Direct, Broad-Spectrum Antimicrobial Activity of Ag+-Doped Hydroxyapatite against Fastidious Anaerobic Periodontal and Aerobic Dental Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AgHAp and HAp Synthesis
2.3. Material Characterization
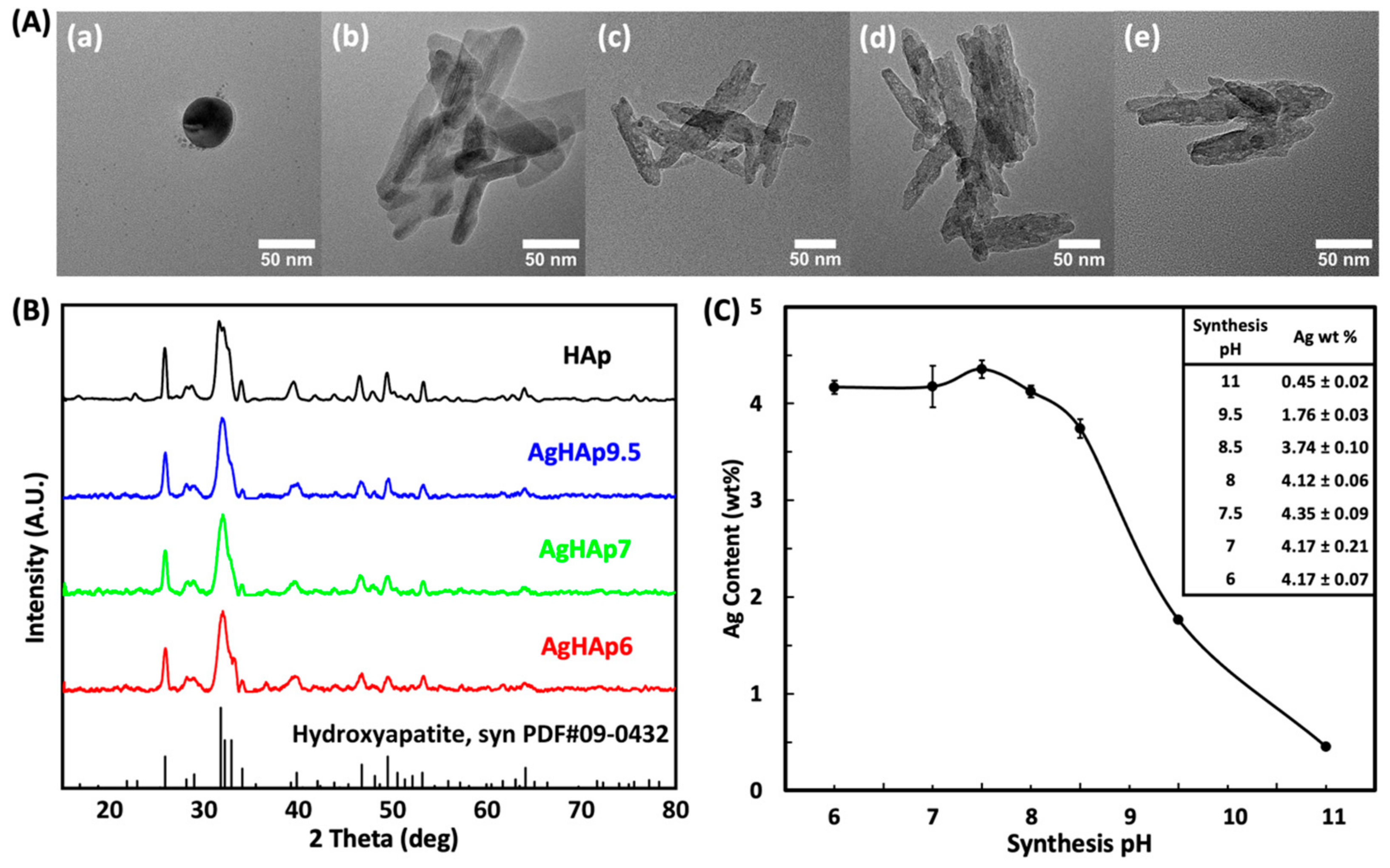
2.3.1. Morphology and Size
2.3.2. Crystal Structure
2.3.3. Total Silver Content of AgHAp NPs
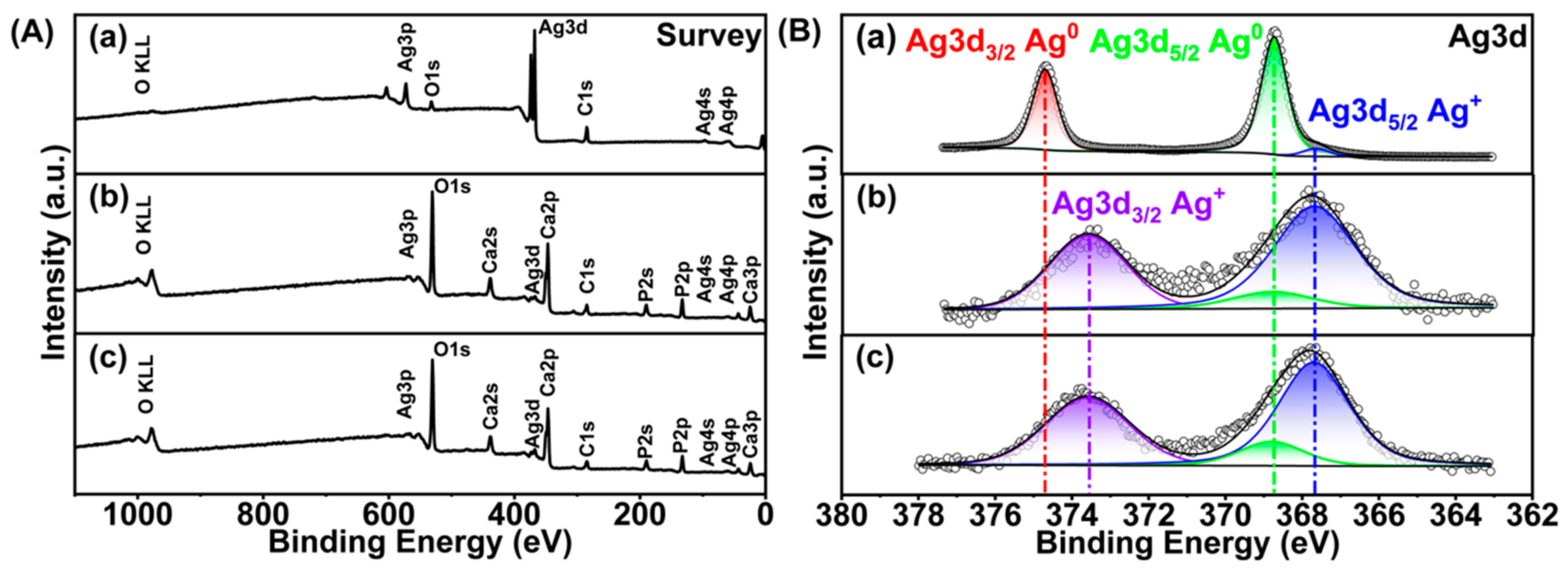
2.3.4. Silver Oxidation State
2.4. Antimicrobial Tests
2.4.1. Bacterial Culture and Growth
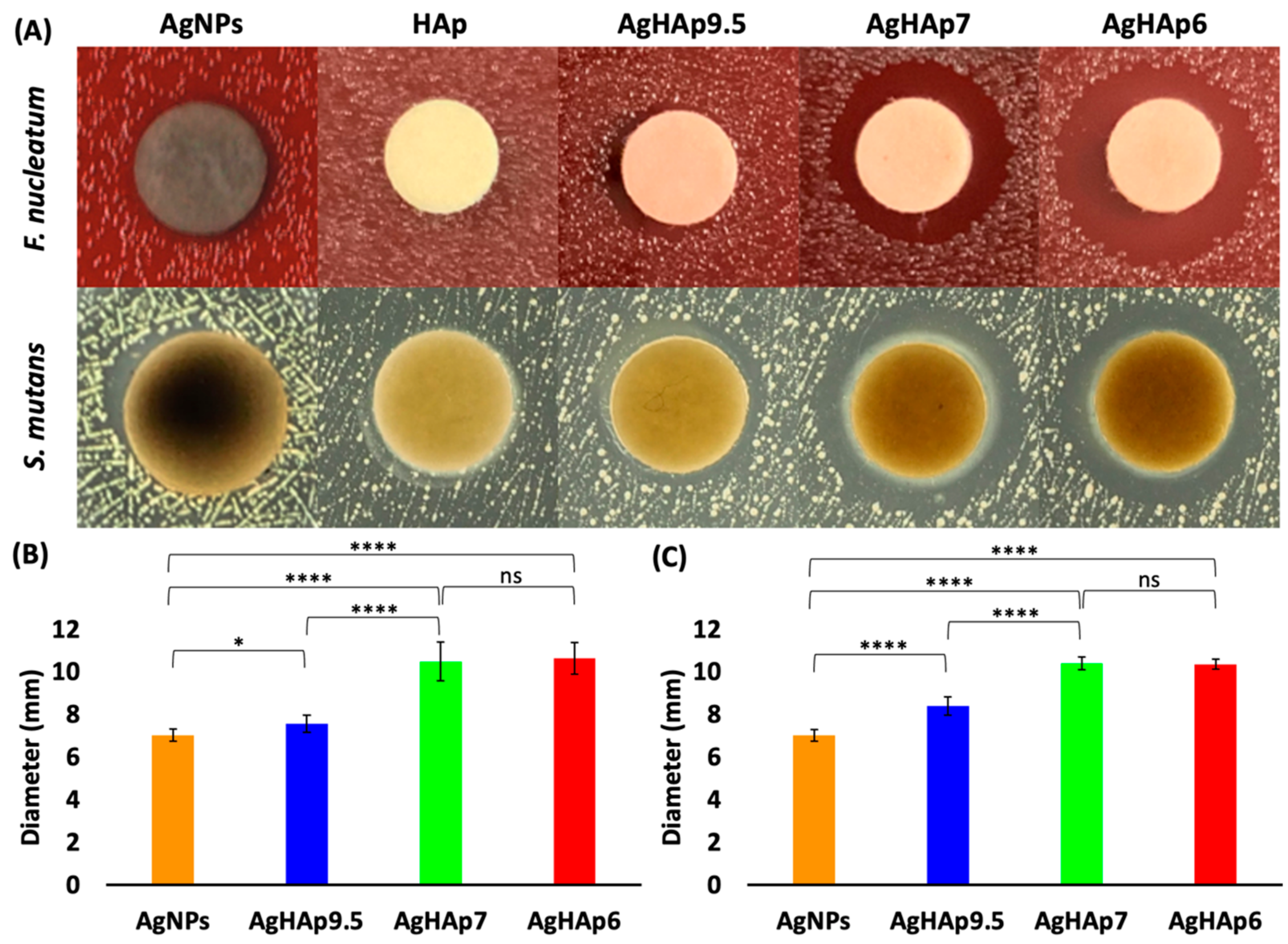
2.4.2. Inhibition Zone Test
2.4.3. Minimum Inhibition Concentration (MIC)
2.5. Silver Complexation
2.6. Fibroblast Cell Cytocompatibility
2.6.1. Fibroblast Cell Culture and Growth
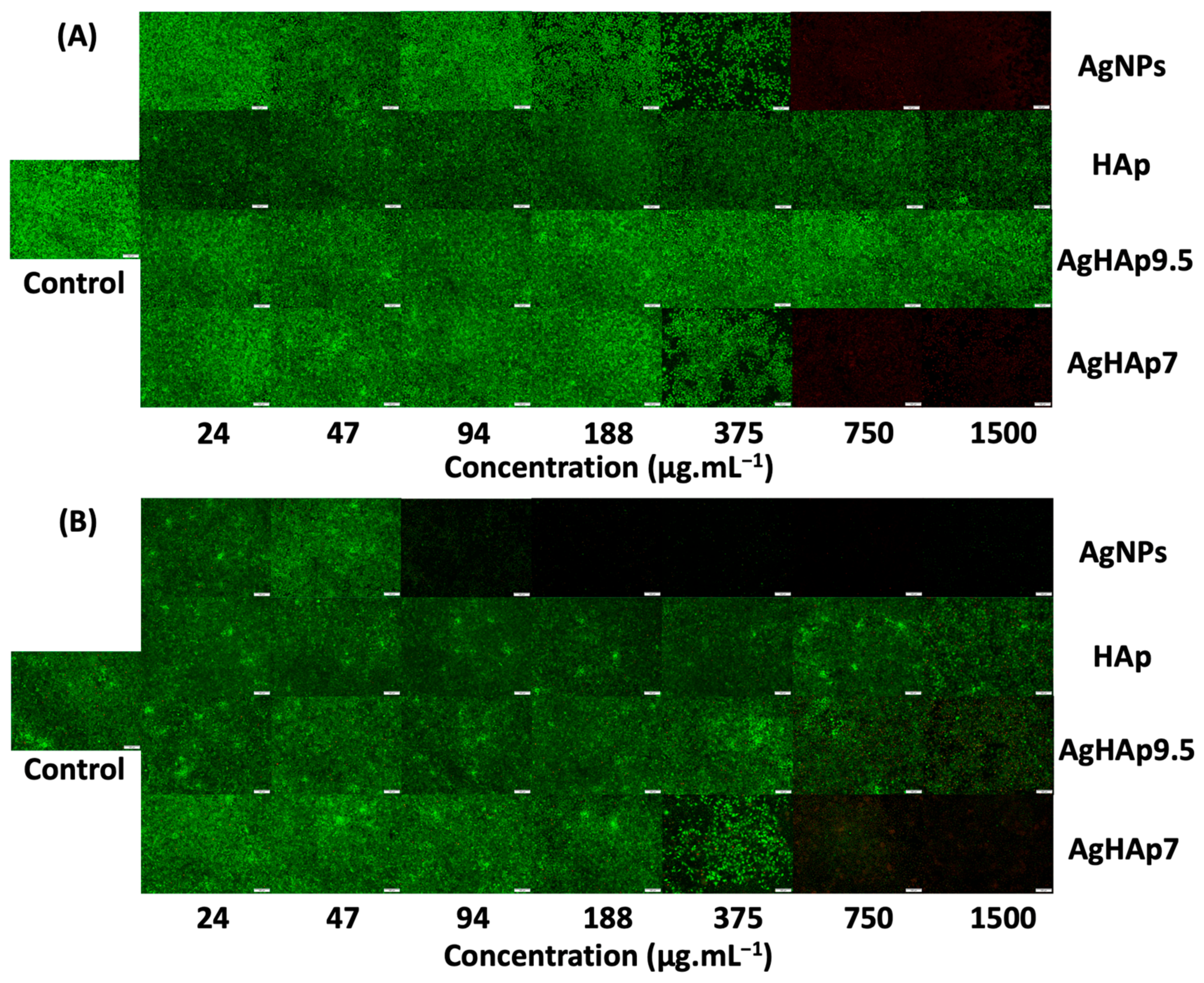
2.6.2. LIVE–DEAD Assay by Fluorescence Microscopy
2.6.3. MTT Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Material Characterization
3.1.1. Appearance of HAp and AgHAp NPs
3.1.2. Crystal Structure
3.1.3. Total Silver Content
3.1.4. Silver Oxidation State
3.2. Antimicrobial Tests
3.2.1. Inhibition Zone
3.2.2. MIC
3.3. Silver Complexation
3.4. Fibroblast Cell Cytocompatibility
3.4.1. Fibroblast Cell Imaging
3.4.2. Fibroblast Cell Viability
4. Discussion
4.1. AgHAp NPs Compared to AgNPs
4.2. Synthesis pH-Dependent Tunability and Tissue-Specific Applications
4.3. Antimicrobial Resistance
4.4. Cost-Effectiveness, Scalability, and Medical Use
4.5. MIC Tests for Strictly Anaerobic Fastidious Bacteria
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valm, A.M. The Structure of Dental Plaque Microbial Communities in the Transition from Health to Dental Caries and Periodontal Disease. J. Mol. Biol. 2019, 431, 2957–2969. [Google Scholar] [CrossRef]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The Role of the Microbiota in Periodontal Disease. Periodontol. 2000 2020, 83, 14–25. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; WHO: Geneva, Switzerland, 2022; ISBN 9789240061484. [Google Scholar]
- Al-Nasser, L.; Lamster, I.B. Prevention and Management of Periodontal Diseases and Dental Caries in the Older Adults. Periodontol. 2000 2020, 84, 69–83. [Google Scholar] [CrossRef]
- Pilot, T. The Periodontal Disease Problem. A Comparison between Industrialised and Developing Countries. Int. Dent. J. 1998, 48, 221–232. [Google Scholar] [CrossRef]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Michaud, D.S.; Lu, J.; Peacock-Villada, A.Y.; Barber, J.R.; Joshu, C.E.; Prizment, A.E.; Beck, J.D.; Offenbacher, S.; Platz, E.A. Periodontal Disease Assessed Using Clinical Dental Measurements and Cancer Risk in the ARIC Study. JNCI J. Natl. Cancer Inst. 2018, 110, 843–854. [Google Scholar] [CrossRef]
- Belstrøm, D.; Constancias, F.; Drautz-Moses, D.I.; Schuster, S.C.; Veleba, M.; Mahé, F.; Givskov, M. Periodontitis Associates with Species-Specific Gene Expression of the Oral Microbiota. NPJ Biofilms Microbiomes 2021, 7, 76. [Google Scholar] [CrossRef]
- Usui, M.; Onizuka, S.; Sato, T.; Kokabu, S.; Ariyoshi, W.; Nakashima, K. Mechanism of Alveolar Bone Destruction in Periodontitis—Periodontal Bacteria and Inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 201–208. [Google Scholar] [CrossRef]
- Holtfreter, B.; Schützhold, S.; Kocher, T. Is Periodontitis Prevalence Declining? A Review of the Current Literature. Curr. Oral. Health Rep. 2014, 1, 251–261. [Google Scholar] [CrossRef]
- Ozmeric, N.; Bissada, N.; da Silva, A.P.B. The Association between Inflammatory Bowel Disease and Periodontal Conditions: Is There a Common Bacterial Etiology? J. Int. Acad. Periodontol. 2018, 20, 40–51. [Google Scholar]
- El Kholy, K.; Genco, R.J.; Van Dyke, T.E. Oral Infections and Cardiovascular Disease. Trends Endocrinol. Metab. 2015, 26, 315–321. [Google Scholar] [CrossRef]
- Meurman, J.; Bascones-Martinez, A. Are Oral and Dental Diseases Linked to Cancer? Oral. Dis. 2011, 17, 779–784. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, Opportunist and Oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial Activity of Silver Nanoparticles: A Surface Science Insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Goudouri, O.-M.; Kontonasaki, E.; Lohbauer, U.; Boccaccini, A.R. Antibacterial Properties of Metal and Metalloid Ions in Chronic Periodontitis and Peri-Implantitis Therapy. Acta Biomater. 2014, 10, 3795–3810. [Google Scholar] [CrossRef]
- Stabryla, L.M.; Johnston, K.A.; Diemler, N.A.; Cooper, V.S.; Millstone, J.E.; Haig, S.-J.; Gilbertson, L.M. Role of Bacterial Motility in Differential Resistance Mechanisms of Silver Nanoparticles and Silver Ions. Nat. Nanotechnol. 2021, 16, 996–1003. [Google Scholar] [CrossRef]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio Silver: Its Interactions with Peptides and Bacteria, and Its Uses in Medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomedicine 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Mulenos, M.R.; Liu, J.; Lujan, H.; Guo, B.; Lichtfouse, E.; Sharma, V.K.; Sayes, C.M. Copper, Silver, and Titania Nanoparticles Do Not Release Ions under Anoxic Conditions and Release Only Minute Ion Levels under Oxic Conditions in Water: Evidence for the Low Toxicity of Nanoparticles. Environ. Chem. Lett. 2020, 18, 1319–1328. [Google Scholar] [CrossRef]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-Dependent Antibacterial Activities of Silver Nanoparticles against Oral Anaerobic Pathogenic Bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef]
- Spacciapoli, P.; Buxton, D.; Rothstein, D.; Friden, P. Antimicrobial Activity of Silver Nitrate against Periodontal Pathogens. J. Periodontal Res. 2001, 36, 108–113. [Google Scholar] [CrossRef]
- Malic, S.; Rai, S.; Redfern, J.; Pritchett, J.; Liauw, C.M.; Verran, J.; Tosheva, L. Zeolite-Embedded Silver Extends Antimicrobial Activity of Dental Acrylics. Colloids Surf. B Biointerfaces 2019, 173, 52–57. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial Resistance to Silver Nanoparticles and How to Overcome It. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. A Mineralogical Perspective on the Apatite in Bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Hu, R.; Sahai, N. Citric Acid-Modified Hydroxyapatite Nanoparticles as an Antibiotic Protein Carrier. In Proceedings of the APS March Meeting Abstracts, Chicago, IL, USA, 14–18 March 2022; Volume 2022, p. S05.009. [Google Scholar]
- Nasar, A. Hydroxyapatite and Its Coatings in Dental Implants. In Applications of Nanocomposite Materials in Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 145–160. ISBN 9780128137420. [Google Scholar]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, Characterization and Antimicrobial Activity of Copper and Zinc-Doped Hydroxyapatite Nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Martínez-Gracida, N.O.; Esparza-González, S.C.; Castillo-Martínez, N.A.; Serrano-Medina, A.; Olivas-Armendariz, I.; Campos-Múzquiz, L.G.; Múzquiz-Ramos, E.M. Synergism in Novel Silver-Copper/Hydroxyapatite Composites for Increased Antibacterial Activity and Biocompatibility. Ceram. Int. 2020, 46, 20215–20225. [Google Scholar] [CrossRef]
- Shi, C.; Gao, J.; Wang, M.; Fu, J.; Wang, D.; Zhu, Y. Ultra-Trace Silver-Doped Hydroxyapatite with Non-Cytotoxicity and Effective Antibacterial Activity. Mater. Sci. Eng. C 2015, 55, 497–505. [Google Scholar] [CrossRef]
- Peng, J.J.-Y.; Botelho, M.G.; Matinlinna, J.P. Silver Compounds Used in Dentistry for Caries Management: A Review. J. Dent. 2012, 40, 531–541. [Google Scholar] [CrossRef]
- Kim, T.N.; Feng, Q.L.; Kim, J.O.; Wu, J.; Wang, H.; Chen, G.C.; Cui, F.Z. Antimicrobial Effects of Metal Ions (Ag+, Cu2+, Zn2+) in Hydroxyapatite. J. Mater. Sci. Mater. Med. 1998, 9, 129–134. [Google Scholar] [CrossRef]
- Feng, Q.L.; Cui, F.Z.; Kim, T.N.; Kim, J.W. Ag-Substituted Hydroxyapatite Coatings with Both Antimicrobial Effects and Biocompatibility. J. Mater. Sci. Lett. 1999, 18, 559–561. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Pasuk, I.; Vasile, B.S.; Lupu, A.R.; Hermenean, A.; Dinischiotu, A.; Predoi, D. Structural Properties of Silver Doped Hydroxyapatite and Their Biocompatibility. Mater. Sci. Eng. C 2013, 33, 1395–1402. [Google Scholar] [CrossRef]
- Hakimi, F.; Hashemikia, S.; Sadighian, S.; Ramazani, A. Nanofibrous Chitosan/Polyethylene Oxide Silver/Hydroxyapatite/Silica Composite as a Potential Biomaterial for Local Treatment of Periodontal Disease. Polym. Bull. 2023, 80, 8703–8723. [Google Scholar] [CrossRef]
- Thurman, R.B.; Gerba, C.P.; Bitton, G. The Molecular Mechanisms of Copper and Silver Ion Disinfection of Bacteria and Viruses. Crit. Rev. Environ. Control. 1989, 18, 295–315. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Wang, X.; Liu, D. Release Strategies of Silver Ions from Materials for Bacterial Killing. ACS Appl. Bio Mater. 2021, 4, 3985–3999. [Google Scholar] [CrossRef]
- Saravanan, M.; Nanda, A. Extracellular Synthesis of Silver Bionanoparticles from Aspergillus Clavatus and Its Antimicrobial Activity against MRSA and MRSE. Colloids Surf. B Biointerfaces 2010, 77, 214–218. [Google Scholar] [CrossRef]
- Haider, A.; Kang, I.-K. Preparation of Silver Nanoparticles and Their Industrial and Biomedical Applications: A Comprehensive Review. Adv. Mater. Sci. Eng. 2015, 2015, 165257. [Google Scholar] [CrossRef]
- Sorinolu, A.J.; Godakhindi, V.; Siano, P.; Vivero-Escoto, J.L.; Munir, M. Influence of Silver Ion Release on the Inactivation of Antibiotic Resistant Bacteria Using Light-Activated Silver Nanoparticles. Mater. Adv. 2022, 3, 9090–9102. [Google Scholar] [CrossRef]
- Elbasuney, S.; El-Sayyad, G.S.; Radwan, S.M.; Correa-Duarte, M.A. Antimicrobial, and Antibiofilm Activities of Silver Doped Hydroxyapatite: A Novel Bioceramic Material for Dental Filling. J. Inorg. Organomet. Polym. Mater. 2022, 32, 4559–4575. [Google Scholar] [CrossRef]
- Sulaeman, U.; Suhendar, S.; Diastuti, H.; Andreas, R.; Yin, S. Design of Defect and Metallic Silver in Silver Phosphate Photocatalyst Using the Hydroxyapatite and Glucose. Indones. J. Chem. 2020, 20, 1441. [Google Scholar] [CrossRef]
- Honda, M.; Kawanobe, Y.; Ishii, K.; Konishi, T.; Mizumoto, M.; Kanzawa, N.; Matsumoto, M.; Aizawa, M. In Vitro and in Vivo Antimicrobial Properties of Silver-Containing Hydroxyapatite Prepared via Ultrasonic Spray Pyrolysis Route. Mater. Sci. Eng. C 2013, 33, 5008–5018. [Google Scholar] [CrossRef] [PubMed]
- Soma, T.; Iwasaki, R.; Sato, Y.; Kobayashi, T.; Ito, E.; Matsumoto, T.; Kimura, A.; Homma, F.; Saiki, K.; Takahashi, Y.; et al. An Ionic Silver Coating Prevents Implant-Associated Infection by Anaerobic Bacteria in Vitro and in Vivo in Mice. Sci. Rep. 2022, 12, 18387. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Reus, M.A.; Memarzadeh, K.; Huang, J.; Ren, G.G.; Allaker, R.P. Antimicrobial Activity of Nanoparticulate Metal Oxides against Peri-Implantitis Pathogens. Int. J. Antimicrob. Agents 2012, 40, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ustriyana, P.; Moore, F.; Sahai, N. Biological Response of and Blood Plasma Protein Adsorption on Silver-Doped Hydroxyapatite. ACS Biomater. Sci. Eng. 2019, 5, 561–571. [Google Scholar] [CrossRef]
- Brook, I.; Wexler, H.M.; Goldstein, E.J.C. Antianaerobic Antimicrobials: Spectrum and Susceptibility Testing. Clin. Microbiol. Rev. 2013, 26, 526–546. [Google Scholar] [CrossRef]
- Kaysner, C.A.; DePaola, A., Jr. Bacteriological Analytical Manual Chapter 9: Vibrio; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2004. [Google Scholar]
- U.S. Food & Drug Administration. BAM Media M20: Blood Agar. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-media-m20-blood-agar (accessed on 29 April 2022).
- Penner, G.H.; Liu, X. Silver NMR Spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 49, 151–167. [Google Scholar] [CrossRef]
- Leung, B.O.; Jalilehvand, F.; Mah, V.; Parvez, M.; Wu, Q. Silver(I) Complex Formation with Cysteine, Penicillamine, and Glutathione. Inorg. Chem. 2013, 52, 4593–4602. [Google Scholar] [CrossRef]
- Alekseev, V.G.; Semenov, A.N.; Pakhomov, P.M. Complexation of Ag+ Ions with L-Cysteine. Russ. J. Inorg. Chem. 2012, 57, 1041–1044. [Google Scholar] [CrossRef]
- Babel, L.; Bonnet-Gómez, S.; Fromm, K. Appropriate Buffers for Studying the Bioinorganic Chemistry of Silver(I). Chemistry 2020, 2, 193–202. [Google Scholar] [CrossRef]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver Nanoparticles and Silver Ions as Potential Antibacterial Agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Vasil’kov, A.; Batsalova, T.; Dzhambazov, B.; Naumkin, A. XPS Study of Silver and Copper Nanoparticles Demonstrated Selective Anticancer, Proapoptotic, and Antibacterial Properties. Surf. Interface Anal. 2022, 54, 189–202. [Google Scholar] [CrossRef]
- Lee, S.U.; Jun, Y.-S.; Lee, E.Z.; Heo, N.S.; Hong, W.H.; Huh, Y.S.; Chang, Y.K. Selective Silver Ion Adsorption onto Mesoporous Graphitic Carbon Nitride. Carbon 2015, 95, 58–64. [Google Scholar] [CrossRef]
- Firet, N.J.; Blommaert, M.A.; Burdyny, T.; Venugopal, A.; Bohra, D.; Longo, A.; Smith, W.A. Operando EXAFS Study Reveals Presence of Oxygen in Oxide-Derived Silver Catalysts for Electrochemical CO2 Reduction. J. Mater. Chem. A Mater. 2019, 7, 2597–2607. [Google Scholar] [CrossRef]
- El Mel, A.A.; Stephant, N.; Hamon, J.; Thiry, D.; Chauvin, A.; Chettab, M.; Gautron, E.; Konstantinidis, S.; Granier, A.; Tessier, P.Y. Creating Nanoporosity in Silver Nanocolumns by Direct Exposure to Radio-Frequency Air Plasma. Nanoscale 2016, 8, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Gaarenstroom, S.W.; Winograd, N. Initial and Final State Effects in the ESCA Spectra of Cadmium and Silver Oxides. J. Chem. Phys. 1977, 67, 3500–3506. [Google Scholar] [CrossRef]
- Joris, S.J.; Amberg, C.H. The Nature of Deficiency in Nonstoichiometric Hydroxyapatites. II. Spectroscopic Studies of Calcium and Strontium Hydroxyapatites. J. Phys. Chem. 1971, 75, 3172–3178. [Google Scholar] [CrossRef]
- Tochon-Danguy, H.J.; Very, J.M.; Geoffroy, M.; Baud, C.A. Calcified Tissue Research Paramagnetic and Crystallographic Effects of Low Temperature Ashing on Human Bone and Tooth Enamel. Calc. Tis Res. 1978, 26, 259–265. [Google Scholar] [CrossRef]
- Bacquet, G.; Truong, V.Q.; Vignoles, M.; Bonel, G. EPR Detection of Acetate Ions Trapping in B-Type Carbonated Fluorapatites. J. Solid. State Chem. 1981, 39, 148–153. [Google Scholar] [CrossRef]
- Rey, C.; Trombe, J.C.; Montel, G. Some Features of the Incorporation of Oxygen in Different Oxidation States in the Apatitic Lattice—III Synthesis and Properties of Some Oxygenated Apatites. J. Inorg. Nucl. Chem. 1978, 40, 27–30. [Google Scholar] [CrossRef]
- Ivanova, T.I.; Frank-Kamenetskaya, O.V.; Kol’tsov, A.B.; Ugolkov, V.L. Crystal Structure of Calcium-Deficient Carbonated Hydroxyapatite. Thermal Decomposition. J. Solid. State Chem. 2001, 160, 340–349. [Google Scholar] [CrossRef]
- Rey, C.; Trombe, J.C.; Montel, G.J. Sur La Fixation de La Glycine Dans Le Réseau Des Phosphates à Structure d’apatite. J. Chem. Res. 1978, 188, 2401–2416. [Google Scholar]
- Choi, Y.; Kim, H.A.; Kim, K.W.; Lee, B.T. Comparative Toxicity of Silver Nanoparticles and Silver Ions to Escherichia coli. J. Environ. Sci. 2018, 66, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Deng, K.K.; Kim, N.J.; Ross, L.; Surampalli, R.Y.; Hu, Z. The Inhibitory Effects of Silver Nanoparticles, Silver Ions, and Silver Chloride Colloids on Microbial Growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Kircheva, N.; Dobrev, S.; Nikolova, V.; Angelova, S.; Dudev, T. Theoretical Insight into the Phosphate-Targeted Silver’s Antibacterial Action: Differentiation between Gram (+) and Gram (−) Bacteria. Inorg. Chem. 2022, 61, 10089–10100. [Google Scholar] [CrossRef]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The Effects of Interfacial Potential on Antimicrobial Propensity of ZnO Nanoparticle. Sci. Rep. 2015, 5, 9578. [Google Scholar] [CrossRef]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta Potential and Membrane Permeability in Bacteria: A Study with Cationic Agents. Springerplus 2015, 4, 672. [Google Scholar] [CrossRef]
- Mankoci, S.; Ewing, J.; Dalai, P.; Sahai, N.; Barton, H.A.; Joy, A. Bacterial Membrane Selective Antimicrobial Peptide-Mimetic Polyurethanes: Structure-Property Correlations and Mechanisms of Action. Biomacromolecules 2019, 20, 4096–4106. [Google Scholar] [CrossRef]
- Mankoci, S.; Kaiser, R.L.; Sahai, N.; Barton, H.A.; Joy, A. Bactericidal Peptidomimetic Polyurethanes with Remarkable Selectivity against Escherichia coli. ACS Biomater. Sci. Eng. 2017, 3, 2588–2597. [Google Scholar] [CrossRef]
- Mirolo, L.; Schmidt, T.; Eckhardt, S.; Meuwly, M.; Fromm, K.M. PH-Dependent Coordination of AgI Ions by Histidine: Experiment, Theory, and a Model for SilE. Chem.—Eur. J. 2013, 19, 1754–1761. [Google Scholar] [CrossRef]
- Santos, E.; Avalle, L.; Pötting, K.; Vélez, P.; Jones, H. Experimental and Theoretical Studies of L-Cysteine Adsorbed at Ag(111) Electrodes. Electrochim. Acta 2008, 53, 6807–6817. [Google Scholar] [CrossRef]
- Luque, N.B.; Vélez, P.; Pötting, K.; Santos, E. Ab Initio Studies of the Electronic Structure of L-Cysteine Adsorbed on Ag(111). Langmuir 2012, 28, 8084–8099. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, L.R.; Jacobs, Z.L.; Dikin, D.; Akula, S.M. Five Nanometer Size Highly Positive Silver Nanoparticles Are Bactericidal Targeting Cell Wall and Adherent Fimbriae Expression. Sci. Rep. 2022, 12, 6729. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Rábago, E.I.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Garcia-Marin, L.E.; Quester, K.; Castro-Longoria, E. Optimized Synthesis of Small and Stable Silver Nanoparticles Using Intracellular and Extracellular Components of Fungi: An Alternative for Bacterial Inhibition. Antibiotics 2022, 11, 800. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Cristóbal, L.F.; Martínez-Castañón, G.A.; Martínez-Martínez, R.E.; Loyola-Rodríguez, J.P.; Patiño-Marín, N.; Reyes-Macías, J.F.; Ruiz, F. Antimicrobial Sensibility of Streptococcus mutans Serotypes to Silver Nanoparticles. Mater. Sci. Eng. C 2012, 32, 896–901. [Google Scholar] [CrossRef]
- Perde-Schrepler, M.; Florea, A.; Brie, I.; Virag, P.; Fischer-Fodor, E.; Vâlcan, A.; Gurzău, E.; Lisencu, C.; Maniu, A. Size-Dependent Cytotoxicity and Genotoxicity of Silver Nanoparticles in Cochlear Cells In Vitro. J. Nanomater. 2019, 2019, 6090259. [Google Scholar] [CrossRef]
- Buckley, J.J.; Lee, A.F.; Olivi, L.; Wilson, K. Hydroxyapatite Supported Antibacterial Ag3PO4 Nanoparticles. J. Mater. Chem. 2010, 20, 8056–8063. [Google Scholar] [CrossRef]
- Terzioğlu, E.; Arslan, M.; Balaban, B.G.; Çakar, Z.P. Microbial Silver Resistance Mechanisms: Recent Developments. World J. Microbiol. Biotechnol. 2022, 38, 158. [Google Scholar] [CrossRef]
- Raad Salh, A.; Hashim Risan, M.; Majeed Jasim, H. Biochemical Characteristics and Antibiotics Susceptibility of Streptococcus mutans Isolates from Dental Caries in Baghdad City. Int. J. Adv. Biol. Biomed. Res. 2022, 10, 32–43. [Google Scholar] [CrossRef]
- Bolstad, A.I.; Jensen, H.B.; Bakken, V. Taxonomy, Biology, and Periodontal Aspects of Fusobacterium nucleatum. Clin. Microbiol. Rev. 1996, 9, 55–71. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Min, C.; Xia, F.; Tang, M.; Li, J.; Hu, Y.; Zou, M. Characterization of Silver Resistance and Coexistence of Sil Operon with Antibiotic Resistance Genes Among Gram-Negative Pathogens Isolated from Wound Samples by Using Whole-Genome Sequencing. Infect. Drug Resist. 2022, 15, 1425–1437. [Google Scholar] [CrossRef]
- Vartoukian, S.R.; Palmer, R.M.; Wade, W.G. Strategies for Culture of “unculturable” Bacteria. FEMS Microbiol. Lett. 2010, 309, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, N.; Kelly, D.J.; Hitchcock, A.; Taylor, A.J. Cysteine Biosynthesis in Campylobacter Jejuni: Substrate Specificity of CysM and the Dualism of Sulfide. Biomolecules 2023, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Kezuka, Y.; Ishida, T.; Yoshida, Y.; Nonaka, T. Structural Insights into the Catalytic Mechanism of Cysteine (Hydroxyl) Lyase from the Hydrogen Sulfide-Producing Oral Pathogen, Fusobacterium nucleatum. Biochem. J. 2018, 475, 733–748. [Google Scholar] [CrossRef] [PubMed]


| Nanoparticle | Stoichiometric Formula | XPS Survey Scan | |||||
|---|---|---|---|---|---|---|---|
| Ca | P | O | C | Ag | Ag | ||
| Ca10−xAgx(PO4)6(OH)2−x | (atom %) | (wt %) | |||||
| AgNPs | - | - | - | 16.6 | 60.7 | 22.7 | - |
| AgHAp9.5 | Ca9.97Ag0.03(PO4)6(OH)1.77 | 16.1 | 11.5 | 56 | 16 | 0.5 | 2.52 |
| AgHAp7 | Ca9.95Ag0.05(PO4)6(OH)0.71 | 15.7 | 12.4 | 56.8 | 14.2 | 0.9 | 4.43 |
| Fitted Area under Ag3d5 Peak | Ag+ (367.7 eV) | Ag0 (368.8 eV) | Ag0 + Ag+ | Ag+/(Ag0 + Ag+) % |
|---|---|---|---|---|
| AgNPs | 779 | 11,931 | 12,710 | 6% |
| AgHAp9.5 | 992 | 203 | 1195 | 83% |
| AgHAp7 | 565 | 88 | 653 | 87% |
| Nanoparticle | F. nucleatum; Medium 1 a | F. nucleatum; Medium 2 b | S. mutans; Medium 3 c | |||
|---|---|---|---|---|---|---|
| MIC | Ag MIC | MIC | Ag MIC | MIC | Ag MIC | |
| AgNPs | >2000 | >2000 | 1500 | 1500 | >1000 | >1000 |
| AgHAp9.5 | >2000 | >35 | 1000 | 18 | 1500 | 26 |
| AgHAp7 | 1500 | 63 | 375 | 16 | 375 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Deng, L.; Hao, X.; Chen, J.; Zhou, X.; Sahai, N. Direct, Broad-Spectrum Antimicrobial Activity of Ag+-Doped Hydroxyapatite against Fastidious Anaerobic Periodontal and Aerobic Dental Bacteria. Materials 2024, 17, 4688. https://doi.org/10.3390/ma17194688
Hu R, Deng L, Hao X, Chen J, Zhou X, Sahai N. Direct, Broad-Spectrum Antimicrobial Activity of Ag+-Doped Hydroxyapatite against Fastidious Anaerobic Periodontal and Aerobic Dental Bacteria. Materials. 2024; 17(19):4688. https://doi.org/10.3390/ma17194688
Chicago/Turabian StyleHu, Ruibo, Leyi Deng, Xiaoying Hao, Jiadong Chen, Xianfeng Zhou, and Nita Sahai. 2024. "Direct, Broad-Spectrum Antimicrobial Activity of Ag+-Doped Hydroxyapatite against Fastidious Anaerobic Periodontal and Aerobic Dental Bacteria" Materials 17, no. 19: 4688. https://doi.org/10.3390/ma17194688







