Assessment of Physico-Chemical Behavior and Sorptivity—Diatomaceous Earth as Support for Paraffinic Phase-Change Materials
Abstract
:1. Introduction
- ✓
- Growth of diatoms: Diatoms, or microscopic algae, grow in aquatic environments such as oceans, lakes, and rivers.
- ✓
- Silica extraction: Diatoms extract silica from water and use it to build complex and porous cell walls, called shells.
- ✓
- Death and accumulation: When diatoms die, their shells sink to the bottom of the water body, accumulating over time. The accumulation of diatom material can result in the formation of layers or sediments.
- ✓
- Consolidation: Over millions of years, accumulated diatomaceous material compacts and consolidates, resulting in diatomite rock.
- ✓
- Mining: Diatomite deposits are usually mined in quarries or pits, and the material is processed to produce the desired product, which is often a fine powder.
2. Materials
2.1. Diatomite
2.2. Paraffinic Materials
3. Experimental Methods
3.1. Laser Particle Size Analysis for Diatomite
3.2. Porosity for Diatomite
3.3. Thermal Conductivity for Diatomite
3.4. Absorption Tests for Diatomite
3.5. SEM Morphology for Diatomite
4. Results
4.1. Laser Particle Size Analysis for Diatomite
4.2. Porosity for Diatomite
4.3. Thermal Conductivity for Diatomite
4.4. Absorption Tests for Diatomite
4.5. SEM Morphology for Diatomite
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zahajská, P.; Opfergelt, S.; Fritz, S.C.; Stadmark, J.; Conley, D.J. What is diatomite? Quat. Res. 2020, 96, 48–52. [Google Scholar] [CrossRef]
- Reka, A.A.; Pavlovski, B.; Fazlija, E.; Berisha, A.; Pacarizi, M.; Daghmehchi, M.; Sacalis, C.; Jovanovski, G.; Makreski, P.; Oral, A. Diatomaceous Earth: Characterization, thermal modification, and application. Open Chem. 2021, 19, 451–461. [Google Scholar] [CrossRef]
- Reka, A.A.; Pavlovski, B.; Boev, B.; Bogoevski, S.; Boškovski, B.; Lazarova, M.; Lamamra, A.; Jašari, A.; Jovanovski, G.; Makreski, P. Diatomite—Evaluation of physico-mechanical, chemical, mineralogical and thermal properties. Geol. Maced. 2021, 35, 5–14. [Google Scholar] [CrossRef]
- Losic, D.; Korunic, Z. CHAPTER 10: Diatomaceous Earth, A Natural Insecticide for Stored Grain Protection: Recent Progress and Perspectives. In Diatom Nanotechnology: Progress and Emerging Applications; Nanoscience & Nanotechnology; The Royal Society of Chemistry: London, UK, 2017; pp. 219–247. [Google Scholar] [CrossRef]
- Ferreira, R.L.S.; Pinto, L.; Nóbrega, A.F.; Carneiro, A.M.P. Diatomaceous earth: A review of its characteristics and effects on the properties of mortars. Constr. Build. Mater. 2024, 421, 135711. [Google Scholar] [CrossRef]
- Mejía, J.M.; de Gutiérrez, R.M.; Montes, C. Rice husk ash and spent diatomaceous earth as a source of silica to fabricate a geopolymeric binary binder. J. Clean. Prod. 2016, 118, 133–139. [Google Scholar] [CrossRef]
- Loganina, V.I.; Simonov, E.E.; Jezierski, W.; Małaszkiewicz, D. Application of activated diatomite for dry lime mixes. Constr. Build. Mater. 2014, 65, 29–37. [Google Scholar] [CrossRef]
- Man, J.; Gao, W.; Yan, S.; Liu, G.; Hao, H. Preparation of porous brick from diatomite and sugar filter mud at lower temperature. Constr. Build. Mater. 2017, 156, 1035–1042. [Google Scholar] [CrossRef]
- Shih, Y.-F.; Wang, C.-H.; Tsai, M.-L.; Jehng, J.-M. Shape-stabilized phase change material/nylon composite based on recycled diatomite. Mater. Chem. Phys. 2020, 242, 122498. [Google Scholar] [CrossRef]
- Harwood, D.M. Diatomite. In The Diatoms: Applications for the Environmental and Earth Sciences; Smol, J.P., Stoermer, E.F., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 570–574. [Google Scholar]
- Ecology: Diatoms. Available online: https://www.ekologia.pl/slownik/okrzemki/ (accessed on 5 August 2024).
- Zachariasse, W.J.; Kontakiotis, G.; Lourens, L.J.; Antonarakou, A. The Messinian of Agios Myron (Crete, Greece): A key to better understanding of diatomite formation on Gavdos (south of Crete). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 581, 110633. [Google Scholar] [CrossRef]
- Ivanov, S.E.; Belyakov, A.V. Diatomite and its application. Glass Ceram. 2008, 65, 1–4. [Google Scholar] [CrossRef]
- GEOLOGYSCIENCE: Diatomite. Available online: https://pl.geologyscience.com/rocks/sedimentary-rocks/non-clastic-sedimentary-rock/diatomite/?amp (accessed on 5 August 2024).
- Sosa, G.L.; Morantes, C.F.; Flores, F.M.; Sánchez, R.M.T.; Zalts, A.; Ramirez, S.A. Characterization of diatomaceous earth modified by organic ligands for enhanced zinc adsorption. J. Environ. Chem. Eng. 2019, 7, 103197. [Google Scholar] [CrossRef]
- ElSayed, E.E. Natural diatomite as an effective adsorbent for heavy metals in water and wastewater treatment (a batch study). Water Sci. 2018, 32, 32–43. [Google Scholar] [CrossRef]
- Maeda, H.; Ishida, E.H. Hydrothermal preparation of diatomaceous earth combined with calcium silicate hydrate gels. J. Hazard. Mater. 2011, 185, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhang, L.; Hao, C.; Qi, P.; Wang, G.; Ma, W. Preparation and characterization of zeolite/diatomite composite materials. Mater. Res. Express 2019, 6, 125516. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Wu, Y.; Wang, T.; Qiu, J. Preparation and characterization of a diatomite hybrid microfiltration carbon membrane for oily wastewater treatment. J. Taiwan Inst. Chem. Eng. 2018, 89, 39–48. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.; Xie, B.; Ma, H.; Chen, J. Enhanced properties of diatomite-based composite phase change materials for thermal energy storage. Renew. Energy 2020, 147, 265–274. [Google Scholar] [CrossRef]
- Hao, L.; Gao, W.; Yan, S.; Niu, M.; Liu, G.; Hao, H. Preparation and characterization of porous ceramics with low-grade diatomite and oyster shell. Mater. Chem. Phys. 2019, 235, 121741. [Google Scholar] [CrossRef]
- Aggrey, P.; Nartey, M.; Kan, Y.; Cvjetinovic, J.; Andrews, A.; Salimon, A.I.; Dragnevski, K.I.; Korsunsky, A.M. On the diatomite-based nanostructure-preserving material synthesis for energy applications. RSC Adv. 2021, 11, 31884–31922. [Google Scholar] [CrossRef]
- Bao, K.; Huang, Y.; Huang, T.; Gu, M.; Wang, L.; Li, Y.; Cheng, X. Preparation of diatomite-based porous ceramics containing interlayer porous MgO by low-temperature sintering for integration of high strength and low thermal conductivity. Mater. Today Commun. 2024, 38, 108028. [Google Scholar] [CrossRef]
- Gondek, K.; Micek, P.; Baran, A.; Bajda, T.; Kowal, J.; Lis, M.; Wyrobisz-Papiewska, A.; Wojtysiak, D.; Smoroń, K. Modified Natural Diatomite with Various Additives and Its Environmental Potential. Materials 2023, 16, 4494. [Google Scholar] [CrossRef]
- Witkowska-Kita, B.; Biel, K.; Blaschke, W.; Orlicka, A. Analysis of the possibility of obtaining scarce mineral resources in Poland. Annu. Set Environ. Prot. 2017, 19, 777–794. [Google Scholar]
- DIATO: Diatomite Sorbent. Available online: https://diato.pl (accessed on 5 August 2024).
- Bońda, R. Diatomite rock. In Balance of Resources of Fossil Deposits in Poland as of December 31, 2023; Szuflicki, M., Malon, A., Tymiński, M., Eds.; National Geological Institute—National Research Institute: Warsaw, Poland, 2024; pp. 80–81. [Google Scholar]
- Lutyński, M.; Sakiewicz, P.; Lutyńska, S. Characterization of Diatomaceous Earth and Halloysite Resources of Poland. Minerals 2019, 9, 670. [Google Scholar] [CrossRef]
- Figarska-Warchoł, B.; Stańczak, G.; Rembiś, M.; Toboła, T. Diatomaceous rocks of the Jawornik deposit (the Polish Outer Carpathians)—Petrophysical and petrographical evaluation. Geol. Geophys. Environ. 2015, 41, 311–331. [Google Scholar] [CrossRef]
- Łach, M.; Pławecka, K.; Marczyk, J.; Ziejewska, C.; Hebdowska-Krupa, M.; Nykiel, M.; Hebda, M.; Miernik, K.; Mierzwiński, D.; Korniejenko, K.; et al. Use of diatomite from Polish fields in sustainable development as a sorbent for petroleum substances. J. Clean. Prod. 2023, 389, 136100. [Google Scholar] [CrossRef]
- Marczyk, J.; Pławecka, K.; Hebdowska-Krupa, M.; Nykiel, M.; Łach, M. Research on diatomite from Polish deposits and the possibilities of its use. J. Achiev. Mater. Manuf. Eng. 2022, 115, 5–15. [Google Scholar] [CrossRef]
- Elden, H.; Morsy, G.; Bakr, M. Diatomite: Its Characterization, Modifications and Applications. Asian J. Mater. Sci. 2010, 2, 121–136. [Google Scholar]
- Ibrahim, S.S.; Selim, A.Q. Producting a micro-porous diatomite by a simple classification—Calcination process. J. Ore Dress. 2010, 12, 24–32. [Google Scholar]
- Seckbach, J.; Gordon, R. Diatoms: Fundamentals and Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2019. [Google Scholar] [CrossRef]
- Perera, H.J.; Goyal, A.; Banu, H.; Alhassan, S.M. Low-cost fluorinated diatomaceous earth polyurethane foam for the absorption of oil. MRS Energy Sustain. 2022, 9, 94–104. [Google Scholar] [CrossRef]
- Galović, I.; Halamić, J.; Grizelj, A.; Rozman, V.; Liška, A.; Korunić, Z.; Lucić, P.; Baličević, R. Croatian diatomites and their possible application as a natural insecticide. Geol. Croat. 2017, 70, 177956. [Google Scholar] [CrossRef]
- Li, C.; Sheng, X.; Lia, N.; Pinga, Q.; Lub, P.; Zhang, J. Insecticidal characteristics and mechanism of a promising natural insecticide against saw-toothed grain beetle. RSC Adv. 2022, 12, 7066–7074. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, R.; Miao, J.; Wang, J.; Jia, D. Effect of diatomite on soil evaporation characteristics. Environ. Earth Sci. 2021, 80, 219. [Google Scholar] [CrossRef]
- Aksakal, E.L.; Angin, I.; Oztas, T. Effects of diatomite on soil consistency limits and soil compactibility. Catena 2013, 101, 157–163. [Google Scholar] [CrossRef]
- Aksakal, E.L.; Angin, I.; Oztas, T. Effects of diatomite on soil physical properties. Catena 2012, 88, 1–5. [Google Scholar] [CrossRef]
- Selim, A.Q.; El-Midany, A.A.; Ibrahim, S.S. Microscopic evaluation of diatomite for advanced applications: Case study. Microsc. Sci. Technol. Appl. Educ. 2010, 3, 2174–2181. [Google Scholar]
- França, S.C.A.; Millqvist, M.T.; Luz, A.B. Beneficiation of Brazilian diatomite for the filtration application industry. Min. Metall. Explor. 2003, 20, 42–46. [Google Scholar] [CrossRef]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-sustainable systems based on poly(lactic acid), diatomite and coffee grounds extract for food packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Z.; Yang, S.; Smith, G.J.; Liu, L. Diatomite encapsulated AgNPs as novel hair dye cosmetics: Preparation, performance, and toxicity. Colloids Surf. B Biointerfaces 2021, 200, 111599. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.; Sheng, X.; Li, N.; Ping, Q. Preparation and application of diatomite-based cleaner. China Surfactant Deterg. Cosmet. 2022, 52, 270. [Google Scholar] [CrossRef]
- Dobrosielska, M.; Dobrucka, R.; Brząkalski, D.; Gloc, M.; Rębiś, J.; Głowacka, J.; Kurzydłowski, K.J.; Przekop, R.E. Methodological Aspects of Obtaining and Characterizing Composites Based on Biogenic Diatomaceous Silica and Epoxy Resins. Materials 2021, 14, 4607. [Google Scholar] [CrossRef]
- Guatame-Garcia, A.; Buxton, M. The Use of Infrared Spectroscopy to Determine the Quality of Carbonate-Rich Diatomite Ores. Minerals 2018, 8, 120. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, G.; Duan, X.; Liang, X.; Meng, J.; Liang, J. Environmental Applications of Diatomite Minerals in Removing Heavy Metals from Water. Ind. Eng. Chem. Res. 2019, 58, 11638–11652. [Google Scholar] [CrossRef]
- Rivera-Ortiz, S.F.; Salazar-Ayala, K.A.; Pena-Rodriguez, G. Treatment of subterranean mining water through filtration using ceramic bilayer membranes based of recycled diatomites and kaolin. Contemp. Eng. Sci. 2018, 11, 4437–4446. [Google Scholar] [CrossRef]
- Piri, M.; Sepehr, E.; Samadi, A.; Farhadi, K.H.; Alizadeh, M. Contaminated soil amendment by diatomite: Chemical fractions of zinc, lead, copper and cadmium. Int. J. Environ. Sci. Technol. 2021, 18, 1191–1200. [Google Scholar] [CrossRef]
- Abdelsalam, M.; Yue, Y.; Khater, A.; Luo, D.; Musanyufu, J.; Qin, X. Laboratory Study on the Performance of Asphalt Mixes Modified with a Novel Composite of Diatomite Powder and Lignin Fiber. Appl. Sci. 2020, 10, 5517. [Google Scholar] [CrossRef]
- Tan, B.; Su, Y.; Fan, Y.; Zhang, W.; Li, Q. Preparation and Road Performance Study of Rubber–Diatomite Composite-Modified Asphalt Mixture. Materials 2023, 16, 7359. [Google Scholar] [CrossRef] [PubMed]
- Pławecka, K.; Bąk, A.; Hebdowska-Krupa, M.; Łach, M. The Use of Calcined Diatomite as an Additive to Geopolymeric Materials. Mater. Proc. 2023, 13, 28. [Google Scholar] [CrossRef]
- Nykiel, M.; Korniejenko, K.; Setlak, K.; Melnychuk, M.; Polivoda, N.; Kozub, B.; Hebdowska-Krupa, M.; Łach, M. The Influence of Diatomite Addition on the Properties of Geopolymers Based on Fly Ash and Metakaolin. Materials 2024, 17, 2399. [Google Scholar] [CrossRef]
- Figarska-Warchoł, B.; Rembiś, M.; Stańczak, G. The impact of calcination on changes in the physical and mechanical properties of the diatomites of the Leszczawka Member (the Outer Carpathians, Poland). Geol. Geophys. Environ. 2019, 45, 269. [Google Scholar] [CrossRef]
- Ediz, N.; Bentli, I.; Tatar, I. Improvement in filtration characteristics of diatomite by calcination. Int. J. Miner. Process. 2010, 94, 129–134. [Google Scholar] [CrossRef]
- Hamed, A. A brief and general overview on diatoms and their applications. Egypt. J. Phycol. 2023, 24, 1–53. [Google Scholar] [CrossRef]
- Rubitherm Phase Change Material. Available online: https://www.rubitherm.eu/en/ (accessed on 5 August 2024).
- PCM Products. Available online: https://www.pcmproducts.net (accessed on 5 August 2024).
- POL-AURA. Available online: https://pol-aura.pl (accessed on 5 August 2024).
- WARCHEM. Available online: https://warchem.pl (accessed on 5 August 2024).
- FLEXOL. Available online: https://www.flexol.pl (accessed on 5 August 2024).
- EN 12664; Thermal Performance of Building Materials and Products. Determination of Thermal Resistance by Means of Guarded Hot Plate and Heat Flow Meter Methods. Dry and Moist Products of Medium and Low Thermal Resistance. European Committee for Standardisation: Brussels, Belgium, 2001.
- ASTM C1784; Standard Test Method for Using a Heat Flow Meter Apparatus for Measuring Thermal Storage Properties of Phase Change Materials and Products. International American Society for Testing of Materials: West Conshohocken, PA, USA, 2020.
- ASTM C518; Standard Test Method for Steady-State Thermal Transmission Properties by Means of the Heat Flow Meter Apparatus. International American Society for Testing of Materials: West Conshohocken, PA, USA, 2021.
- ISO 8301; Thermal Insulation—Determination of Steady-State Thermal Resistance and Related Properties—Heat Flow Meter Apparatus. International Standards Organization: London, UK, 1991.
- Stempkowska, A.; Gawenda, T.; Smoroń, K. The Diatomite Grinding Technology Concept for the Protection of Diatomite Shells and the Control of Product Grading. Materials 2024, 17, 3662. [Google Scholar] [CrossRef] [PubMed]
- Kobylinska, N.; Dudarko, O.; Gładysz-Płaska, A.; Tertykh, V.A.; Majdan, M. Optimal Synthesis of Novel Phosphonic Acid Modified Diatomite Adsorbents for Effective Removal of Uranium(VI) Ions from Aqueous Solutions. Materials 2023, 16, 5263. [Google Scholar] [CrossRef] [PubMed]
- Fajdek-Bieda, A.; Wróblewska, A.; Miądlicki, P.; Konstanciak, A. Conversion of Geraniol into Useful Value-Added Products in the Presence of Catalysts of Natural Origin: Diatomite and Alum. Materials 2022, 15, 2449. [Google Scholar] [CrossRef] [PubMed]
- Bushell, M.; Beauchemin, S.; Kunc, F.; Gardner, D.; Ovens, J.; Toll, F.; Kennedy, D.; Nguyen, K.; Vladisavljevic, D.; Rasmussen, P.E.; et al. Characterization of Commercial Metal Oxide Nanomaterials: Crystalline Phase, Particle Size and Specific Surface Area. Nanomaterials 2020, 10, 1812. [Google Scholar] [CrossRef]
- Arvaniti, E.C.; Juenger, M.C.G.; Bernal, S.A.; Duchesne, J.; Courard, L.; Leroy, S.; Provis, J.L.; Klemm, A.; De Belie, N. Determination of particle size, surface area, and shape of supplementary cementitious materials by different techniques. Mater. Struct. 2015, 48, 3687–3701. [Google Scholar] [CrossRef]
- Cordeiro, G.C.; Filho, R.D.T.; Tavares, L.M.; de Moraes Rego Fairbairn, E.; Hempel, S. Influence of particle size and specific surface area on the pozzolanic activity of residual rice husk ash. Cem. Concr. Compos. 2011, 33, 529–534. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Duong, X.Q.; Rowinski, L.; Nguyen, X.P. Advanced super-hydrophobic polymer-based porous absorbents for the treatment of oil-polluted water. Chemosphere 2021, 277, 130274. [Google Scholar] [CrossRef]
- Du, W.; Dai, G.; Wang, B.; Li, Z.; Li, L. Biodegradable porous organosilicone-modified collagen fiber matrix: Synthesis and high oil absorbency. J. Appl. Polym. Sci. 2018, 135, 46264. [Google Scholar] [CrossRef]
- Yadav, S.; Illa, M.P.; Rastogi, T.; Sharma, C.S. High absorbency cellulose acetate electrospun nanofibers for feminine hygiene application. Appl. Mater. Today 2016, 4, 62–70. [Google Scholar] [CrossRef]
- Benayache, S.; Alleg, S.; Mebrek, A.; Suñol, J.J. Thermal and microstructural properties of paraffin/diatomite composite. Vacuum 2018, 157, 136–144. [Google Scholar] [CrossRef]
- Cavargna, A.; Mongibello, L.; Iasiello, M.; Bianco, N. Analysis of a Phase Change Material-Based Condenser of a Low-Scale Refrigeration System. Energies 2023, 16, 3798. [Google Scholar] [CrossRef]
- Nandan, R.; Arumuru, V.; Rath, P.; Das, M.K. Experimental study of PCM based hybrid heat sink for electronic cooling. J. Enhanc. Heat Transf. 2022, 29, 1–15. [Google Scholar] [CrossRef]
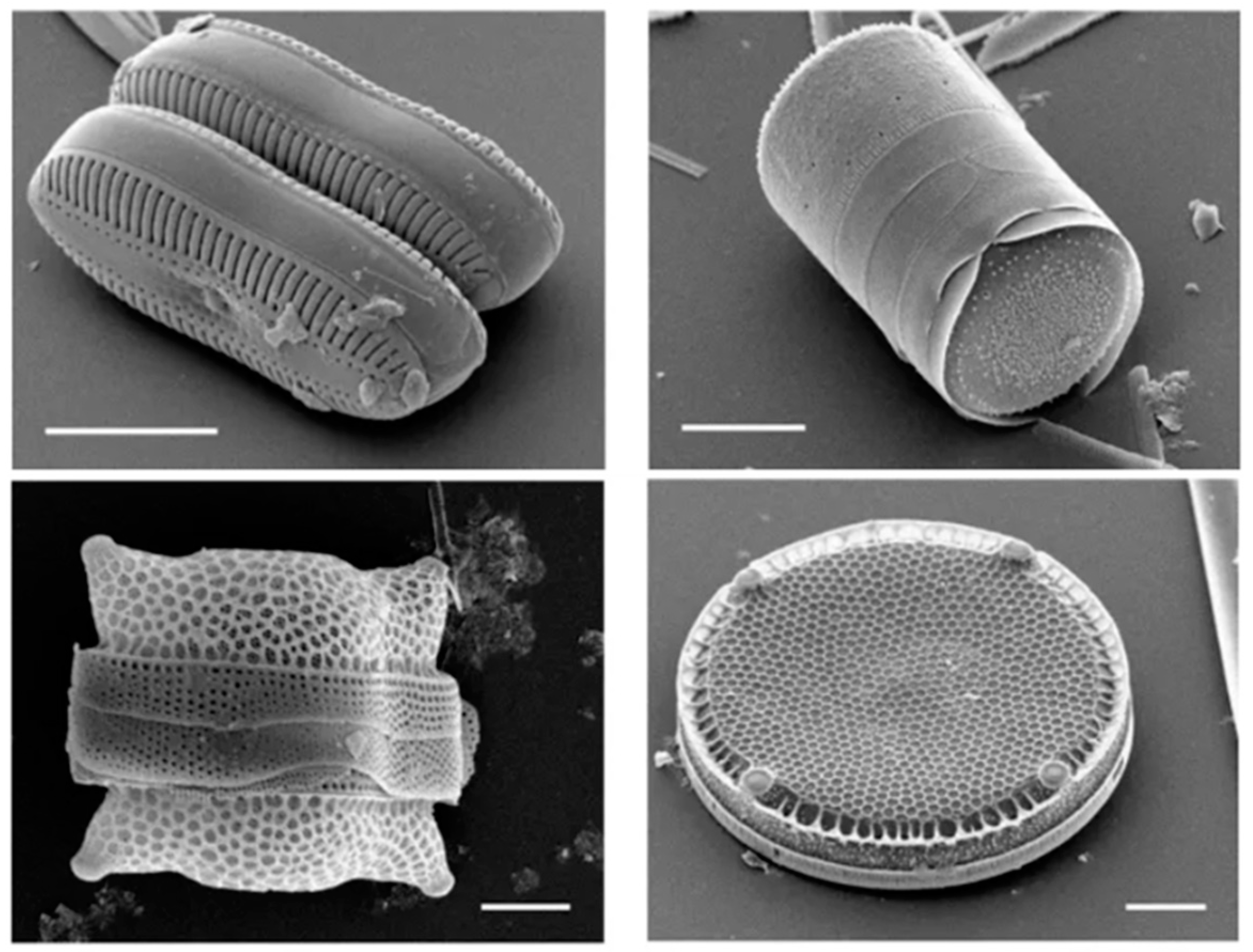




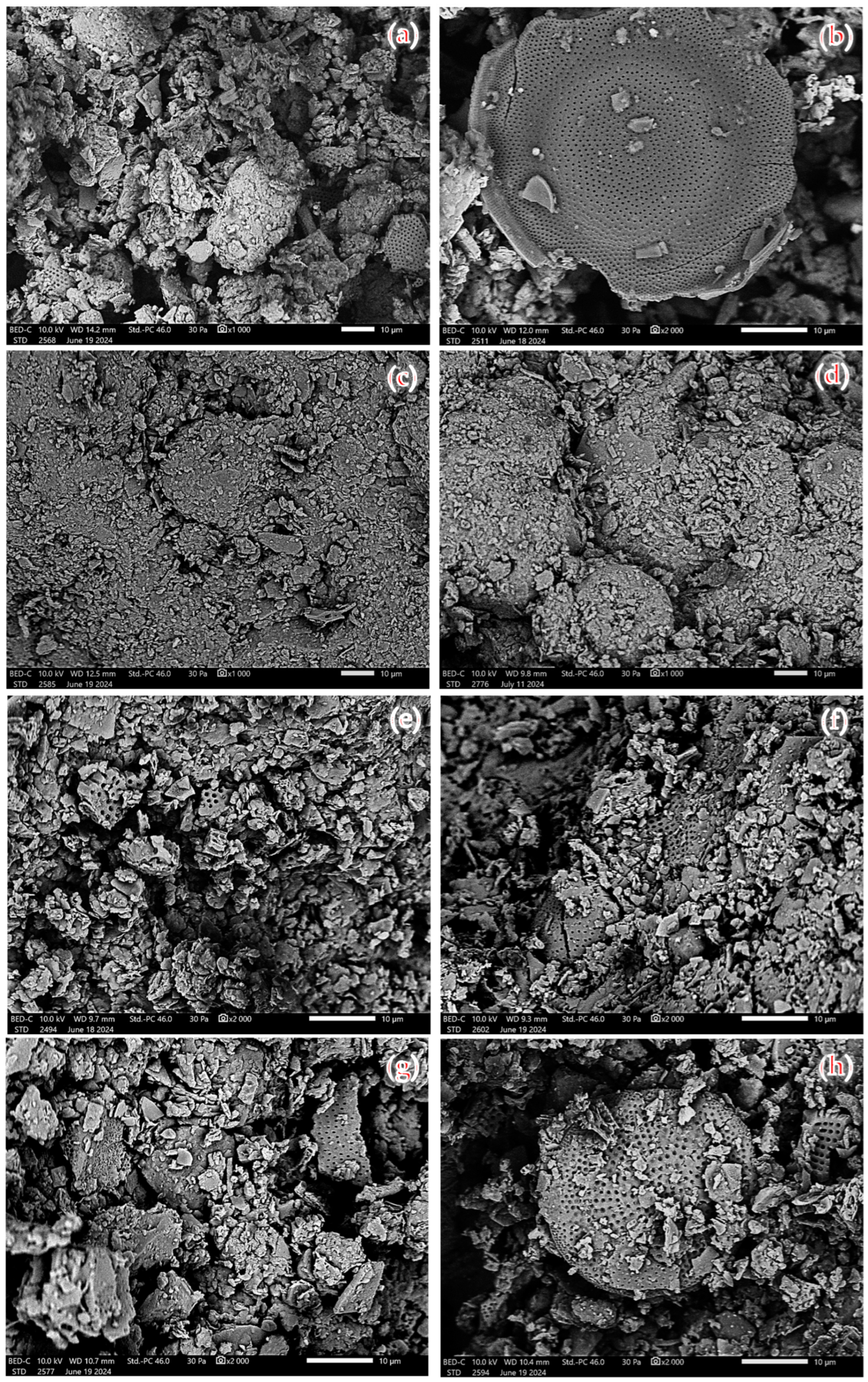
| Type of Diatomite | Oxide Composition (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | K2O | SO3 | TiO2 | CaO | |
| Powder | 78.697 | 15.991 | 2.752 | 1.427 | 0.447 | 0.367 | 0.235 |
| Powder 850 °C | 80.331 | 15.286 | 2.318 | 1.264 | 0.174 | 0.329 | 0.220 |
| Granulate | 74.117 | 14.849 | 6.120 | 2.480 | 0.930 | 0.610 | 0.610 |
| Granulate 850 °C | 74.920 | 15.320 | 5.660 | 2.370 | 0.350 | 0.580 | 0.560 |
| Aggregate 0.5–3 mm | 78.244 | 14.275 | 2.328 | 1.348 | 2.515 | 0.350 | 0.863 |
| Aggregate 0.5–3 mm 850 °C | 78.459 | 12.487 | 4.050 | 2.030 | 1.390 | 0.530 | 0.930 |
| Aggregate 2–5 mm | 79.420 | 15.558 | 2.090 | 1.416 | 0.624 | 0.365 | 0.447 |
| Aggregate 2–5 mm 850 °C | 77.048 | 14.057 | 4.680 | 2.160 | 0.750 | 0.540 | 0.630 |
| Type of Diatomite | Quantitative Share of Phase [%] | |||
|---|---|---|---|---|
| Quartz | Illite | Kaolinite | Albite | |
| 01-075-8321 | 00-026-0911 | 00-058-2001 | 00-001-0739 | |
| SiO2 | Al2Si3AlO10(OH)2 | Al2Si2O5(OH)4 | NaAlSi3O8 | |
| Powder | 19.0 | 23.7 | 39.9 | 17.4 |
| Powder 850 °C | 21.9 | 13.6 | 48.2 | 16.3 |
| Granulate | 26.3 | 28.5 | 28.4 | 16.8 |
| Granulate 850 °C | 22.2 | 12.2 | 47.6 | 17.9 |
| Aggregate 0.5–3 mm | 15.3 | 20.0 | 46.7 | 18.1 |
| Aggregate 0.5–3 mm 850 °C | 30.2 | 27.2 | 27.4 | 15.2 |
| Aggregate 2–5 mm | 17.3 | 24.2 | 40.2 | 18.3 |
| Aggregate 2–5 mm 850 °C | 26.9 | 24.0 | 22.2 | 27.0 |
| Material | Phase Change Area [°C] | Thermal Conductivity [W/m × K] | Specific Heat Capacity [kJ/kg × K] | Flash Point [°C] | Density [g/cm3] | Viscosity [mPa × s] |
|---|---|---|---|---|---|---|
| RT0 | −1 to 2 | 0.2 | 2 | 110 | 0.770–0.880 | - |
| RT25HC | 22 to 26 | 0.2 | 2 | 150 | 0.770–0.880 | - |
| PusICE A18 | 18 | 0.22 | 2.18 | 200 | 0.765 | - |
| PEG 600 | 17 to 22 | 0.187 | - | 252 | 1.127 | 16–19 |
| Light liquid paraffin | −20 to −15 | 0.1–0.3 | - | - | 0.810–0.875 | 25–80 |
| Heavy liquid paraffin | −12 to −10 | 0.1–0.3 | - | - | 0.845–0.880 | 110–230 |
| Paraffin oil | −30 to −3 | 0.1–0.3 | - | 260 | 0.862 | - |
| Material | D10 [μm] | D50 [μm] | D90 [μm] | Average Particle Size [μm] | Standard Deviation [μm] |
|---|---|---|---|---|---|
| Powder | 2.641 | 9.850 | 20.205 | 11.305 | 0.196 |
| Powder 850 °C | 3.974 | 13.708 | 24.371 | 14.691 | 0.127 |
| Material | Total Porosity [%] | Pore Diameter Range [μm] | Total Surface Area [m2/g] | Total Intruded Volume [cm3/g] |
|---|---|---|---|---|
| Granulate | 52.61 | 4.26–1068.83 | 0.0088 | 0.3175 |
| Granulate 850 °C | 55.92 | 4.26–1068.83 | 0.0086 | 0.3321 |
| Aggregate 0.5–3 mm | 32.40 | 4.27–1051.31 | 0.0089 | 0.2810 |
| Aggregate 0.5–3 mm 850 °C | 35.83 | 4.25–1073.31 | 0.0104 | 0.2331 |
| Aggregate 2–5 mm | 11.67 | 4.30–906.43 | 0.0054 | 0.1498 |
| Aggregate 2–5 mm 850 °C | 9.67 | 4.26–1100.94 | 0.0034 | 0.0602 |
| Type of Diatomite | Thermal Conductivity Coefficient λ [W/m × K] |
|---|---|
| Powder | 0.092 |
| Powder 850 °C | 0.070 |
| Granulate | 0.152 |
| Granulate 850 °C | 0.137 |
| Aggregate 0.5–3 mm | 0.152 |
| Aggregate 0.5–3 mm 850 °C | 0.121 |
| Aggregate 2–5 mm | 0.143 |
| Aggregate 2–5 mm 850 °C | 0.123 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybek, A. Assessment of Physico-Chemical Behavior and Sorptivity—Diatomaceous Earth as Support for Paraffinic Phase-Change Materials. Materials 2024, 17, 4691. https://doi.org/10.3390/ma17194691
Przybek A. Assessment of Physico-Chemical Behavior and Sorptivity—Diatomaceous Earth as Support for Paraffinic Phase-Change Materials. Materials. 2024; 17(19):4691. https://doi.org/10.3390/ma17194691
Chicago/Turabian StylePrzybek, Agnieszka. 2024. "Assessment of Physico-Chemical Behavior and Sorptivity—Diatomaceous Earth as Support for Paraffinic Phase-Change Materials" Materials 17, no. 19: 4691. https://doi.org/10.3390/ma17194691






