behavior of MnWO4 and MnWO4@C on Li+ Storage
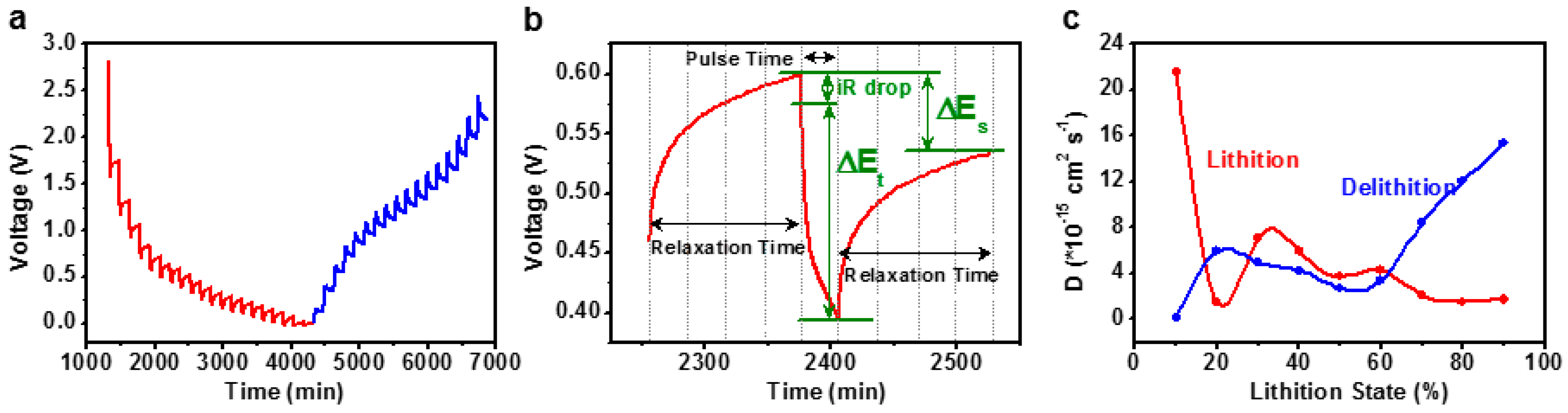
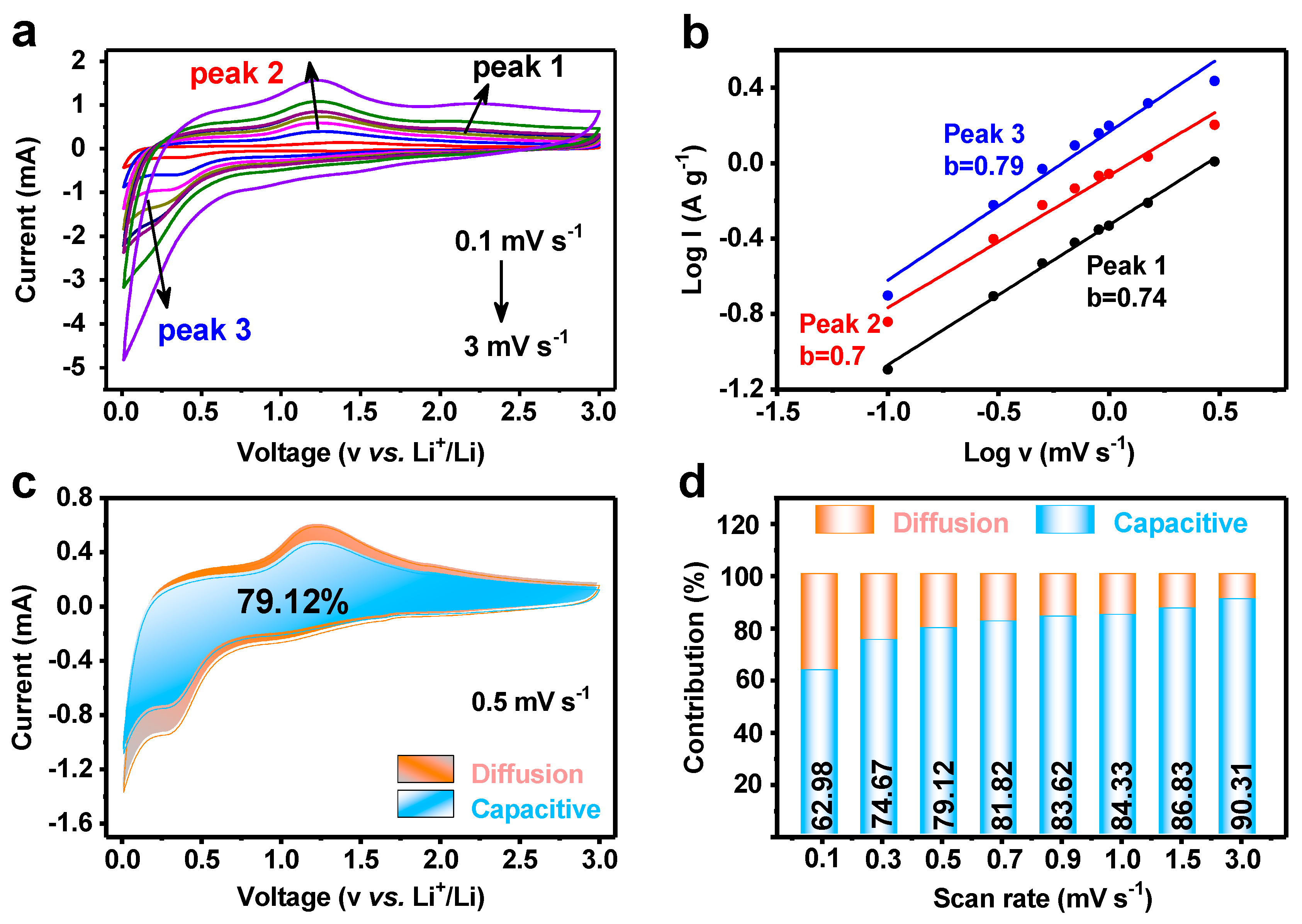
3.3. Kinetics Comparison of MnWO4 and MnWO4@C
3.4. The Influence of an Electrolyte Additive
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Liu, C.; Qiu, Y.; Liu, Y. Novel 3D grid porous Li4Ti5O12 thick electrodes fabricated by 3D printing for high performance lithium-ion batteries. J. Adv. Ceram. 2022, 11, 295–307. [Google Scholar] [CrossRef]
- Chen, C.; Lee, C.S.; Tang, Y.B. Fundamental Understanding and Optimization Strategies for Dual-Ion Batteries: A Review. Nano-Micro Lett. 2023, 15, 121. [Google Scholar] [CrossRef]
- Hou, J.B.; Zhang, K.; Xiao, J.H.; Xu, Z.Q.; Gao, W.J.; Gao, X.Y.; Zhou, S.K.; Jiao, Z.Z.; Yi, M.R.; Yin, Y.H.; et al. Modified tungsten oxide as a binder-free anode in lithium-ion battery for improving electrochemical stability. Tungsten 2022, 4, 356–369. [Google Scholar] [CrossRef]
- Montemayor, S.M.; Fuentes, A.F. Electrochemical characteristics of lithium insertion in several 3D metal tungstates (MWO4, M = Mn, Co, Ni and Cu) prepared by aqueous reactions. Ceram. Int. 2004, 30, 393–400. [Google Scholar] [CrossRef]
- Tiwari, A.; Singh, V.; Nagaiah, T.C. Tuning the MnWO4 morphology and its electrocatalytic activity towards oxygen reduction reaction. J. Mater. Chem. A 2018, 6, 2681–2692. [Google Scholar] [CrossRef]
- Amina, M.; Amna, T.; Hassan, M.S.; Al Musayeib, N.M.; Al-Deyab, S.S.S.; Khil, M.-S. Low temperature synthesis of Manganese tungstate nanoflowers with antibacterial potential: Future material for water purification. Korean J. Chem. Eng. 2016, 33, 3169–3174. [Google Scholar] [CrossRef]
- Jiao, L.; Sun, G.R.; Wang, Y.; Zhang, Z.X.; Wang, Z.; Wang, H.R.; Li, H.B.; Feng, M. Defective TiO2 hollow nanospheres as photo-electrocatalysts for photo-assisted Li-O2 batteries. Chin. Chem. Lett. 2022, 33, 4008–4012. [Google Scholar] [CrossRef]
- Muthamizh, S.; Suresh, R.; Giribabu, K.; Manigandan, R.; Kumar, S.P.; Munusamy, S.; Narayanan, V. MnWO4 nanocapsules: Synthesis, characterization and its electrochemical sensing property. J. Alloys Compd. 2015, 619, 601–609. [Google Scholar] [CrossRef]
- Liu, H.; Di, J.; Wang, P.; Gao, R.; Tian, H.; Ren, P.F.; Yuan, Q.X.; Huang, W.X.; Liu, R.P.; Liu, Q.; et al. A novel design of 3D carbon host for stable lithium metal anode. Carbon Energy 2022, 4, 654–664. [Google Scholar] [CrossRef]
- Raj, B.G.S.; Acharya, J.; Seo, M.-K.; Khil, M.-S.; Kim, H.-Y.; Kim, B.-S. One-pot sonochemical synthesis of hierarchical MnWO4 microflowers as effective electrodes in neutral electrolyte for high performance asymmetric supercapacitors. Int. J. Hydrog. Energy 2019, 44, 10838–10851. [Google Scholar] [CrossRef]
- Rani, B.J.; Ravi, G.; Ravichandran, S.; Ganesh, V.; Ameen, F.; Al-Sabri, A.; Yuvakkumar, R. Electrochemically active XWO4 (X = Co, Cu, Mn, Zn) nanostructure for water splitting applications. Appl. Nanosci. 2018, 8, 1241–1258. [Google Scholar] [CrossRef]
- Xiang, H.; Xiang, Y.; Dai, F.Z. High-entropy ceramics: Present status, challenges, and a look forward. J. Adv. Ceram. 2021, 10, 385–441. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, L.; Yen, H.Y.; Zhou, Y.C.; Jang, G.; Yuan, S.L.; Wang, J.H.; Xiong, P.X.; Liu, M.L.; Park, H.S.; et al. Single-Phase Ternary Compounds with a Disordered Lattice and Liquid Metal Phase for High-Performance Li-Ion Battery Anodes. Nano-Micro Lett. 2023, 15, 63. [Google Scholar] [CrossRef]
- Dai, Y.M.; Chen, Q.J.; Hu, C.C.; Huang, Y.Y.; Wu, W.Y.; Yu, M.L.; Sun, D.; Luo, W. Copper fluoride as a low-cost sodium-ion battery cathode with high capacity. Chin. Chem. Lett. 2022, 33, 1435–1438. [Google Scholar] [CrossRef]
- Park, G.D.; Lee, J.-K.; Kang, Y.C. Synthesis of Uniquely Structured SnO2 Hollow Nanoplates and Their Electrochemical Properties for Li-Ion Storage. Adv. Funct. Mater. 2017, 27, 1603399–1603408. [Google Scholar] [CrossRef]
- Kong, D.; Wang, Y.; Liu, B.; Huang, Z.; Yang, H.Y. Seed-assisted growth of α-Fe2O3 nanorod arrays on reduced graphene oxide: A superior anode for high-performance Li-ion and Na-ion batteries. J. Mater. Chem. A 2016, 4, 11800–11811. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; He, T.; Hu, L.; Wang, R.; Mai, L.; Mu, S. Hierarchical three-dimensional MnO nanorods/carbon anodes for ultralong-life lithium-ion batteries. J. Mater. Chem. A 2016, 4, 16936–16945. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Wang, B.; Liu, H.; Wu, H.; Liu, H.; Dou, S. Nitrogen-Doped Graphene Ribbon Assembled Core–Sheath MnO@Graphene Scrolls as Hierarchically Ordered 3D Porous Electrodes for Fast and Durable Lithium Storage. Adv. Funct. Mater. 2016, 26, 7754–7765. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Z.Y.; Wang, P.; Wang, S.M.; Feng, M. Synthesis of MOF-derived nitrogen-doped carbon microtubules via template self-consumption. Rare Met. 2022, 41, 2582–2587. [Google Scholar] [CrossRef]
- Zhang, K.; Li, P.; Ma, M.; Park, J.H. Core-Shelled Low-Oxidation State Oxides@Reduced Graphene Oxides Cubes via Pressurized Reduction for Highly Stable Lithium Ion Storage. Adv. Funct. Mater. 2016, 26, 2959–2965. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, J.; Zhang, T.; Ouyang, H.; Li, A.; Zhang, Z. Effect of variation Mn/W molar ratios on phase composition, morphology and optical properties of MnWO4. Ceram. Int. 2013, 39, 5159–5163. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Wang, Y.; Wang, X.; Han, G. Morphology-controlled synthesis and characterization of MnWO4 nanocrystals via a facile, additive-free hydrothermal process. J. Alloys Compd. 2016, 654, 246–250. [Google Scholar] [CrossRef]
- Wang, W.; Wu, N.; Zhou, J.M.; Li, F.; Wei, Y.; Li, T.H.; Wu, X.L. MnWO4 nanoparticles as advanced anodes for lithium-ion batteries: F-doped enhanced lithiation/delithiation reversibility and Li-storage properties. Nanoscale 2018, 10, 6832–6836. [Google Scholar] [CrossRef]
- Yu, S.H.; Liu, B.; Mo, M.S.; Huang, J.H.; Liu, X.M.; Qian, Y.T. General Synthesis of Single-Crystal Tungstate Nanorods/Nanowires: A Facile, Low-Temperature Solution Approach. Adv. Funct. Mater. 2003, 13, 639–647. [Google Scholar] [CrossRef]
- Chen, S.-J.; Chen, X.-T.; Xue, Z.; Zhou, J.-H.; Li, J.; Hong, J.-M.; You, X.-Z. Morphology control of MnWO4 nanocrystals by a solvothermal route. J. Mater. Chem. 2003, 13, 1132–1135. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Wang, Y.; Wang, X.; Wang, L.; Han, G. Shape-controlled synthesis of MnWO4 nanocrystals via a simple hydrothermal method. J. Alloys Compd. 2013, 578, 215–219. [Google Scholar] [CrossRef]
- Gao, G.; Dang, W.; Wu, H.; Zhang, G.; Feng, C. Synthesis of MnWO4@C as novel anode material for lithium ion battery. J. Mater. Sci. Mater. Electron. 2018, 29, 12804–12812. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Abdu Raheem, V.P.; Jayakrishnan, P.; Pradyumnan, P.P. Acrylonitrile butadiene rubber (NBR)/manganous tungstate (MnWO4) nanocomposites: Characterization, mechanical and electrical properties. AIP Conf. Proc. 2014, 1620, 3–9. [Google Scholar]
- En Zhang, Zheng Xing, Ji Wang, Zhicheng Ju, Yitai Qian, Enhanced energy storage and rate performance induced by dense nanocavities inside MnWO4 nanobars. RSC Adv. 2012, 2, 6748–6751. [CrossRef]
- Peng, J.M.; Chen, Z.Q.; Li, Y.; Hu, S.J.; Pan, Q.C.; Zheng, F.H.; Wang, H.Q.; Li, Q.Y. Conducting network interface modulated rate performance in LiFePO4/C cathode materials. Rare Met. 2022, 41, 951–959. [Google Scholar] [CrossRef]
- Wang, Y.T.; Chen, X. Single crystal growth and electrochemical studies of garnet-type fast Li-ion conductors. Tungsten 2022, 4, 263–268. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Zhang, H.; Xu, H.; He, X. Revelation of the transition-metal doping mechanism in lithium manganese phosphate for high performance of lithium-ion batteries. Battery Energy 2022, 1, 20220020. [Google Scholar] [CrossRef]
- Yao, S.; Xing, L.; Dong, Y.; Wu, X. Hierarchical WO3@MnWO4 core-shell structure for asymmetric supercapacitor with ultrahigh cycling performance at low temperature. J. Colloid Interface Sci. 2018, 531, 216–224. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Y.; Liu, B.; Yang, W.; Li, J.; Niu, P.; Jiang, X. High-performance phototransistor based on individual high electron mobility MnWO4 nanoplate. J. Alloys Compd. 2018, 762, 933–940. [Google Scholar] [CrossRef]
- Petnikota, S.; Srikanth, V.V.S.S.; Nithyadharseni, P.; Reddy, M.V.; Adams, S.; Chowdari, B.V.R. Sustainable Graphenothermal Reduction Chemistry to Obtain MnO Nanonetwork Supported Exfoliated Graphene Oxide Composite and its Electrochemical Characteristics. ACS Sustain. Chem. Eng. 2015, 3, 3205–3213. [Google Scholar] [CrossRef]
- Choi, M.J.; Baek, J.H.; Kim, J.Y.; Jang, H.W. Highly textured and crystalline materials for rechargeable Li-ion batteries. Battery Energy 2023, 2, 20230010. [Google Scholar] [CrossRef]
- Gu, X.X.; Yang, Z.G.; Qiao, S.; Shao, C.B.; Ren, X.L.; Yang, J.J. Exploiting methylated amino resin as a multifunctional binder for high-performance lithium-sulfur batteries. Rare Met. 2021, 40, 529–536. [Google Scholar] [CrossRef]
- Hyun-Woo Shim, Ah-Hyeon Lim, Jae-Chan Kim, Gwang-Hee Lee, Dong-Wan Kim, Hydrothermal realization of a hierarchical, flowerlike MnWO4@MWCNTs nanocomposite with enhanced reversible Li storage as a new anode material. Chem. Asian J. 2013, 8, 2851–2858. [CrossRef]
- Wei, J.; Ma, J.; Wang, W.; Li, T.; Wu, N.; Zhang, D. Study of the effect of F-doping on lithium electrochemical behavior in MnWO4 anode nanomaterials. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3175–3182. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, S.; Zhou, L.; Chang, L.; Liu, W.; Yin, D.; Yi, Z.; Wang, L. SnO2 quantum dots: Rational design to achieve highly reversible conversion reaction and stable capacities for lithium and sodium storage. Small 2020, 16, 2000681. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.-S.; Yew, K.H.; Lee, K.Y.; Sung, M.; Kim, H.; Kim, S.-S. Effect of fluoroethylene carbonate additive on interfacial properties of silicon thin-film electrode. J. Power Sources 2006, 161, 1254–1259. [Google Scholar] [CrossRef]
- Komaba, S.; Ishikawa, T.; Yabuuchi, N.; Murata, W.; Ito, A.; Ohsawa, Y. Fluorinated Ethylene Carbonate as Electrolyte Additive for Rechargeable Na Batteries. ACS Appl. Mater. Interfaces 2011, 3, 4165–4168. [Google Scholar] [CrossRef]
- Song, S.P.; Yang, C.; Jiang, C.Z.; Wu, Y.M.; Guo, R.; Sun, H.; Yang, J.L.; Xiang, Y.; Zhang, X.K. Increasing ionic conductivity in Li0.33La0.56TiO3 thin-films via optimization of processing atmosphere and temperature. Rare Met. 2022, 41, 179–188. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Wang, Z.; Wang, C.; Yin, D.; Liang, Y.; Wang, L.; Cheng, Y.; Feng, M. Synergistically Boosting Li Storage Performance of MnWO4 Nanorods Anode via Carbon Coating and Additives. Materials 2024, 17, 4682. https://doi.org/10.3390/ma17194682
Wang D, Wang Z, Wang C, Yin D, Liang Y, Wang L, Cheng Y, Feng M. Synergistically Boosting Li Storage Performance of MnWO4 Nanorods Anode via Carbon Coating and Additives. Materials. 2024; 17(19):4682. https://doi.org/10.3390/ma17194682
Chicago/Turabian StyleWang, Duo, Zhaomin Wang, Chunli Wang, Dongming Yin, Yao Liang, Limin Wang, Yong Cheng, and Ming Feng. 2024. "Synergistically Boosting Li Storage Performance of MnWO4 Nanorods Anode via Carbon Coating and Additives" Materials 17, no. 19: 4682. https://doi.org/10.3390/ma17194682




