Hydration and Mechanical Properties of High-Volume Fly Ash Cement under Different Curing Temperatures
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Mixture Proportions
2.3. Methods
3. Results and Discussion
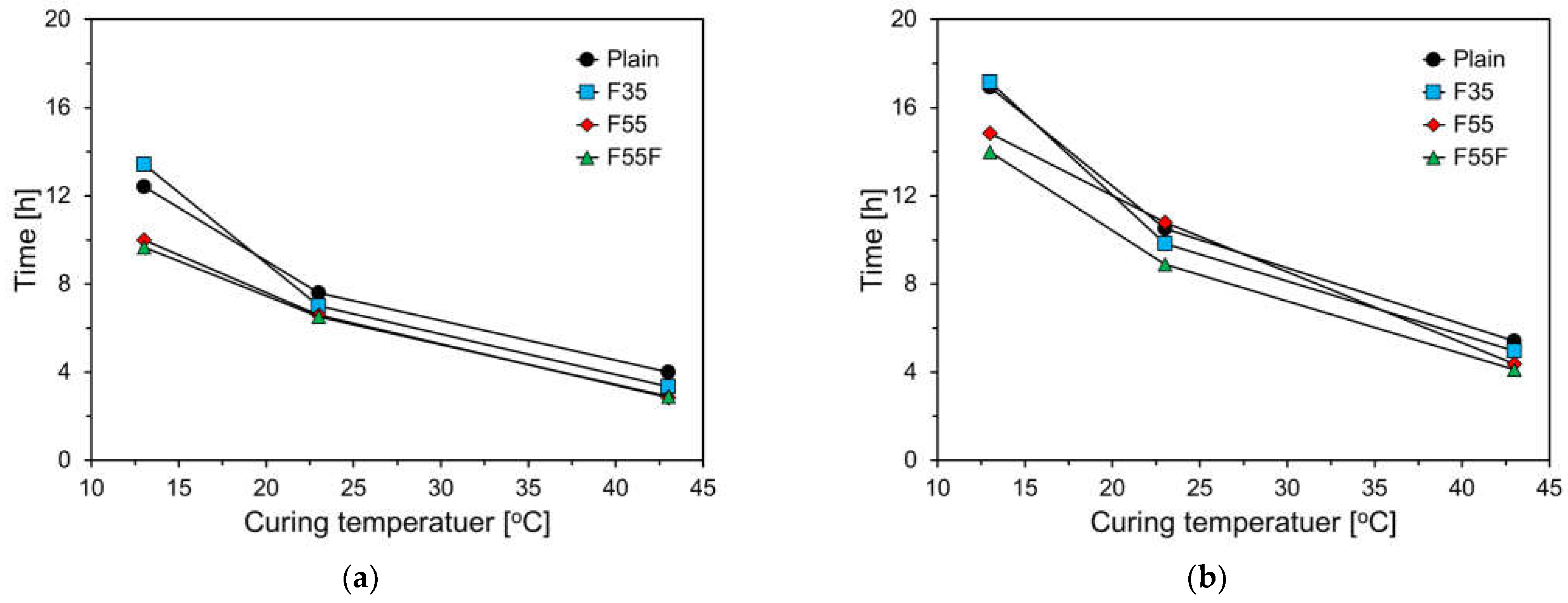
3.1. Setting Behavior of Specimens
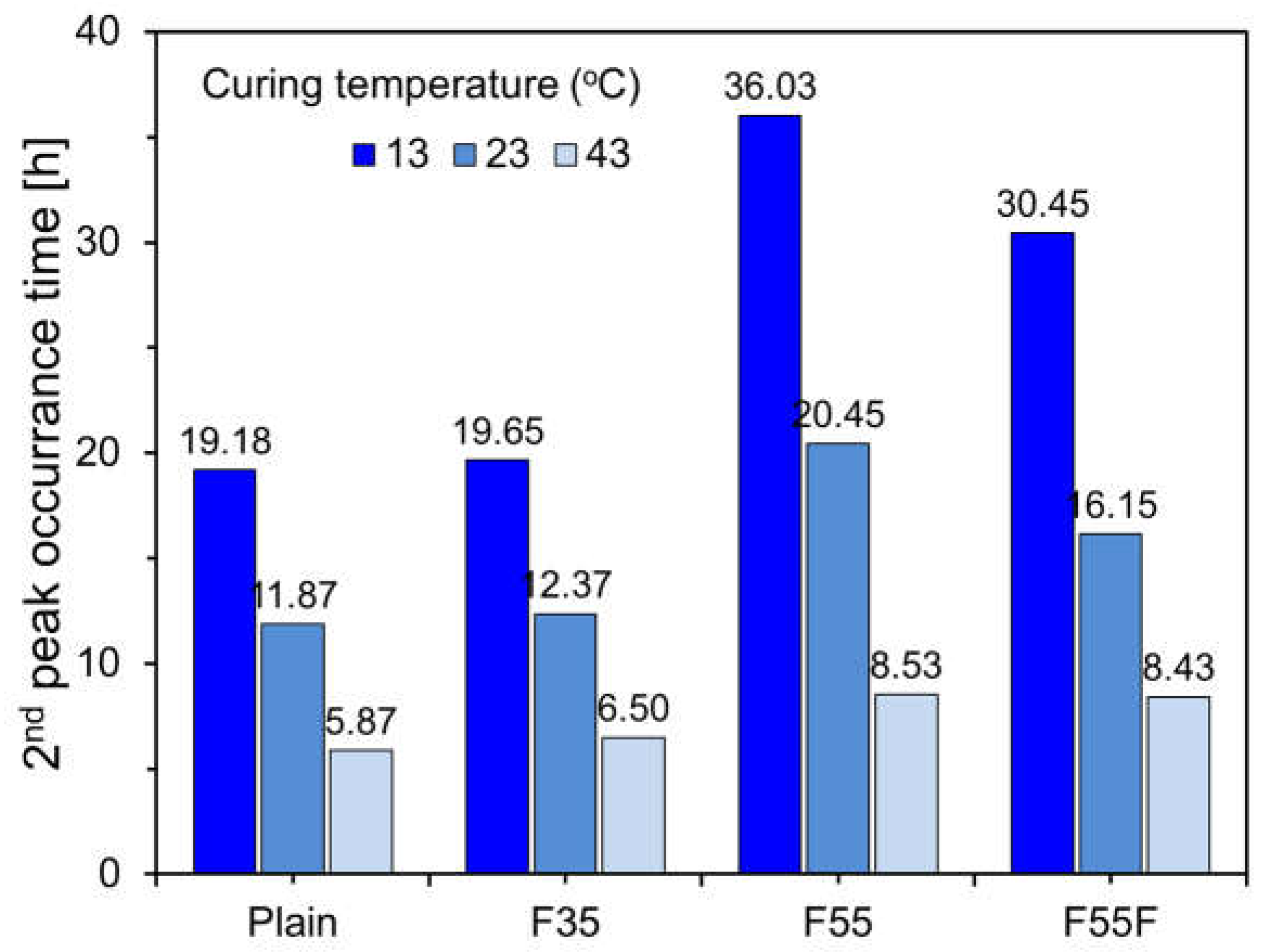
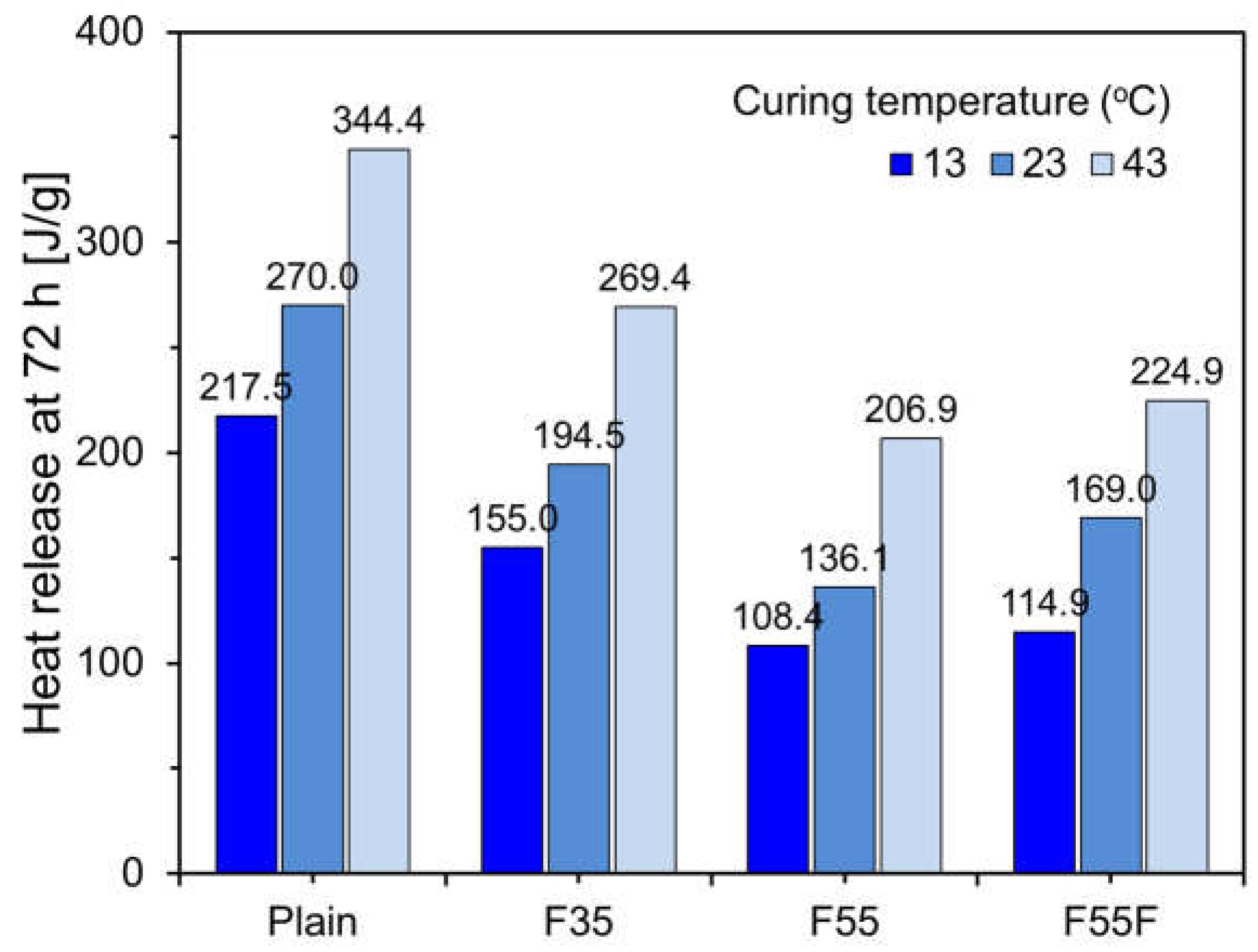
3.2. Effect of Curing Temperature on Heat of Hydration
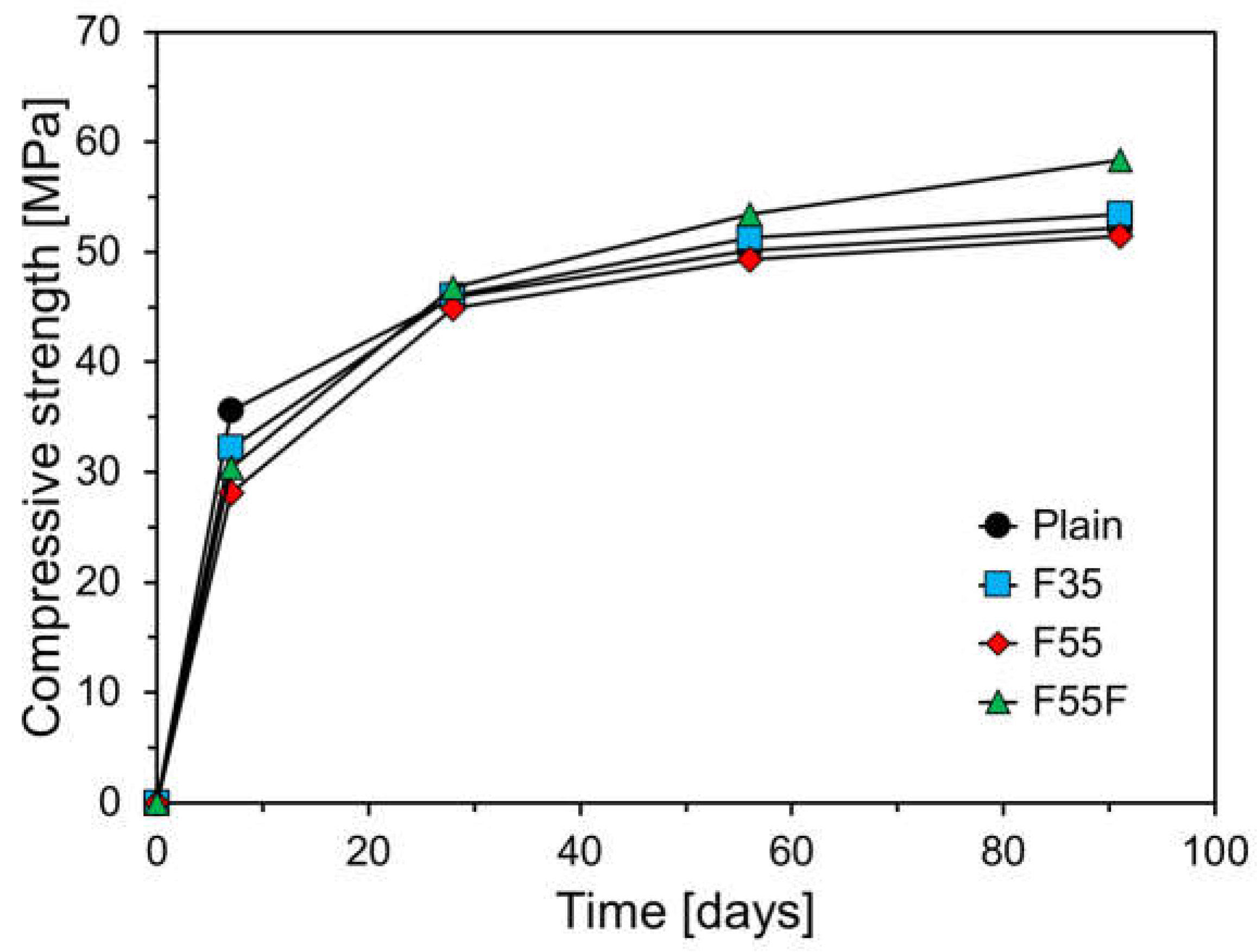
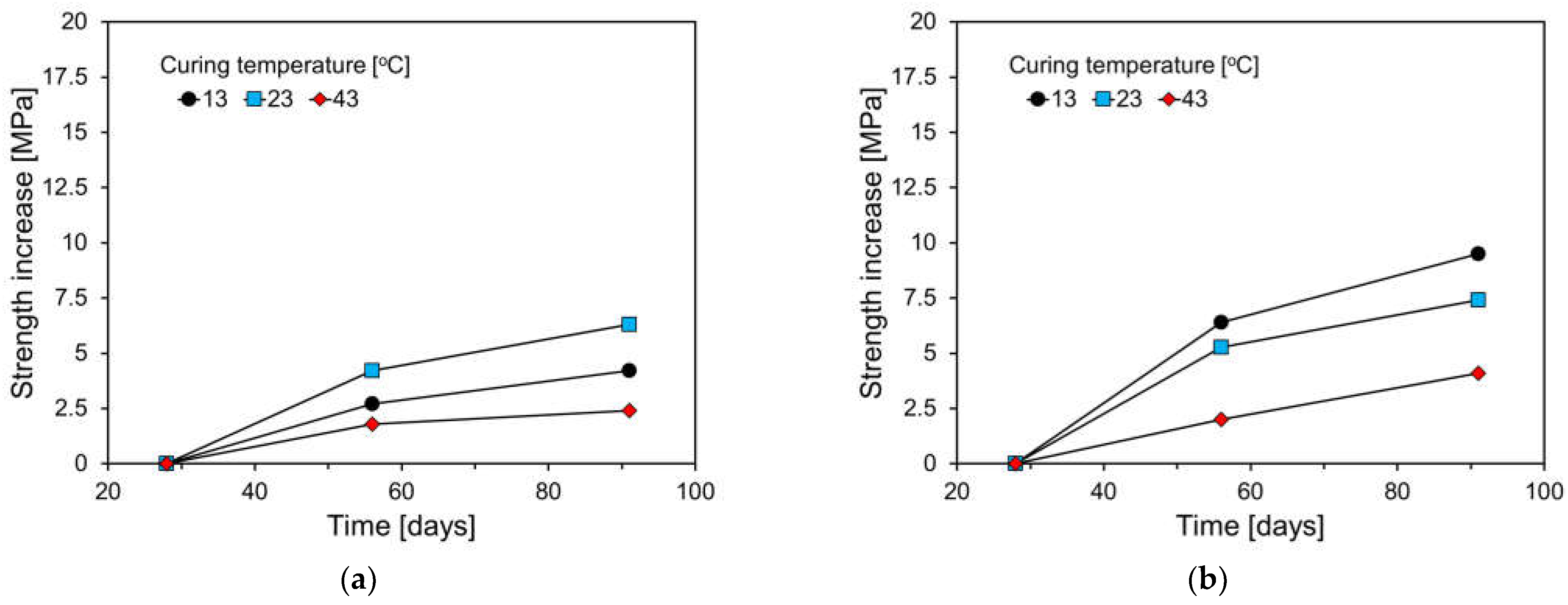
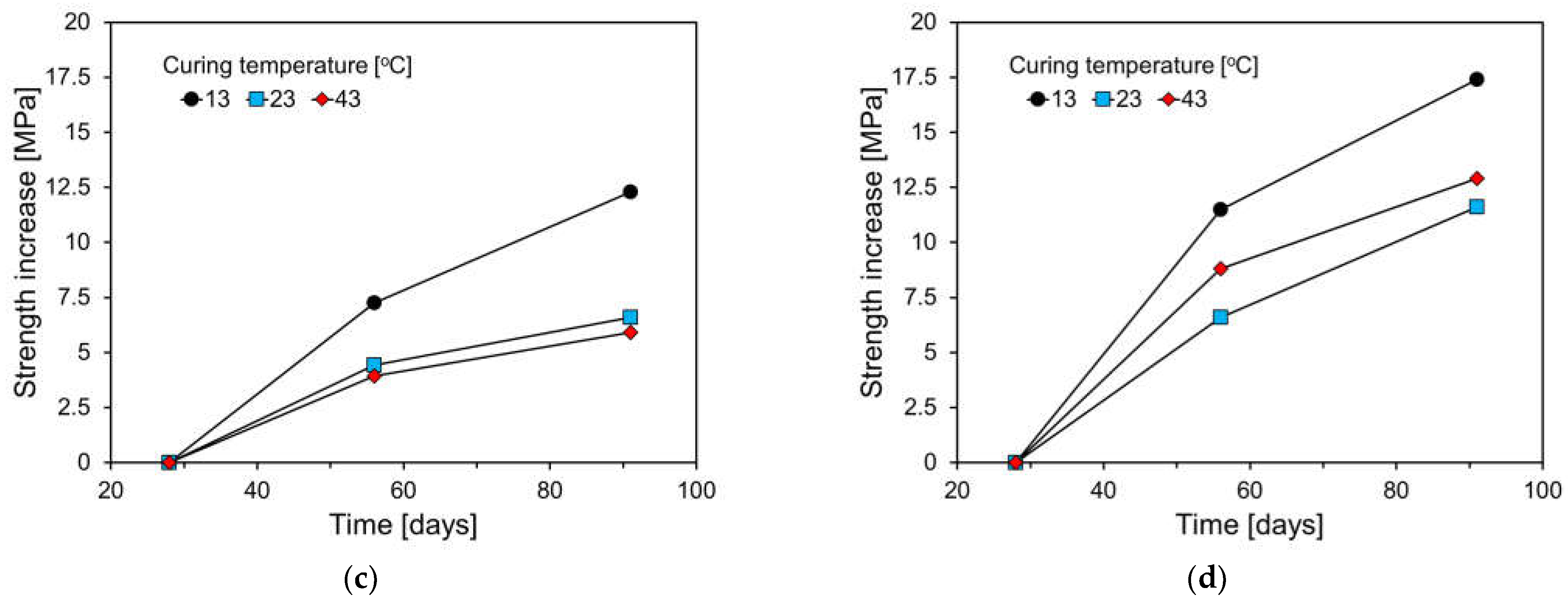
3.3. Compressive Strength Results
4. Conclusions
- Both OPC and FA cement showed a longer induction period as the curing temperature decreased, consistent with the setting time results. This effect was stronger with a higher FA content, as FA adsorbs calcium ions, lowering the Ca/Si ratio and producing calcium silicate hydrates with a reduced Ca/Si ratio.
- The heat of hydration decreased as the FA content increased. Hydration reactions were inhibited at low temperatures and accelerated at high temperatures, with finely ground FA providing nucleation sites, enhancing the filler effect, and shortening the induction period.
- Compared to the plain specimen, the higher FA content resulted in a greater decrease in compressive strength at a low temperature. The plain specimen showed a gradual strength increase after 28 days, while FA-containing specimens showed a larger increase due to the pozzolanic reaction of FA.
- Specimens cured at 43 °C had a significant increase in 7-day compressive strength, particularly in FA-containing specimens. Plain specimens showed slow strength increases after 28 days, but FA-containing specimens experienced substantial gains, attributed to FA’s pozzolanic reaction and temperature sensitivity.
- High FA content specimens had slower initial hydration and lower early strength, but the pozzolanic reaction led to significant long-term strength gains. FA’s high temperature sensitivity requires careful consideration when using HVFA concrete.
- FA is more temperature-sensitive than other materials, making HVFA concrete highly susceptible to changes in properties due to climate or curing temperature. Environmental factors, such as temperature, should be considered in the design and maintenance of HVFA concrete structures.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sivakrishna, A.; Adesina, A.; Awoyera, P.O.; Kumar, K.R. Green concrete: A review of recent developments. Mater. Today Proc. 2020, 27, 54–58. [Google Scholar] [CrossRef]
- Coffetti, D.; Crotti, E.; Gazzaniga, G.; Carrara, M.; Pastore, T.; Coppola, L. Pathways towards sustainable concrete. Cem. Concr. Res. 2022, 154, 106718. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C.G. Synthesis and hydration of calcium sulfoaluminatebelite cements with varied phase compositions. J. Mater. Sci. 2011, 46, 2568–2577. [Google Scholar] [CrossRef]
- Trauchessec, R.; Mechling, J.M.; Lecomte, A.; Roux, A.; Le Rolland, B. Hydration of ordinary Portland cement and calcium sulfoaluminate cement blends. Cem. Concr. Compos. 2015, 56, 106–114. [Google Scholar] [CrossRef]
- Knight, K.A.; Cunningham, P.R.; Miller, S.A. Optimizing supplementary cementitious material replacement to minimize the environmental impacts of concrete. Cem. Concr. Compos. 2023, 139, 105049. [Google Scholar] [CrossRef]
- Gil, D.M.; Golewski, G.L. Potential of siliceous fly ash and silica fume as a substitute for binder in cementitious concretes. E3S Web Conf. 2018, 49, 00030. [Google Scholar] [CrossRef]
- Shi, M.; Wang, Q.; Zhou, Z. Comparison of the properties between high-volume fly ash concrete and high-volume steel slag concrete under temperature matching curing condition. Constr. Build. Mater. 2015, 98, 649–655. [Google Scholar] [CrossRef]
- Langan, B.W.; Weng, K.; Ward, M.A. Effect of silica fume and fly ash on heat of hydration of Portland cement. Cem. Concr. Res. 2002, 32, 1045–1051. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Nonat, A. Hydration of cementitious materials, present and future. Cem. Concr. Res. 2011, 41, 651–665. [Google Scholar] [CrossRef]
- Skibsted, J.; Snellings, R. Reactivity of supplementary cementitious materials (SCMs) in cement blends. Cem. Concr. Res. 2019, 124, 105799. [Google Scholar] [CrossRef]
- Sumer, M. Compressive strength and sulfate resistance properties of concretes containing Class F and Class C fly ashes. Constr. Build. Mater. 2012, 34, 531–536. [Google Scholar] [CrossRef]
- Yan, X.; Jiang, L.; Guo, M.; Chen, Y.; Song, Z.; Bian, R. Evaluation of sulfate resistance of slag contained concrete under steam curing. Constr. Build. Mater. 2019, 195, 231–237. [Google Scholar] [CrossRef]
- Ortiz Lozano, J.A.; Aguado de Cea, A.; Agulló, L.; García Vicente, T.; Zermeño de León, M.E. Experimental study of the effect of temperature on the strength of ready-mixed concrete. Theory. Mater. Constr. 2008, 58, 7–22. [Google Scholar] [CrossRef]
- Liu, Z.; Sha, A.; Hu, L.; Zou, X. A laboratory study of Portland cement hydration under low temperatures. Road. Mater. Pavement Des. 2017, 18, 12–22. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Gao, J.; Li, Y.; Lou, B.; Diego, M.B.; Ye, T.; Sha, A. Frost-related damage of portland cement pastes at early age. J. Mater. Civ. Eng. 2023, 35, 04023020. [Google Scholar] [CrossRef]
- Barkhordari, M.S.; Armaghani, D.J.; Mohammed, A.S.; Ulrikh, D.V. Data-Driven Compressive Strength Prediction of Fly Ash Concrete Using Ensemble Learner Algorithms. Buildings 2022, 12, 132. [Google Scholar] [CrossRef]
- Abdalla, A.; Salih, A. Microstructure and chemical characterizations with soft computing models to evaluate the influence of calcium oxide and silicon dioxide in the fly ash and cement kiln dust on the compressive strength of cement mortar. Resour. Conserv. Recycl. Adv. 2022, 15, 200090. [Google Scholar] [CrossRef]
- Jaf, D.K.I.; Abdulrahman, A.S.; Abdulrahman, P.I.; Mohammed, A.S.; Kurda, R.; Ahmed, H.U.; Faraj, R.H. Effitioned soft computing models to evaluate the impact of silicon dioxide (SiO2) to calcium oxide (CaO) ratio in fly ash on the compressive strength of concrete. J. Build. Eng. 2023, 74, 106820. [Google Scholar] [CrossRef]
- Han, F.; He, X.; Zhang, Z.; Liu, J. Hydration heat of slag or fly ash in the composite binder at different temperatures. Thermochim. Acta 2017, 655, 202–210. [Google Scholar] [CrossRef]
- Narmluk, M.; Nawa, T. Effect of fly ash on the kinetics of Portland cement hydration at different curing temperatures. Cem. Concr. Res. 2011, 41, 579–589. [Google Scholar] [CrossRef]
- Elkhadiri, I.; Puertas, F. The effect of curing temperature on sulphate-resistant cement hydration and strength. Constr. Build. Mater. 2008, 22, 1331–1341. [Google Scholar] [CrossRef]
- Parry-Jones, G.; Al-Tayyib, A.H.J.; Al-Dulaijin, S.U.; Al-Mana, A.I.S. MAS-NMR hydration and compressive strength study in cement paste. Cem. Concr. Res. 1989, 19, 228–234. [Google Scholar] [CrossRef]
- Escalante-García, J.I.; Sharp, J.H. Effect of temperature on the hydration of the main clinker phases in portland cements: Part ii, blended cements. Cem. Concr. Res. 1998, 28, 1259–1274. [Google Scholar] [CrossRef]
- Dittrich, S.; Neubauer, J.; Goetz-Neunhoeffer, F. The influence of fly ash on the hydration of OPC within the first 44h—A quantitative in situ XRD and heat flow calorimetry study. Cem. Concr. Res. 2014, 56, 129–138. [Google Scholar] [CrossRef]
- ISO 9597; Cement—Test Methods—Determination of Setting Time and Soundness. International Organization for Standardization: Geneva, Switzerland, 2008.
- Hou, D.; Ma, H.; Li, Z. Morphology of calcium silicate hydrate (C-S-H) gel: A molecular dynamic study. Adv. Cem. Res. 2015, 27, 135–146. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, D.; Jiang, J.; Wang, P. Chloride ions transport and adsorption in the nano-pores of silicate calcium hydrate: Experimental and molecular dynamics studies. Constr. Build. Mater. 2016, 126, 991–1001. [Google Scholar] [CrossRef]
- Hou, D.; Ma, H.; Zhu, Y.; Li, Z. Calcium silicate hydrate from dry to saturated state: Structure, dynamics and mechanical properties. Acta Mater. 2014, 67, 81–94. [Google Scholar] [CrossRef]
- Vydra, V.; Kapičková, O.; Demo, P.; Semerák, P. Influence of temperature on induction period and on ultimate porosity of hardening cement paste. Constr. Build. Mater. 2007, 21, 1262–1266. [Google Scholar] [CrossRef]
- Baert, G.; Hoste, S.; De Schutter, G.; De Belie, N. Reactivity of fly ash in cement paste studied by means of thermogravimetry and isothermal calorimetry. J. Therm. Anal. Calorim. 2008, 94, 485–492. [Google Scholar] [CrossRef]
- Dyer, T.D.; Dhir, R.K. Hydration reactions of cement combinations containing vitrified incinerator fly ash. Cem. Concr. Res. 2004, 34, 849–856. [Google Scholar] [CrossRef]
- Huang, H.; Huang, T.; Yuan, Q.; Zhou, D.; Deng, D.; Zhang, L. Temperature dependence of structural build-up and its relation with hydration kinetics of cement paste. Constr. Build. Mater. 2019, 201, 553–562. [Google Scholar] [CrossRef]
- Park, B.; Choi, Y.C. Effects of fineness and chemical activators on the hydration and physical properties of high-volume fly-ash cement pastes. J. Build. Eng. 2022, 51, 104274. [Google Scholar] [CrossRef]
- Poole, J.L.; Riding, K.A.; Folliard, K.J.; Juenger, M.C.; Schindler, A.K. Methods for calculating activation energy for Portland cement. ACI Mater. J. 2007, 104, 303–311. [Google Scholar] [CrossRef]
- Schindler, A.K. Effect of temperature on hydration of cementitious materials. ACI Mater. J. 2004, 101, 72–81. [Google Scholar] [CrossRef]
- Rahhal, V.; Talero, R. Influence of two different fly ashes on the hydration of Portland cements. J. Therm. Anal. Calorim. 2004, 78, 191–205. [Google Scholar] [CrossRef]
- Yu, Z.; Ye, G. The pore structure of cement paste blended with fly ash. Constr. Build. Mater. 2013, 45, 30–35. [Google Scholar] [CrossRef]
- Wang, S.; Liamazos, E.; Baxter, L.; Fonseca, F. Durability of biomass fly ash concrete: Freezing and thawing and rapid chloride permeability tests. Fuel 2008, 87, 359–364. [Google Scholar] [CrossRef]
- Hou, P.K.; Kawashima, S.; Wang, K.J.; Corr, D.J.; Qian, J.S.; Shah, S.P. Effects of colloidal nanosilica on rheological and mechanical properties of fly ash–cement mortar. Cem. Concr. Compos. 2013, 35, 12–22. [Google Scholar] [CrossRef]
- Escalante-Garcia, J.I. Nonevaporable water from neat OPC and replacement materials in composite cements hydrated at different temperatures. Cem. Concr. Res. 2003, 33, 1883–1888. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Yang, J. Hydration mechanisms of composite binders containing phosphorus slag at different temperatures. Constr. Build. Mater. 2017, 147, 720–732. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, J.K.; Park, Y.D. Prediction of compressive strength of fly ash concrete by new apparent activation energy function. Cem. Concr. Res. 2003, 33, 965–971. [Google Scholar] [CrossRef]
- Bentz, D.P. Activation energies of high-volume fly ash ternary blends: Hydration and setting. Cem. Concr. Compos. 2014, 53, 214–223. [Google Scholar] [CrossRef]

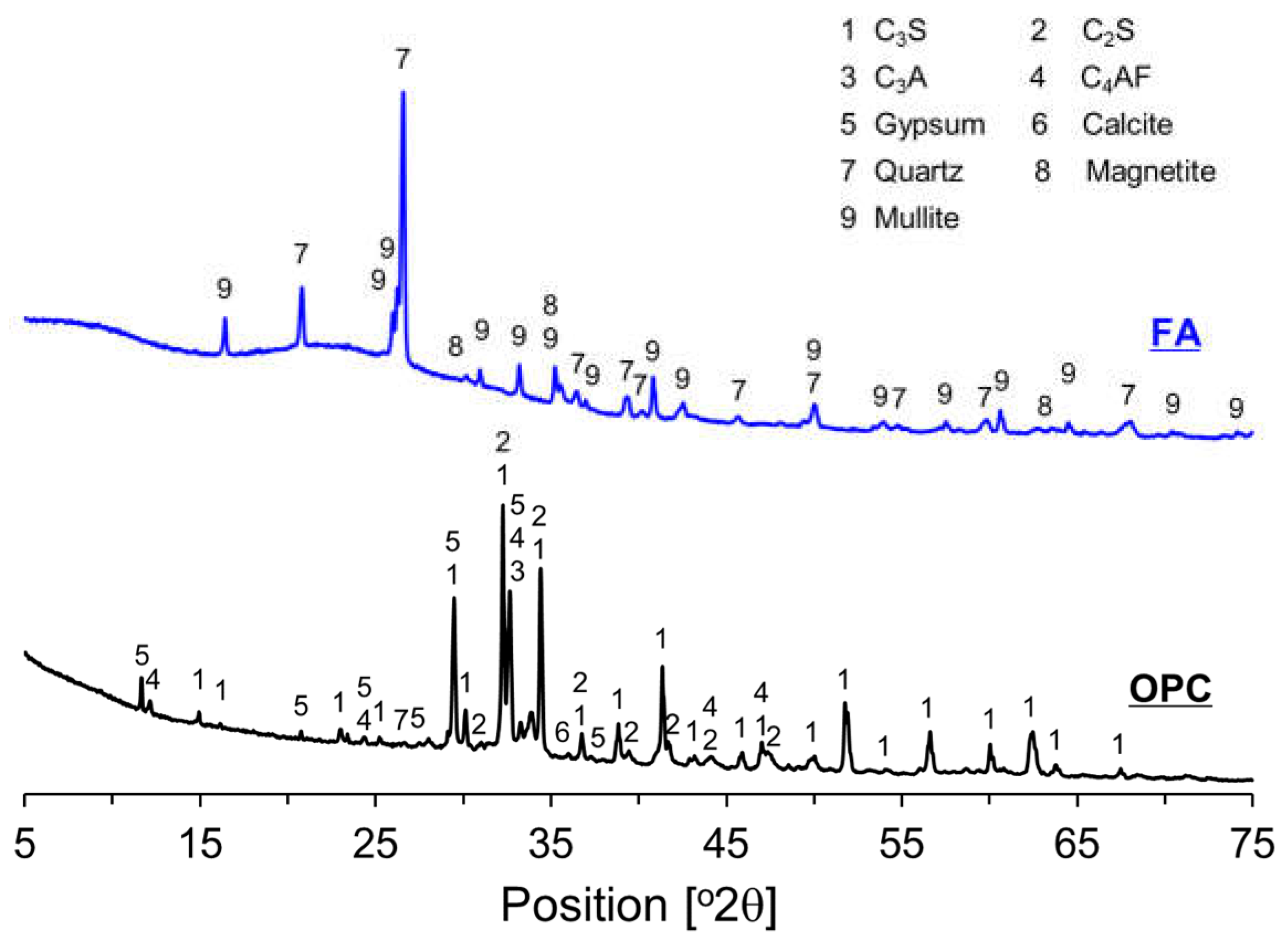
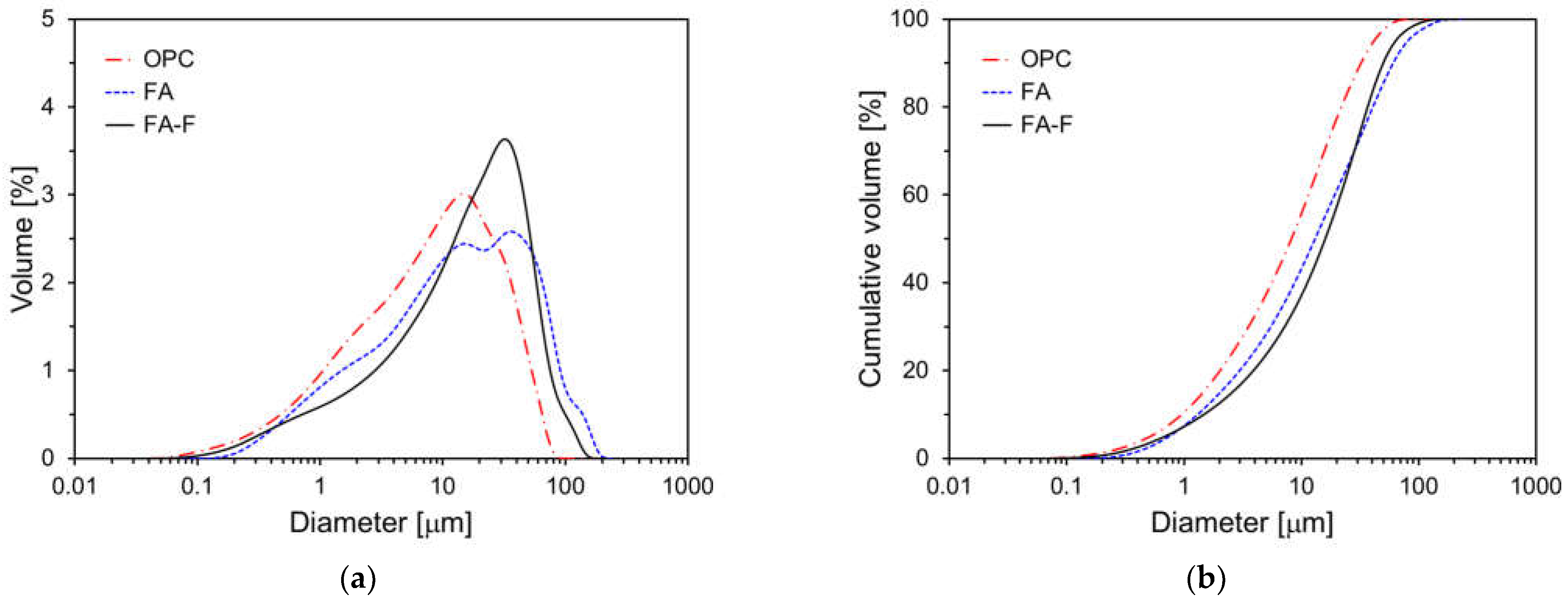




| Chemical Compositions (% by Mass) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | LOI | |
| OPC | 20.8 | 4.6 | 3.7 | 61.8 | 2.9 | 0.8 | 0.4 | 2.3 | 1.1 |
| FA | 51.3 | 23.7 | 7.8 | 4.9 | 1.7 | 1.2 | 1.5 | 0.4 | 3.6 |
| Variables | Water (kg/m3) | Water Binder Ratio (-) | Binder (kg/m3) | Aggregate (kg/m3) | |||
|---|---|---|---|---|---|---|---|
| OPC | FA | FA-F | Fine | Coarse | |||
| Plain | 170 | 0.45 | 380 | - | - | 720 | 1033 |
| FC35 | 165 | 0.39 | 273 | 147 | - | 690 | 989 |
| FC55 | 125 | 0.28 | 200 | 244 | - | 710 | 1019 |
| FC55F | 125 | 0.28 | 200 | 122 | 122 | 710 | 1019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-C. Hydration and Mechanical Properties of High-Volume Fly Ash Cement under Different Curing Temperatures. Materials 2024, 17, 4716. https://doi.org/10.3390/ma17194716
Choi Y-C. Hydration and Mechanical Properties of High-Volume Fly Ash Cement under Different Curing Temperatures. Materials. 2024; 17(19):4716. https://doi.org/10.3390/ma17194716
Chicago/Turabian StyleChoi, Young-Cheol. 2024. "Hydration and Mechanical Properties of High-Volume Fly Ash Cement under Different Curing Temperatures" Materials 17, no. 19: 4716. https://doi.org/10.3390/ma17194716





