Influence of Heat Treatment Temperature on Microstructure, Hardness and Sensitization of UNS S32205 Duplex Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Microstructural Analysis
2.3. Nanomechanical Analysis
2.4. Electrochemical Analysis
3. Results and Discussion
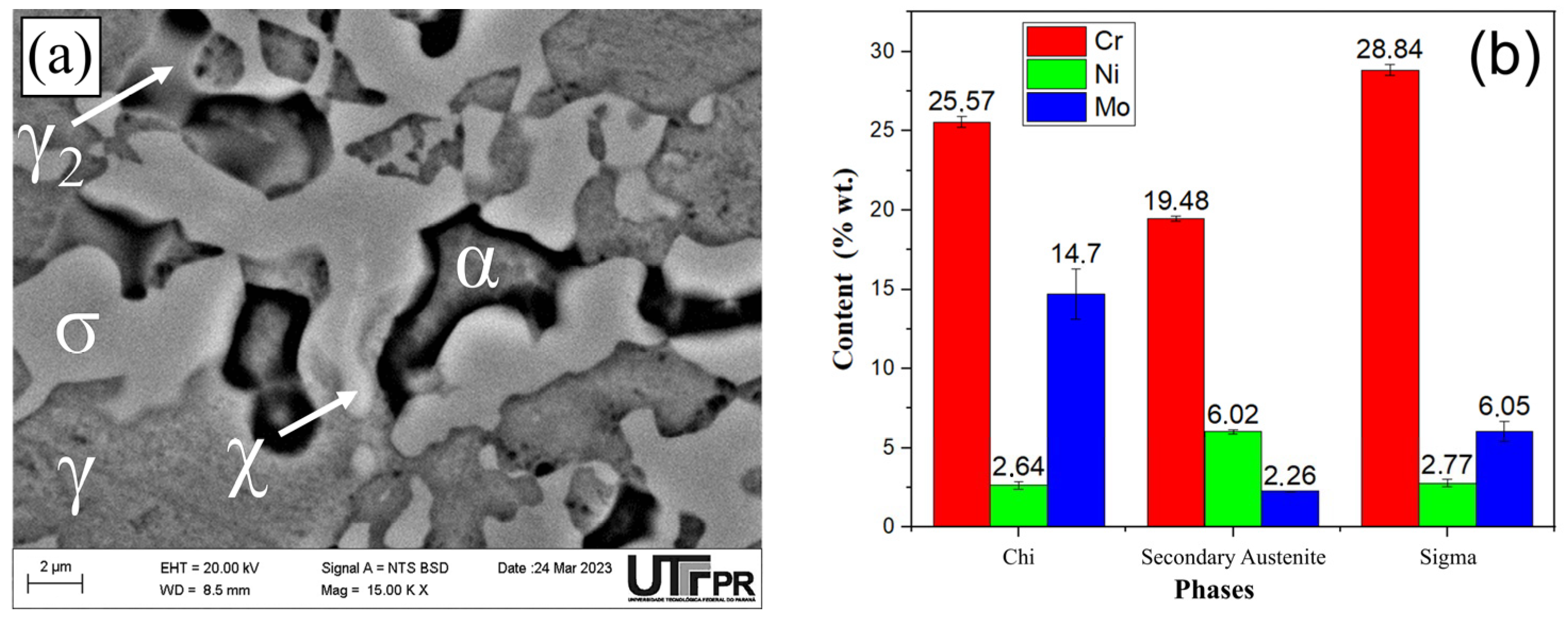
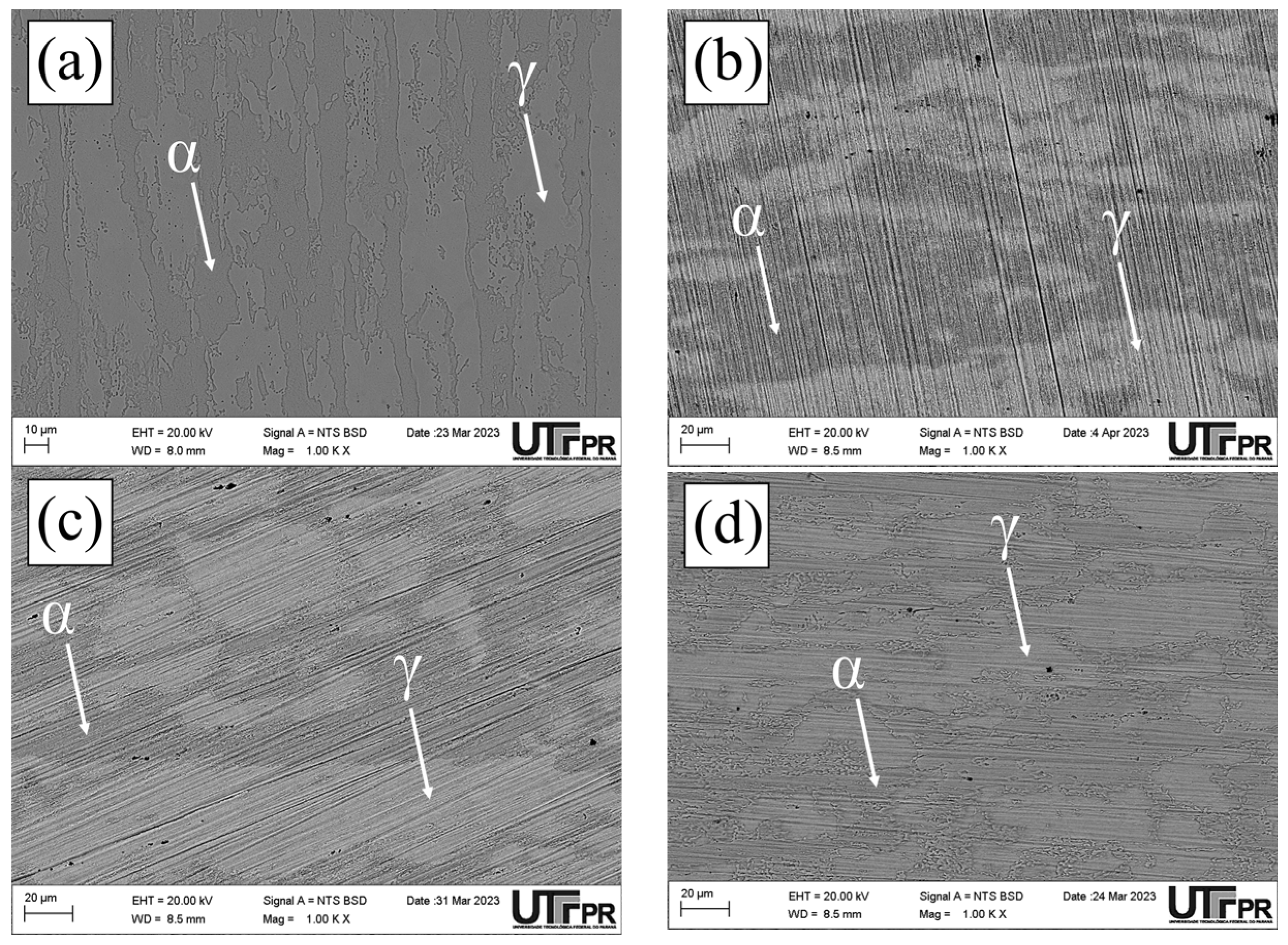
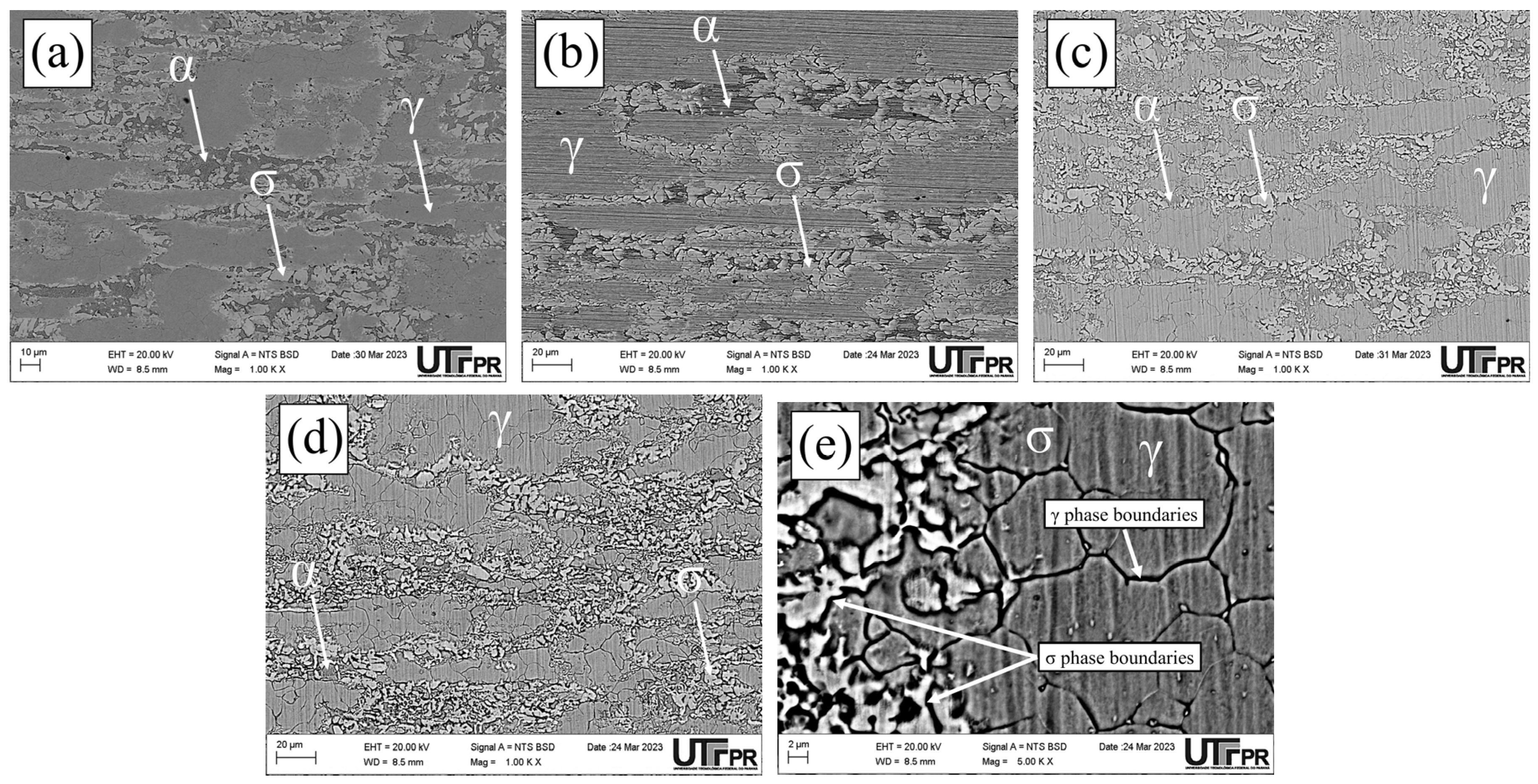
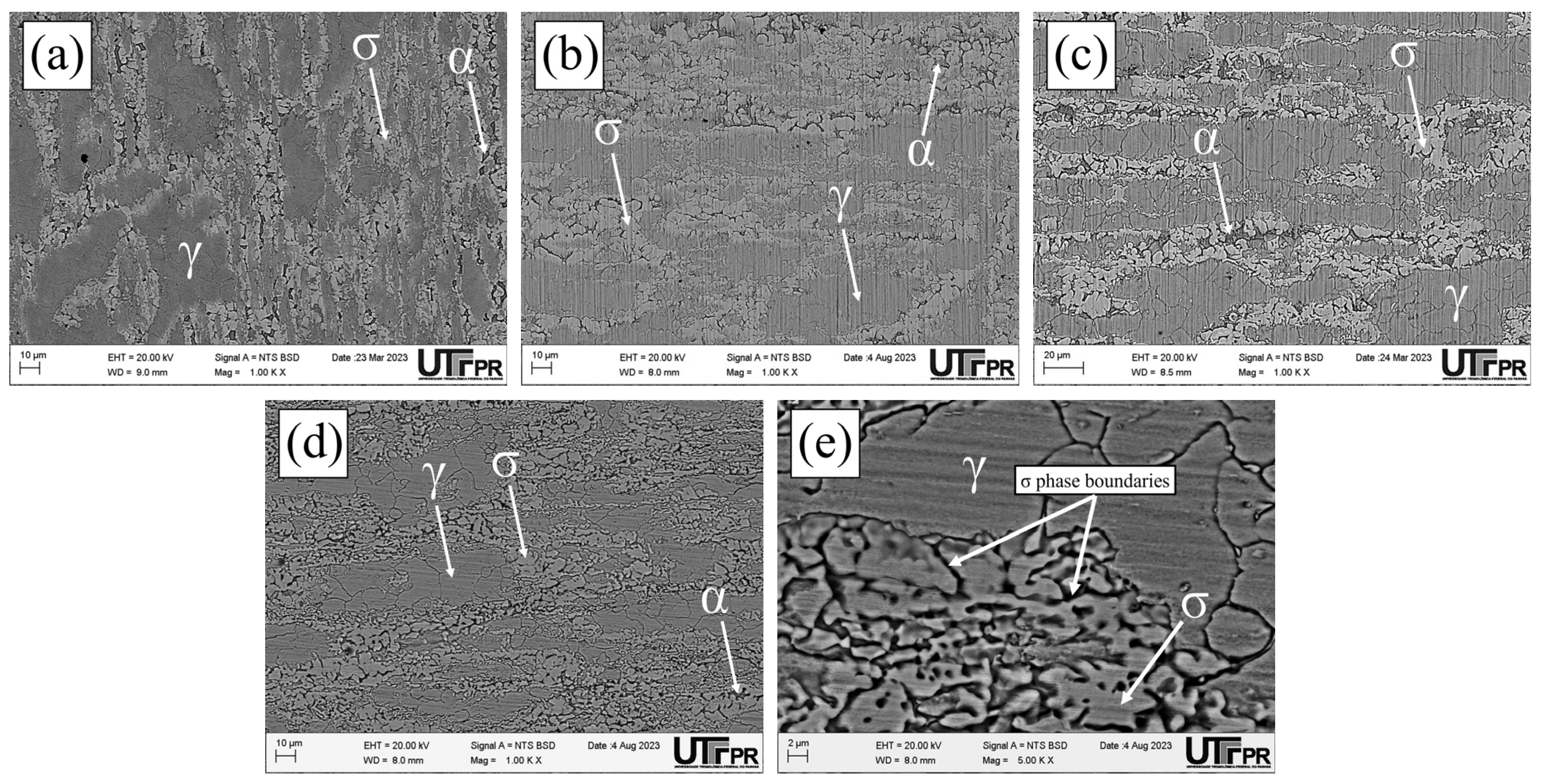
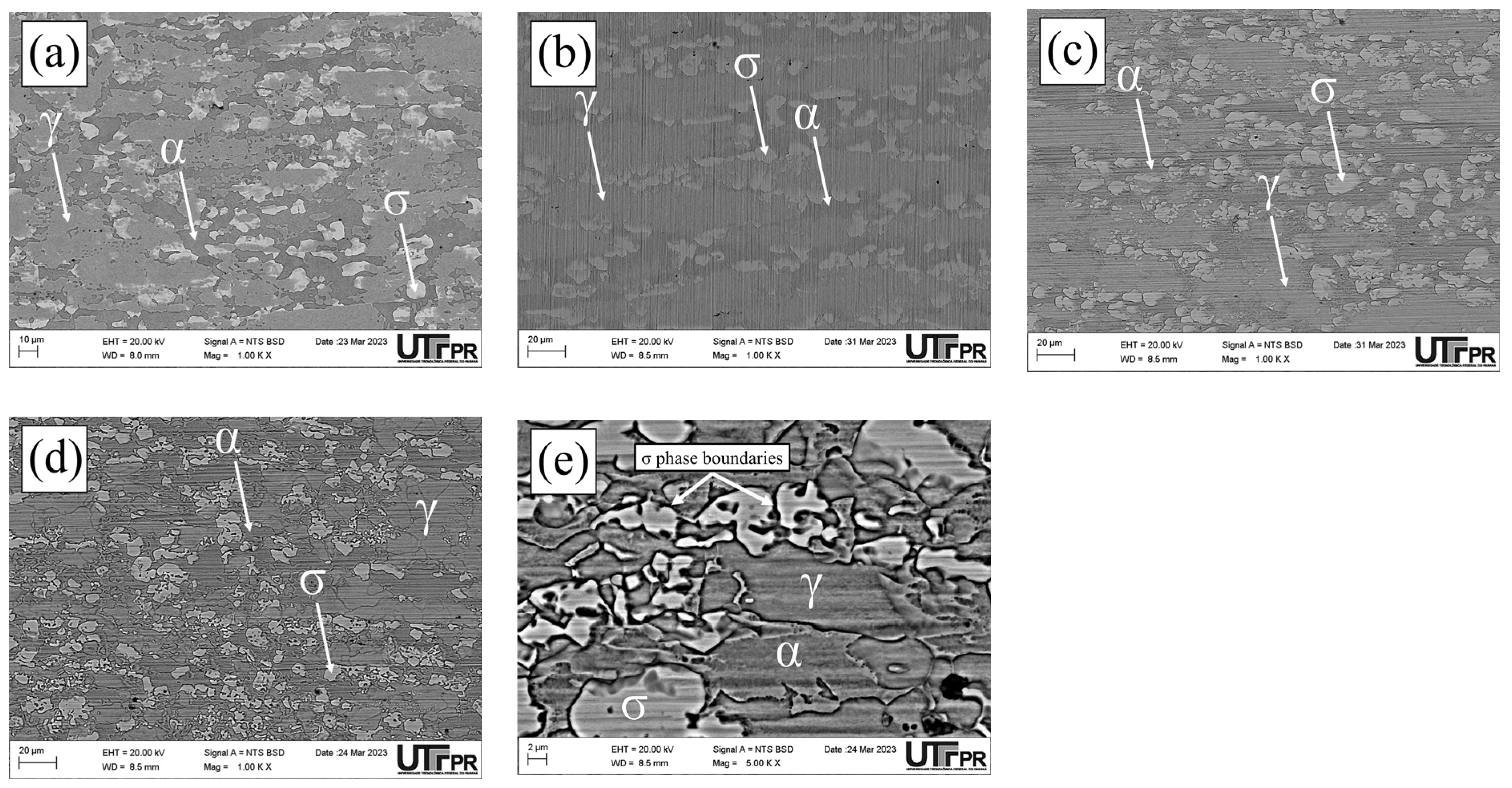
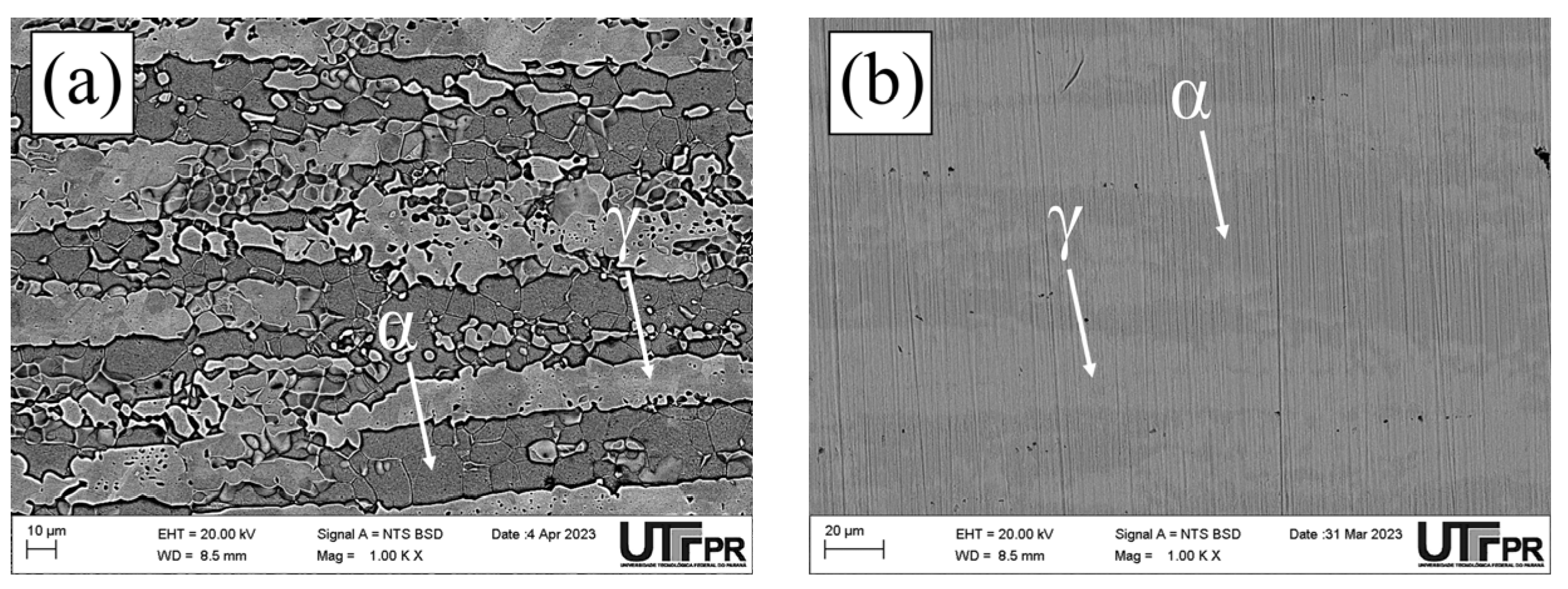
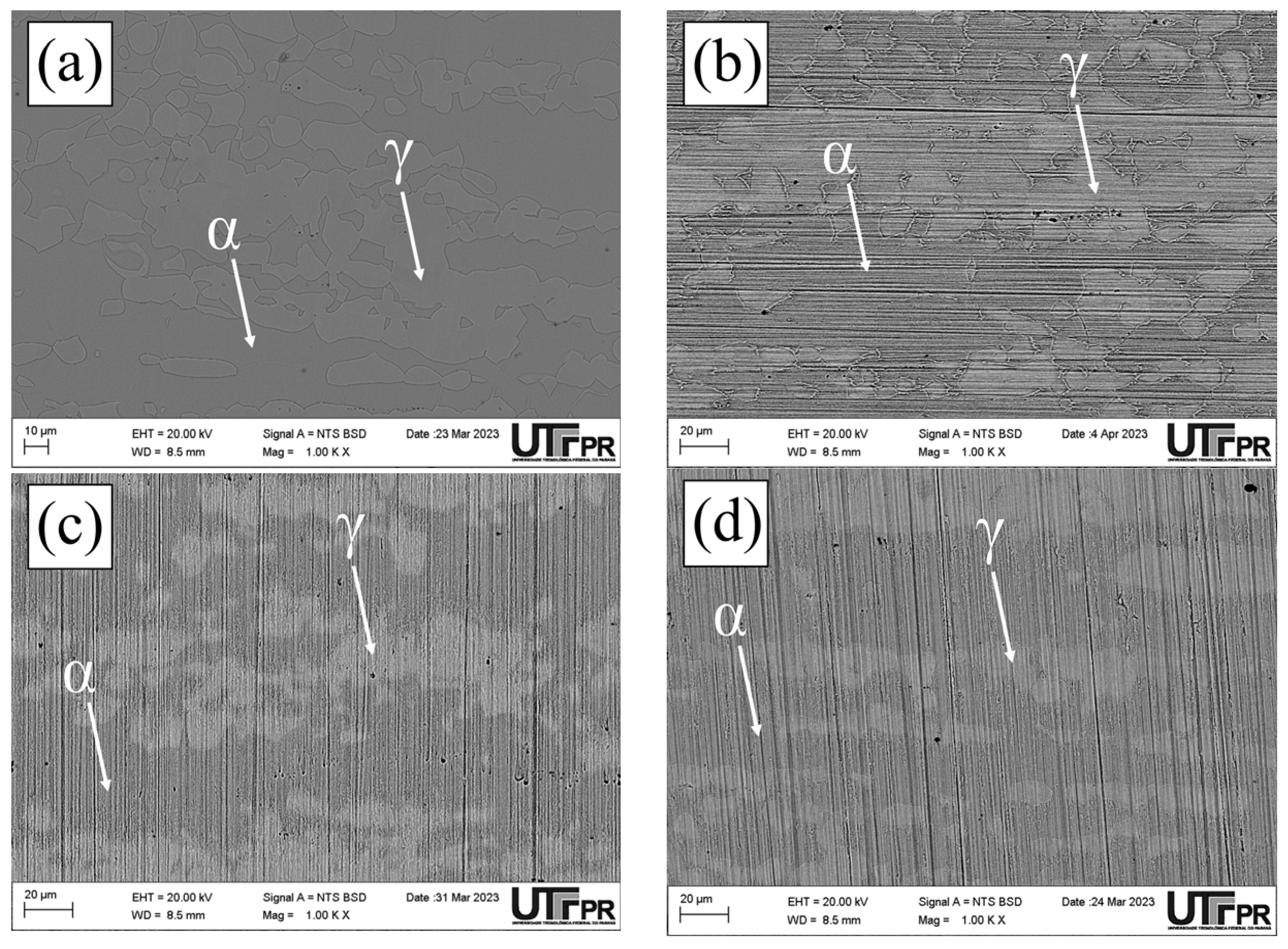
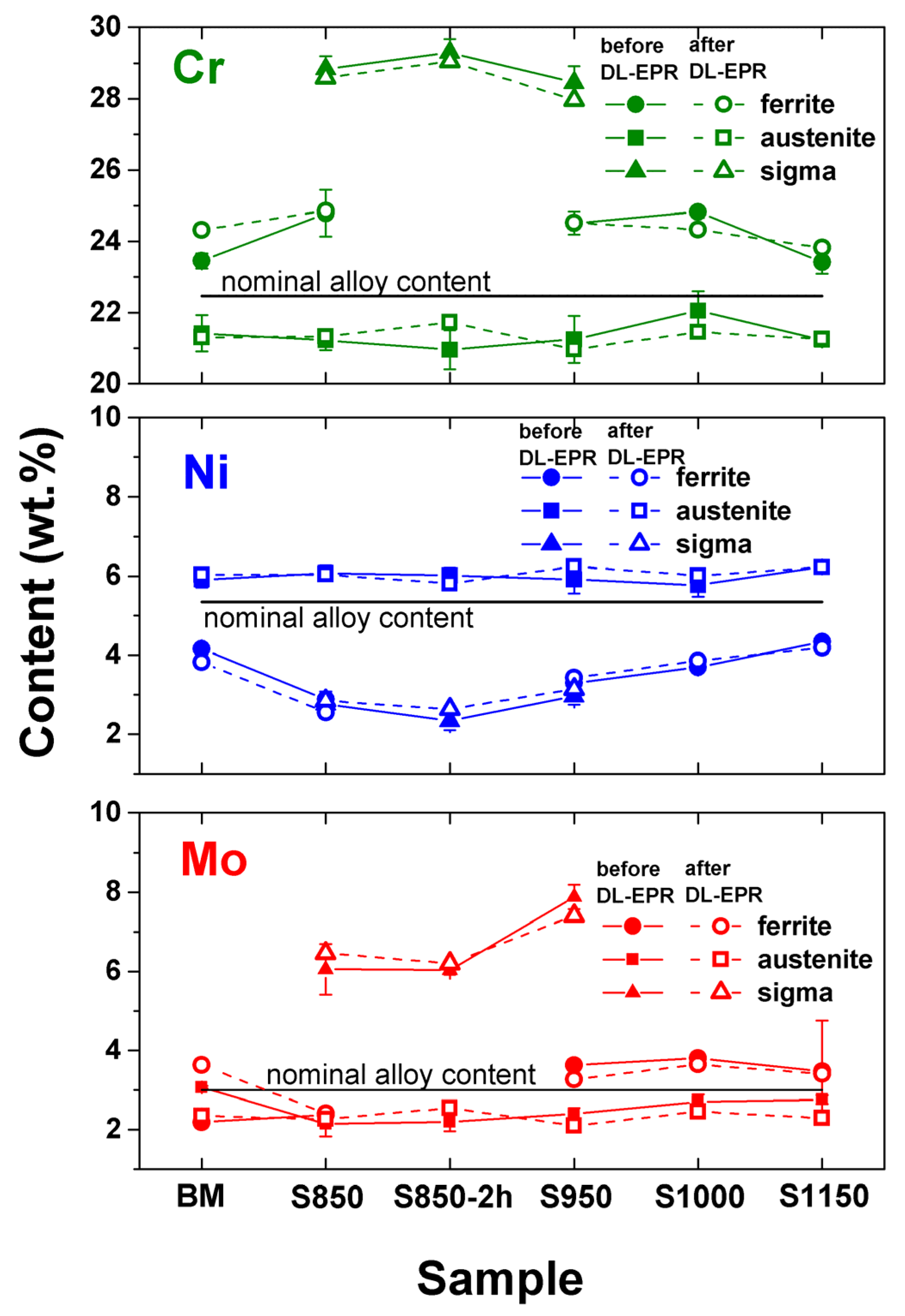
3.1. Microstructural Characterization
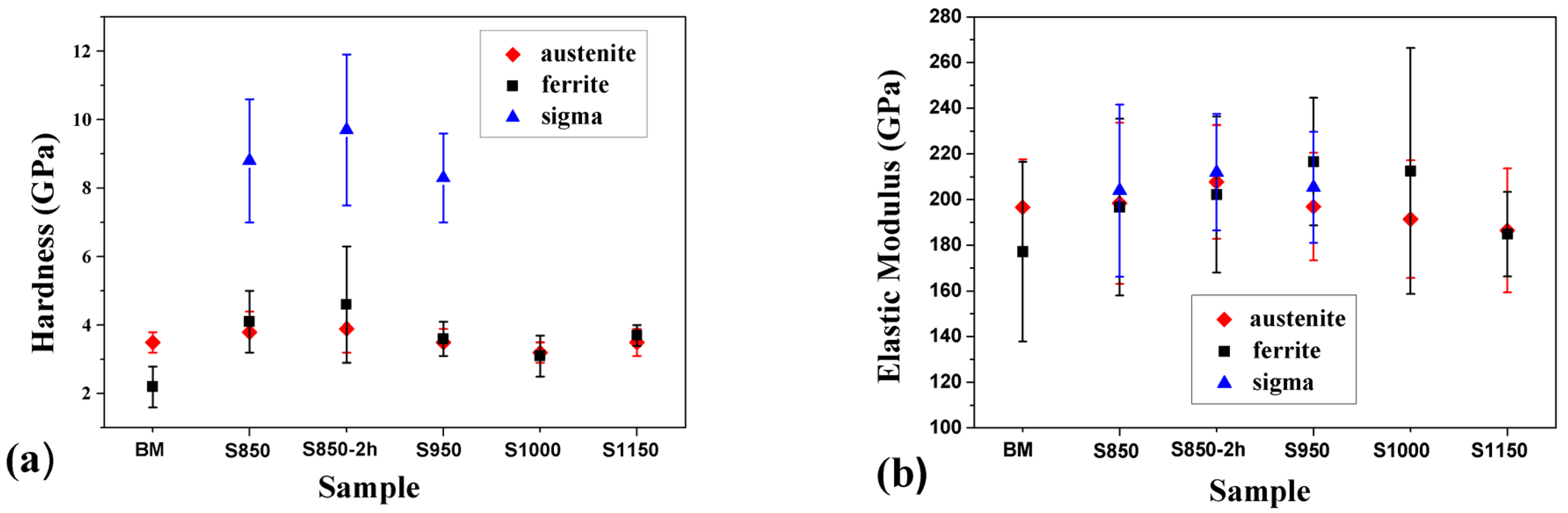
3.2. Hardness and Elastic Modulus Measurements
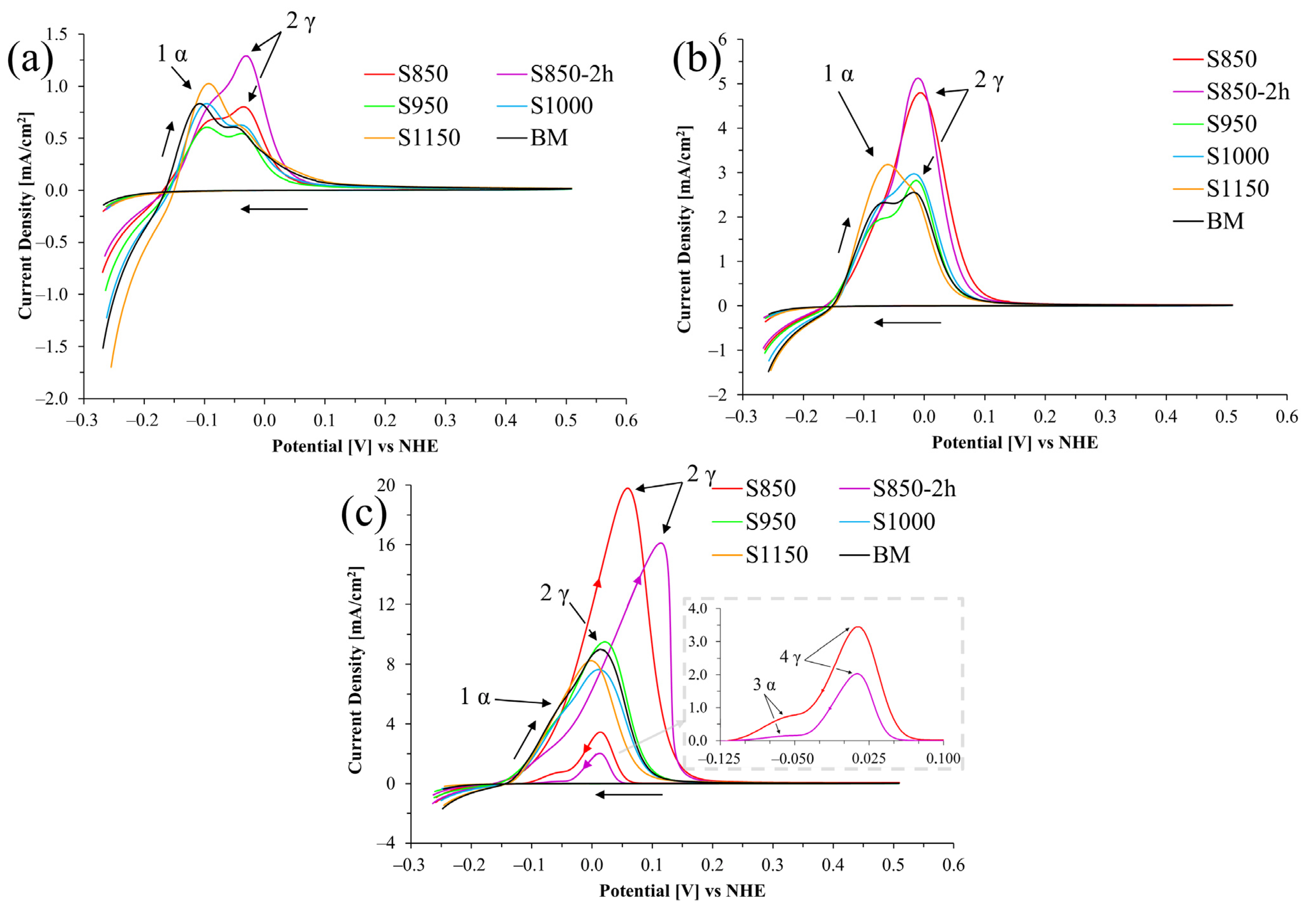
3.3. Electrochemical Characterization
4. Conclusions
- The microstructural analysis allowed the following conclusions:
- According to the nanomechanical analysis:
- According to the electrochemical analysis:
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, R.; Liu, E.; Zhang, Y.; Zhu, L.; Zeng, Z. Tribocorrosion Behavior of SAF 2205 Duplex Stainless Steel in Artificial Seawater. J. Mater. Eng. Perform. 2019, 28, 414–422. [Google Scholar] [CrossRef]
- Charles, J. Duplex Stainless Steels—A Review after DSS ‘07 Held in Grado. Steel Res. Int. 2008, 79, 455–465. [Google Scholar] [CrossRef]
- Sieurin, H.; Sandström, R. Sigma Phase Precipitation in Duplex Stainless Steel 2205. Mater. Sci. Eng. A 2007, 444, 271–276. [Google Scholar] [CrossRef]
- Filho, A.I.; Rollo, J.M.D.A.; Silva, R.V.; Martinez, G. Alternative Process to Manufacture Austenitic-Ferritic Stainless Steel Wires. Mater. Lett. 2005, 59, 1192–1194. [Google Scholar] [CrossRef]
- de la Rosa, Y.E.N.; Calabokis, O.P.; Borges, P.C.; Ballesteros, V.B. Effect of Low-Temperature Plasma Nitriding on Corrosion and Surface Properties of Duplex Stainless Steel UNS S32205. J. Mater. Eng. Perform. 2020, 29, 2612–2622. [Google Scholar] [CrossRef]
- Badji, R.; Bouabdallah, M.; Bacroix, B.; Kahloun, C.; Belkessa, B.; Maza, H. Phase Transformation and Mechanical Behavior in Annealed 2205 Duplex Stainless Steel Welds. Mater. Charact. 2008, 59, 447–453. [Google Scholar] [CrossRef]
- Fargas, G.; Mestra, A.; Mateo, A. Effect of Sigma Phase on the Wear Behavior of a Super Duplex Stainless Steel. Wear 2013, 303, 584–590. [Google Scholar] [CrossRef]
- Michalska, J.; Sozańska, M. Qualitative and Quantitative Analysis of σ and χ Phases in 2205 Duplex Stainless Steel. Mater. Charact. 2006, 56, 355–362. [Google Scholar] [CrossRef]
- Sathirachinda, N.; Pettersson, R.; Pan, J. Depletion Effects at Phase Boundaries in 2205 Duplex Stainless Steel Characterized with SKPFM and TEM/EDS. Corros. Sci. 2009, 51, 1850–1860. [Google Scholar] [CrossRef]
- Chaves, R.; Costa, I.; De Melo, H.G.; Wolynec, S. Evaluation of Selective Corrosion in UNS S31803 Duplex Stainless Steel with Electrochemical Impedance Spectroscopy. Electrochim. Acta 2006, 51, 1842–1846. [Google Scholar] [CrossRef]
- Lo, K.H.; Kwok, C.T.; Chan, W.K. Characterisation of Duplex Stainless Steel Subjected to Long-Term Annealing in the Sigma Phase Formation Temperature Range by the DLEPR Test. Corros. Sci. 2011, 53, 3697–3703. [Google Scholar] [CrossRef]
- Dainezi, I.; Borges, S.H.; Mariano, N.A. Effect of Precipitation of Alpha Line and Sigma Phases on the Microstructure and Corrosion Resistance of the Duplex Stainless Steel SAF 2205. Mater. Res. 2023, 26, e20220571. [Google Scholar] [CrossRef]
- ISO 12732; Corrosion of Metals and Alloys Electrochemical Potentiokinetic Reactivation Measurement Using the Double Loop Method (Based on Cihal’s Method). International Organization for Standardization: Geneva, Switzerland, 2006.
- Číhal, V. A Potentiokinetic Reactivation Method for Predicting the I.C.C. and I.G.S.C.C. Sensitivity of Stainless Steels and Alloys. Corros. Sci. 1980, 20, 737–744. [Google Scholar] [CrossRef]
- Cronemberger, M.E.R.; Nakamatsu, S.; Mariano, N.A.; della Rovere, C.A.; Kuri, S.E. Effect of Cooling Rate on the Corrosion Behavior of As-Cast SAF 2205 Duplex Stainless Steel after Solution Annealing Treatment. Mater. Sci. Forum 2016, 869, 620–624. [Google Scholar] [CrossRef]
- Park, C.J.; Rao, V.S.; Kwon, H.S. Effects of Sigma Phase on the Initiation and Propagation of Pitting Corrosion of Duplex Stainless Steel. Corrosion 2005, 61, 76–83. [Google Scholar] [CrossRef]
- Duret-Thual, C.; Bonis, M.; Crolet, J.L. Application of the EPR Method to Duplex Stainless Steels. Werkst. Korros. 2001, 52, 37–44. [Google Scholar] [CrossRef]
- Del Abra-Arzola, J.L.; García-Rentería, M.A.; Cruz-Hernández, V.L.; García-Guerra, J.; Martínez-Landeros, V.H.; Falcón-Franco, L.A.; Curiel-López, F.F. Study of the Effect of Sigma Phase Precipitation on the Sliding Wear and Corrosion Behaviour of Duplex Stainless Steel AISI 2205. Wear 2018, 400–401, 43–51. [Google Scholar] [CrossRef]
- Symniotis, E. Galvanic Effects on the Active Dissolution of Duplex Stainless Steels. Corrosion 1990, 46, 2–12. [Google Scholar] [CrossRef]
- Gong, J.; Jiang, Y.M.; Deng, B.; Xu, J.L.; Hu, J.P.; Li, J. Evaluation of Intergranular Corrosion Susceptibility of UNS S31803 Duplex Stainless Steel with an Optimized Double Loop Electrochemical Potentiokinetic Reactivation Method. Electrochim. Acta 2010, 55, 5077–5083. [Google Scholar] [CrossRef]
- Lo, I.H.; Fu, Y.; Lin, C.J.; Tsai, W.T. Effect of Electrolyte Composition on the Active-to-Passive Transition Behavior of 2205 Duplex Stainless Steel in H2SO4/HCl Solutions. Corros. Sci. 2006, 48, 696–708. [Google Scholar] [CrossRef]
- Dos Santos, D.C.; Magnabosco, R. Kinetic Study to Predict Sigma Phase Formation in Duplex Stainless Steels. Metall. Mater. Trans. A 2016, 47, 1554–1565. [Google Scholar] [CrossRef]
- Magnabosco, R. Kinetics of Sigma Phase Formation in a Duplex Stainless Steel. Mater. Res. 2009, 12, 321–327. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, X.; Lin, P.; Chou, K. Effect of Solution Annealing on the Microstructure Evolution and Corrosion Behavior of 2205 Duplex Stainless Steel. Mater. Corros. 2019, 70, 676–687. [Google Scholar] [CrossRef]
- Ribeiro, J.J.K.; Turin, A.R.; de la Rosa, Y.E.N.; Quadros, P.V.C.A.; Calabokis, O.P.; Lepienski, C.M.; Brunatto, S.F.; Borges, P.C. Mechanical Characterization at Nanoscale of Austenite, Ferrite, and Sigma Phases via Hardness Measurement and Fretting Wear Behavior of a Duplex Stainless Steel. Metals 2023, 13, 864. [Google Scholar] [CrossRef]
- ISO 14577-1; Metallic Materials Instrumented Indentation Test for Hardness and Materials Parameters Part 1: Test Method. International Organization for Standardization: Geneva, Switzerland, 2002.
- Cavalcanti, D.A.; Muterlle, P.V.; Reinke, G. Effect of Sigma Phase Precipitated at 850 °C on Corrosion Behaviour of UNS S82441 Duplex Stainless Steel. Rev. Mater. 2019, 24, e12459. [Google Scholar] [CrossRef]
- Escriba, D.M.; Materna-Morris, E.; Plaut, R.L.; Padilha, A.F. Chi-Phase Precipitation in a Duplex Stainless Steel. Mater. Charact. 2009, 60, 1214–1219. [Google Scholar] [CrossRef]
- Elmer, J.W.; Palmer, T.A.; Specht, E.D. In Situ Observations of Sigma Phase Dissolution in 2205 Duplex Stainless Steel Using Synchrotron X-Ray Diffraction. Mater. Sci. Eng. A 2007, 459, 151–155. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Riquier, Y. δ Phase Precipitation and Its Effect on the Mechanical Properties of a Super Duplex Stainless Steel. Mater. Sci. Eng. A 1994, 174, 149–156. [Google Scholar] [CrossRef]
- Pohl, M.; Storz, O.; Glogowski, T. Effect of Intermetallic Precipitations on the Properties of Duplex Stainless Steel. Mater. Charact. 2007, 58, 65–71. [Google Scholar] [CrossRef]
- Gao, T.; Wang, J.; Sun, Q.; Han, P. Corrosion Behavior Difference in Initial Period for Austenite and Ferrite Phases of 2205 Duplex Stainless Steel in Chloride Containing Solution. Mater. Des. 2017, 116, 346–352. [Google Scholar] [CrossRef]
- Dos Santos, D.C.; Magnabosco, R.; de Moura-Neto, C. Influence of Sigma Phase Formation on Pitting Corrosion of an Aged UNS S31803 Duplex Stainless Steel. Corrosion 2013, 69, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, J.; Li, H.-B.; Li, Y.; Hu, Y.-D.; Bai, J.-G.; Han, P.-D. Chi Phase after Short-Term Aging and Corrosion Behavior in 2205 Duplex Stainless Steel. J. Iron Steel Res. Int. 2016, 23, 1071–1079. [Google Scholar] [CrossRef]
- Argandoña, G.; Palacio, J.F.; Berlanga, C.; Biezma, M.V.; Rivero, P.J.; Peña, J.; Rodriguez, R. Effect of the Temperature in the Mechanical Properties of Austenite, Ferrite and Sigma Phases of Duplex Stainless Steels Using Hardness, Microhardness and Nanoindentation Techniques. Metals 2017, 7, 219. [Google Scholar] [CrossRef]
- Maamache, B.; Cheniti, B.; Belkessa, B.; Tahar-chaouch, K.; Kouba, R. Effect of Aging Temperature on the Microstructure, Local Mechanical Properties, and Wear Behavior of a UNS S32750 Super Duplex Stainless Steel. J. Mater. Eng. Perform. 2021, 30, 546–555. [Google Scholar] [CrossRef]
- Meyers, M.; Chawla, C. Mechanical Behavior of Materials; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Fischer-Cripps, A. Elastic–Plastic Contact. In Nanoindentation; Springer: New York, NY, USA, 2004. [Google Scholar]
- Tao, P.; Gong, J.M.; Wang, Y.F.; Jiang, Y.; Li, Y.; Cen, W.W. Characterization on Stress-Strain Behavior of Ferrite and Austenite in a 2205 Duplex Stainless Steel Based on Nanoindentation and Finite Element Method. Results Phys. 2018, 11, 377–384. [Google Scholar] [CrossRef]
- Gadelrab, K.R.; Li, G.; Chiesa, M.; Souier, T. Local Characterization of Austenite and Ferrite Phases in Duplex Stainless Steel Using MFM and Nanoindentation. J. Mater. Res. 2012, 27, 1573–1579. [Google Scholar] [CrossRef]
- Sun, L.; Sun, Y.; Lv, C.; Liu, Y.; Dai, N.; Jiang, Y.; Li, J.; Macdonald, D.D. Studies on the Degree of Sensitization of Hyper-Duplex Stainless Steel 2707 at 900 °C Using a Modified DL-EPR Test. Corros. Sci. 2021, 185, 109432. [Google Scholar] [CrossRef]
- De Rezende, S.C.; Cronemberger, M.E.R.; Silva, R.; Della Rovere, C.A.; Kuri, S.E.; de Sousa, L.L.; Mariano, N.A. Effect of Solution Annealing Time on the Microstructure and Corrosion Resistance of Duplex Stainless Steel. Mater. Sci. Forum 2018, 930, 374–379. [Google Scholar] [CrossRef]
- Lee, J.S.; Jeon, S.H.; Park, Y.S. Effects of Solution Annealing Temperature on the Galvanic Corrosion Behavior of the Super Duplex Stainless Steels. J. Mater. Eng. Perform. 2013, 22, 557–562. [Google Scholar] [CrossRef]
- Tsai, W.T.; Chen, J.R. Galvanic Corrosion between the Constituent Phases in Duplex Stainless Steel. Corros. Sci. 2007, 49, 3659–3668. [Google Scholar] [CrossRef]
- Wu, T.F.; Tsai, W.T. Effect of KSCN and Its Concentration on the Reactivation Behavior of Sensitized Alloy 600 in Sulfuric Acid Solution. Corros. Sci. 2003, 45, 267–280. [Google Scholar] [CrossRef]
- Alvarez, T.R.; Pardal, J.M.; de Almeida, B.B.; Louzada, P.; Tavares, S.S.M.; Ponzio, E.A.; de Macedo, M.C.S. Nondestructive Microstructural Characterization of Superduplex Stainless Steel by Double Loop Electrochemical Polarization Reactivation Portable Test. Mater. Res. 2017, 20, 440–446. [Google Scholar] [CrossRef]
- Zanotto, F.; Grassi, V.; Merlin, M.; Balbo, A.; Zucchi, F. Effect of Brief Heat Treatments Performed between 650 and 850 °C on Corrosion Behaviour of a Lean Duplex Stainless Steel. Corros. Sci. 2015, 94, 38–47. [Google Scholar] [CrossRef]
- Silva, R.; Vacchi, G.S.; Kugelmeier, C.L.; Santos, I.G.R.; Filho, A.A.M.; Magalhães, D.C.C.; Afonso, C.R.M.; Sordi, V.L.; Rovere, C.A.D. New Insights into the Hardening and Pitting Corrosion Mechanisms of Thermally Aged Duplex Stainless Steel at 475 °C: A Comparative Study between 2205 and 2101 Steels. J. Mater. Sci. Technol. 2022, 98, 123–135. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Mondal, S. High Temperature Ageing Behaviour of a Duplex Stainless Steel. Mater. Charact. 2008, 59, 1776–1783. [Google Scholar] [CrossRef]
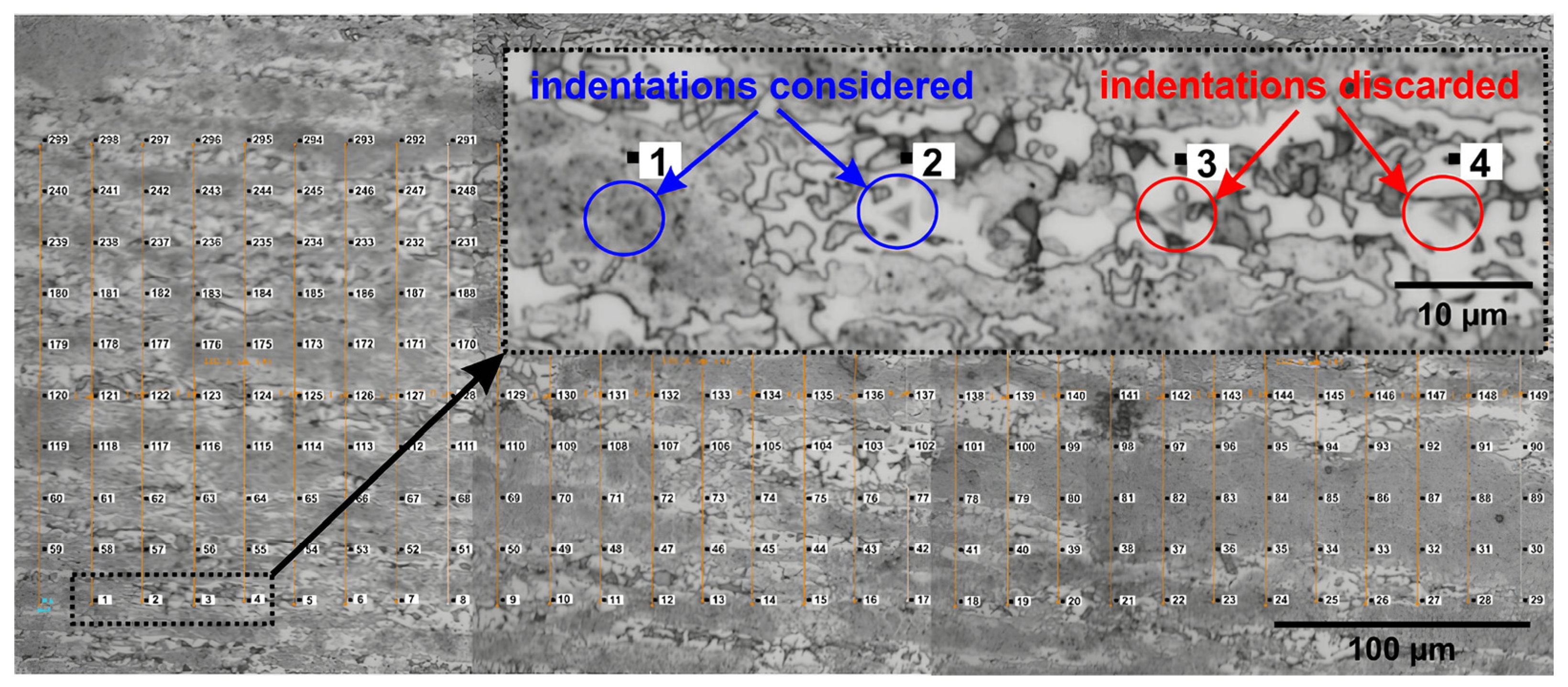
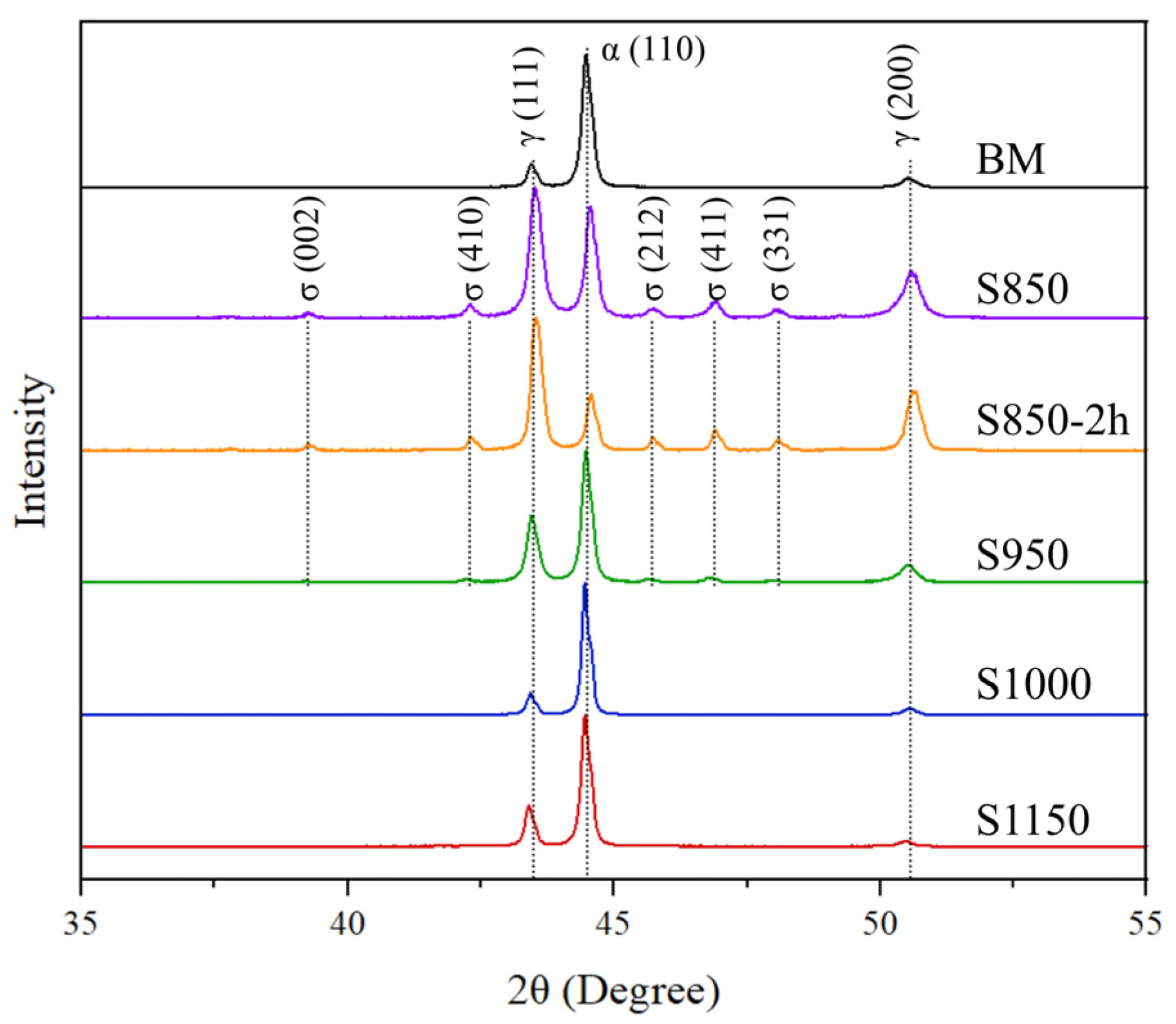
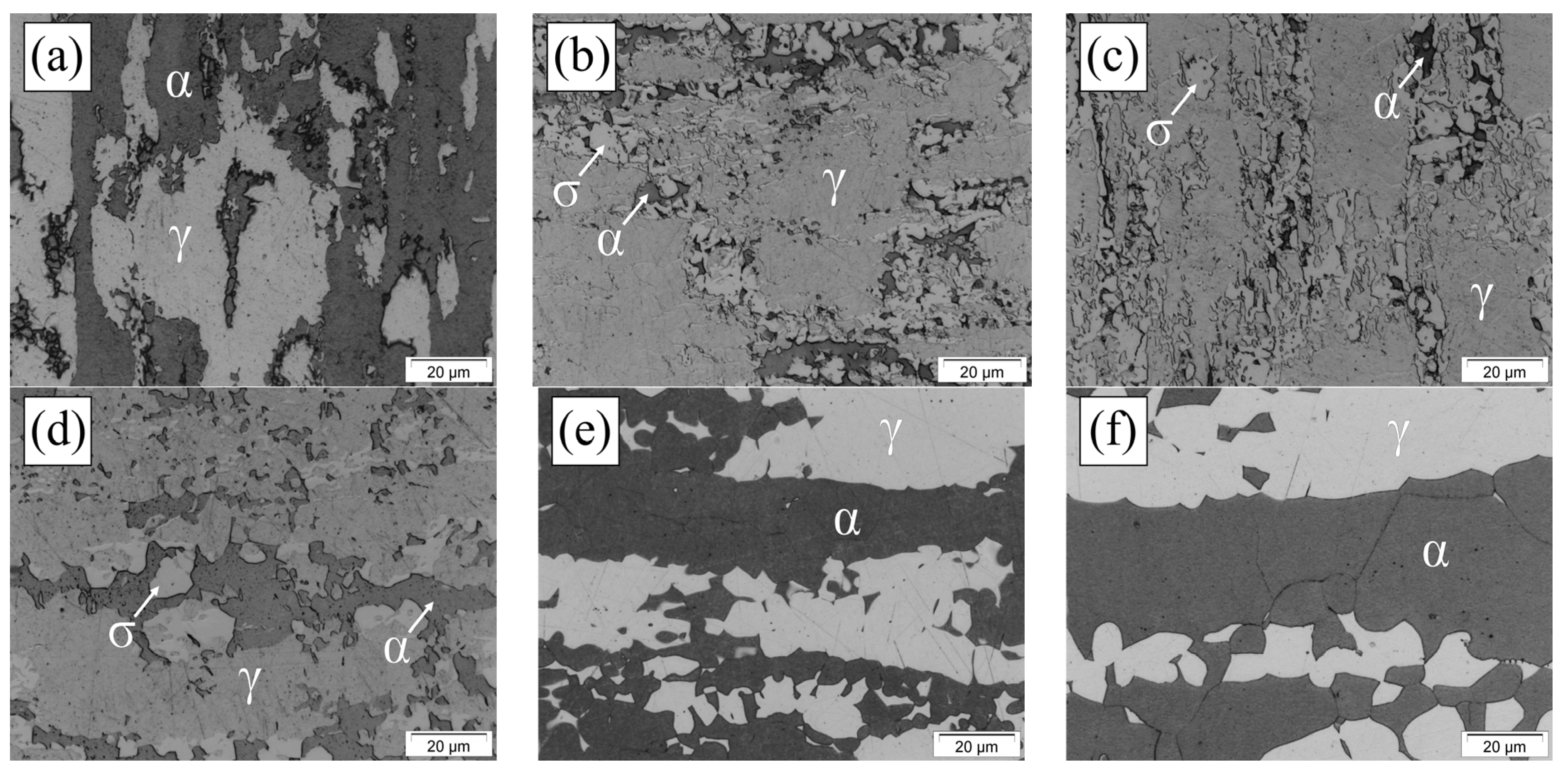
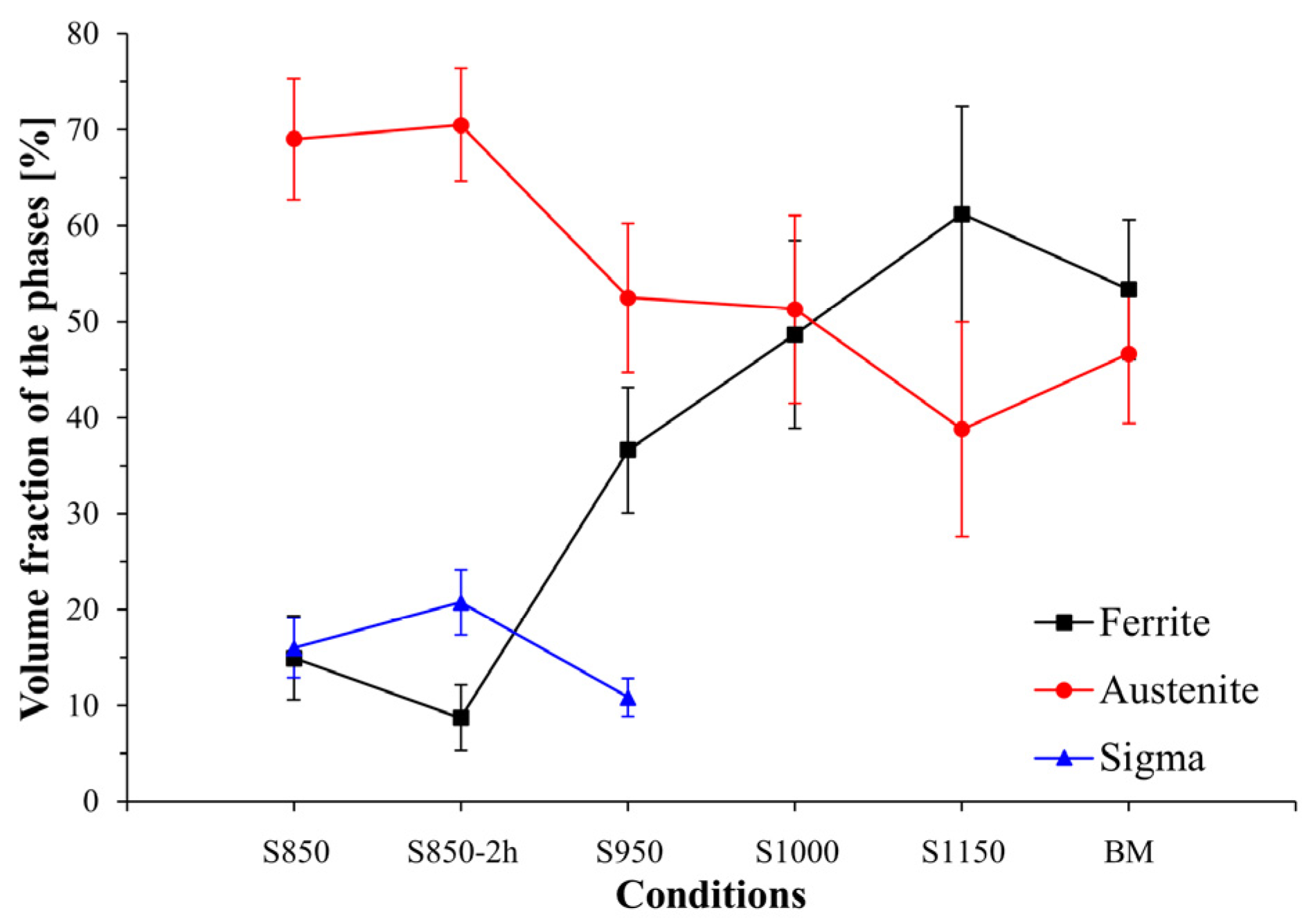




| DSS | Electrolyte | Surface Preparation Methods | Polarization | Thermal Treatment (Time) | Ref. |
|---|---|---|---|---|---|
| SAF 2205 | 0.5 M H2SO4 + 0.001 M TA | Paper grit was not informed | −0.5 V until 0.3 V (SCE) | 1100 °C (240 min) | [15] |
| 22% Cr | 2 M H2SO4 + 1 M HCl | Grounded until 600-grit paper | −0.5 V until 0.4 V (SCE) | 850 °C (2–30 min) and 1070–1150 °C (not informed) | [17] |
| AISI 2205 | 2 M H2SO4 + 1 M HCl | Paper grit was not informed | Estart at OCP * until 0.6 V vs. OCP * | 850 °C for 5–60 min | [18] |
| UNS S31803 | 2 M H2SO4 + 0.1, 0.5, 1, and 2 M HCl | Grounded until 1000-grit paper | Estart at OCP * until 0.3 V (SCE) | 800 °C for 0.5–48 h | [20] |
| Chemical Element | C | Cr | Ni | Mo | Mn | Si | Cu | N | P | S |
|---|---|---|---|---|---|---|---|---|---|---|
| wt% | 0.016 | 22.458 | 5.345 | 3.004 | 1.691 | 0.393 | 0.211 | 0.146 | 0.029 | 0.0004 |
| Identification Code | Study Condition |
|---|---|
| BM (Base Metal) | Industrial condition (1060 °C for 30 s) |
| S850 | BM + Aging at 850 °C for 30 min |
| S850-2h | BM + Aging at 850 °C for 2 h |
| S950 | BM + Aging at 950 °C for 30 min |
| S1000 | BM + Solubilization at 1000 °C for 30 min |
| S1150 | BM + Solubilization at 1150 °C for 30 min |
| Electrolyte | H2SO4 0.5 mol/L HCl 1 mol/L | H2SO4 1.5 mol/L HCl 1 mol/L | H2SO4 2.5 mol/L HCl 1 mol/L | ||||
|---|---|---|---|---|---|---|---|
| Phase | Ferrie | Austenite | Ferrite | Austenite | Ferrite | Austenite | |
| Condition | |||||||
| BM | −103 ± 8 | −45 ± 10 | −66 ± 2 | −17 ± 2 | −50 ± 0 | 13 ± 2 | |
| S850 (Activation) | −79 ± 7 | −35 ± 1 | −82 ± 5 | 1 ± 10 | 26 ± 2 | 60 ± 18 | |
| S850 (Reactivation) | - | - | - | - | −57 ± 8 | 15 ± 13 | |
| S850-2h (Activation) | −95 ± 3 | −29 ± 1 | −90 ± 8 | −10 ± 6 | 26 ± 6 | 63 ± 4 | |
| S850-2h (Reactivation) | - | - | - | - | −52 ± 3 | −2 ± 6 | |
| S950 | −93 ± 5 | −38 ± 6 | −67 ± 2 | −14 ± 2 | −35 ± 9 | 31 ± 33 | |
| S1000 | −97 ± 1 | −39 ± 1 | −76 ± 5 | −20 ± 4 | −62 ± 10 | 11 ± 18 | |
| S1150 | −93 ± 2 | −44 ± 8 | −61 ± 11 | −30 ± 4 | −75 ± 7 | −10 ± 6 | |
| Sample | DOS (Ir/Ip) | Classification |
|---|---|---|
| S850 | 0.174 ± 0.077 | Sensitized |
| S850-2h | 0.126 ± 0.096 | Sensitized |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quadros, P.V.C.A.; Ribeiro, J.J.K.; Schibicheski Kurelo, B.C.E.; Palma Calabokis, O.; Nuñez de la Rosa, Y.E.; Turin, A.R.; Borges, P.C. Influence of Heat Treatment Temperature on Microstructure, Hardness and Sensitization of UNS S32205 Duplex Stainless Steel. Materials 2024, 17, 4715. https://doi.org/10.3390/ma17194715
Quadros PVCA, Ribeiro JJK, Schibicheski Kurelo BCE, Palma Calabokis O, Nuñez de la Rosa YE, Turin AR, Borges PC. Influence of Heat Treatment Temperature on Microstructure, Hardness and Sensitization of UNS S32205 Duplex Stainless Steel. Materials. 2024; 17(19):4715. https://doi.org/10.3390/ma17194715
Chicago/Turabian StyleQuadros, Pedro Victorio Caetano Abrantes, Jomar José Knaip Ribeiro, Bruna Corina Emanuely Schibicheski Kurelo, Oriana Palma Calabokis, Yamid E. Nuñez de la Rosa, Alba Regina Turin, and Paulo César Borges. 2024. "Influence of Heat Treatment Temperature on Microstructure, Hardness and Sensitization of UNS S32205 Duplex Stainless Steel" Materials 17, no. 19: 4715. https://doi.org/10.3390/ma17194715







