Improvement of the Stability of Quantum-Dot Light Emitting Diodes Using Inorganic HfOx Hole Transport Layer
Abstract
:1. Introduction
2. Materials and Method
2.1. Solution Synthesis
2.2. Fabrication of the Device
2.3. Characterization and Measurement of Films and Devices
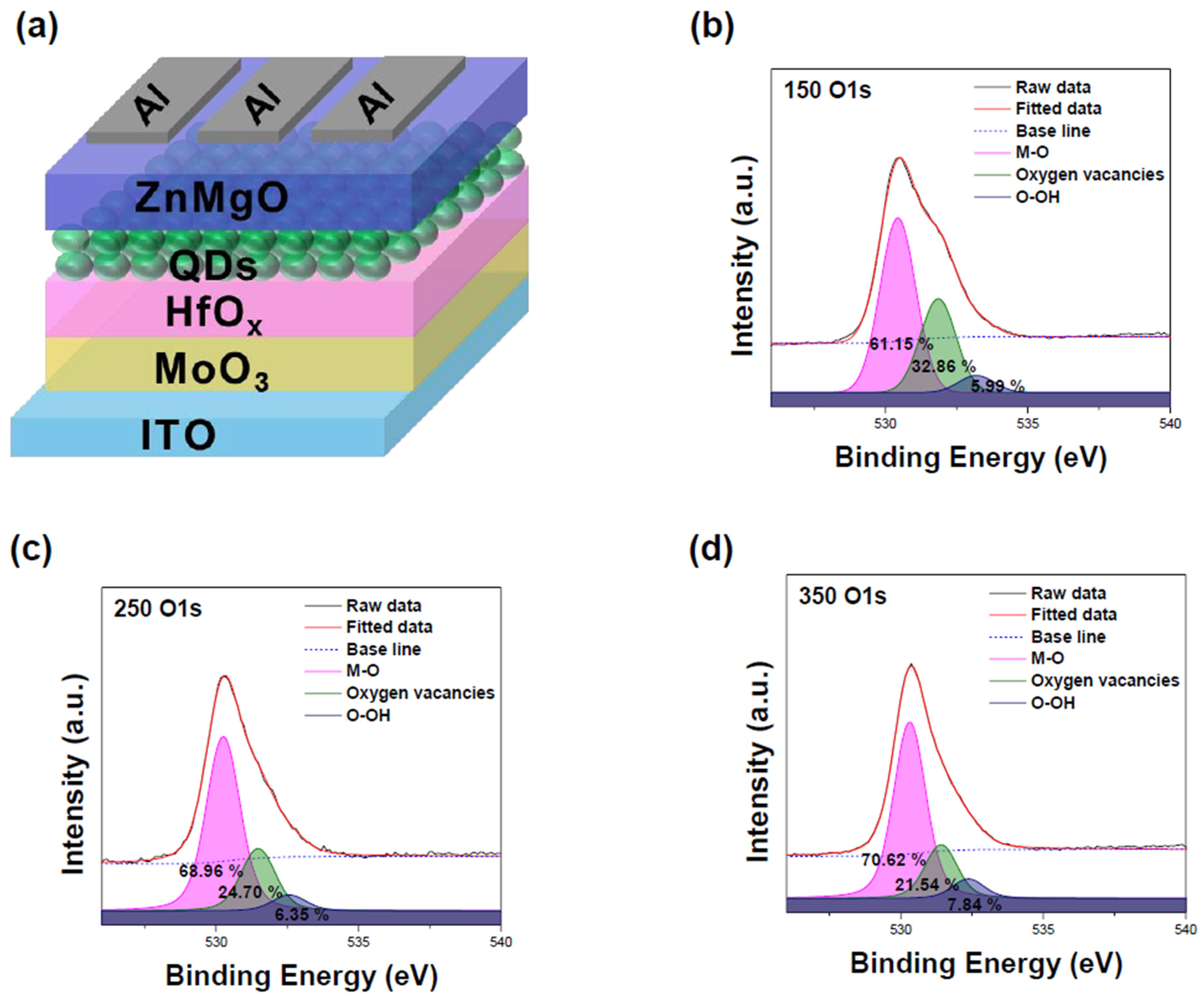
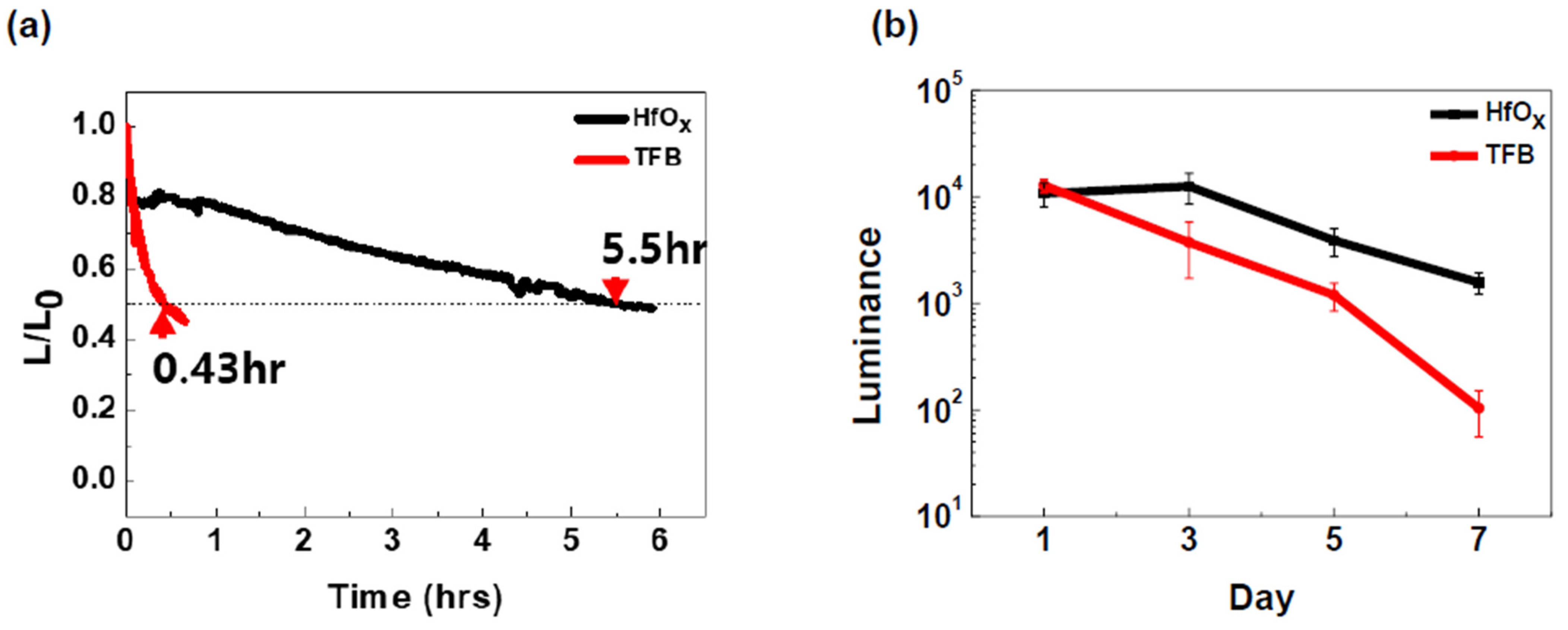
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jang, E.; Jang, H. Quantum dot light-emitting diodes. Chem. Rev. 2023, 123, 4663–4692. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, M.G.; Ma, J.H.; Park, M.H.; Ha, H.J.; Kang, S.J.; Maeng, M.-J.; Kim, Y.D.; Park, Y.; Kang, S.J. Improving the Performance of Solution−Processed Quantum Dot Light−Emitting Diodes via a HfOx Interfacial Layer. Materials 2022, 15, 8977. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Anaya, M.; Abfalterer, A.; Stranks, S.D. Halide perovskite light-emitting diode technologies. Adv. Opt. Mater. 2021, 9, 2002128. [Google Scholar] [CrossRef]
- Moon, H.; Lee, C.; Lee, W.; Kim, J.; Chae, H. Stability of quantum dots, quantum dot films, and quantum dot light-emitting diodes for display applications. Adv. Mater. 2019, 31, 1804294. [Google Scholar] [CrossRef] [PubMed]
- Sudheendran Swayamprabha, S.; Dubey, D.K.; Shahnawaz; Yadav, R.A.K.; Nagar, M.R.; Sharma, A.; Tung, F.C.; Jou, J.H. Approaches for long lifetime organic light emitting diodes. Adv. Sci. 2021, 8, 2002254. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, C.; He, T.; Jiang, Y.; Wei, J.; Huang, Y.; Yuan, M. High-performance quasi-2D perovskite light-emitting diodes: From materials to devices. Light: Sci. Appl. 2021, 10, 61. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.; Yuan, C.; Gao, P.; Su, Q.; Chen, S. Color Revolution: Prospects and Challenges of Quantum-Dot Light-Emitting Diode Display Technologies. Small Methods 2024, 8, 2300359. [Google Scholar] [CrossRef]
- Vasudevan, D.; Gaddam, R.R.; Trinchi, A.; Cole, I. Core–shell quantum dots: Properties and applications. J. Alloys Compd. 2015, 636, 395–404. [Google Scholar] [CrossRef]
- Cao, F.; Wu, Q.; Sui, Y.; Wang, S.; Dou, Y.; Hua, W.; Kong, L.; Wang, L.; Zhang, J.; Jiang, T. All-Inorganic Quantum Dot Light-Emitting Diodes with Suppressed Luminance Quenching Enabled by Chloride Passivated Tungsten Phosphate Hole Transport Layers. Small 2021, 17, 2100030. [Google Scholar] [CrossRef]
- Wang, L.; Pan, J.; Qian, J.; Liu, C.; Zhang, W.; Akram, J.; Lei, W.; Chen, J. Performance enhancement of all-inorganic quantum dot light-emitting diodes via surface modification of nickel oxide nanoparticles hole transport layer. ACS Appl. Electron. Mater. 2019, 1, 2096–2102. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, B.; Wang, S.; Yuan, Q.; Zhang, H.; Kang, Z.; Wang, R.; Zhang, H.; Ji, W. Influence of shell thickness on the performance of NiO-based all-inorganic quantum dot light-emitting diodes. ACS Appl. Mater. Interfaces 2018, 10, 14894–14900. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Z.-H.; Ding, T.; Wang, N.; Chen, G.; Dang, C.; Demir, H.V.; Sun, X.W. High-efficiency all-inorganic full-colour quantum dot light-emitting diodes. Nano Energy 2018, 46, 229–233. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.; Ma, W.; Sun, X.; Wu, L.; Lin, X.; Yang, Y.; Li, R.; Shen, D.; Chen, Y. Highly stable SnO2-based quantum-dot light-emitting diodes with the conventional device structure. ACS Nano 2022, 16, 9631–9639. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Lv, Y.; Jing, P.; Zhang, H.; Wang, J.; Zhang, H.; Zhao, J. Highly efficient and low turn-on voltage quantum dot light-emitting diodes by using a stepwise hole-transport layer. ACS Appl. Mater. Interfaces 2015, 7, 15955–15960. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.; Kim, B.; Hwang, B.; Kim, C.K. Improved performance of quantum dot light-emitting diodes by introducing WO3 hole injection layers. Mol. Cryst. Liq. Cryst. 2022, 735, 51–60. [Google Scholar] [CrossRef]
- Kim, T.Y.; Park, S.; Kim, B.J.; Heo, S.B.; Yu, J.H.; Shin, J.S.; Hong, J.-A.; Kim, B.-S.; Kim, Y.D.; Park, Y. Dual-functional quantum-dots light emitting diodes based on solution processable vanadium oxide hole injection layer. Sci. Rep. 2021, 11, 1700. [Google Scholar] [CrossRef]
- Nie, L.; Fan, J.; Li, Y.; Xiang, C.; Zhang, T. Direct Optical Patterning of MoO3 Nanoparticles and Their Application as a Hole Injection Layer for Solution-Processed Quantum Dot Light-Emitting Diodes. ACS Appl. Nano Mater. 2024, 7, 9499–9506. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, W.; Wu, Y.; He, Z.; Zhang, S.; Chen, S. A low-temperature-annealed and UV-ozone-enhanced combustion derived nickel oxide hole injection layer for flexible quantum dot light-emitting diodes. Nanoscale 2019, 11, 1021–1028. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Zhu, X.; Bai, Y.; Yang, Y.; Hu, S.; Liu, Y.; You, B.; Wang, J.; Li, Y. Highly efficient and super stable full-color quantum dots light-emitting diodes with solution-processed all-inorganic charge transport layers. Small 2021, 17, 2007363. [Google Scholar] [CrossRef]
- Ji, W.; Liu, S.; Zhang, H.; Wang, R.; Xie, W.; Zhang, H. Ultrasonic spray processed, highly efficient all-inorganic quantum-dot light-emitting diodes. ACS Photonics 2017, 4, 1271–1278. [Google Scholar] [CrossRef]
- Ji, W.; Shen, H.; Zhang, H.; Kang, Z.; Zhang, H. Over 800% efficiency enhancement of all-inorganic quantum-dot light emitting diodes with an ultrathin alumina passivating layer. Nanoscale 2018, 10, 11103–11109. [Google Scholar] [CrossRef] [PubMed]
- Hadacek, N.; Nosov, A.; Ranno, L.; Strobel, P.; Galéra, R.-M. Magnetic properties of HfO2 thin films. J. Phys. Condens. Matter 2007, 19, 486206. [Google Scholar] [CrossRef]
- Kaiser, N.; Vogel, T.; Zintler, A.; Petzold, S.; Arzumanov, A.; Piros, E.; Eilhardt, R.; Molina-Luna, L.; Alff, L. Defect-stabilized substoichiometric polymorphs of hafnium oxide with semiconducting properties. ACS Appl. Mater. Interfaces 2021, 14, 1290–1303. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.B.; Park, J.H.; Lee, K.H.; Lee, H.W.; Song, K.M.; Lee, S.J.; Baik, H.K. Solution-processed high-k HfO2 gate dielectric processed under softening temperature of polymer substrates. J. Mater. Chem. C 2013, 1, 1651–1658. [Google Scholar] [CrossRef]
- Krishna, D.N.G.; Philip, J. Review on surface-characterization applications of X-ray photoelectron spectroscopy (XPS): Recent developments and challenges. Appl. Surf. Sci. Adv. 2022, 12, 100332. [Google Scholar] [CrossRef]
- Yun, H.W.; Lee, J.; Kim, R.N.; Ji, S.H.; Ryu, S.O.; Kim, W.-B. Dipole formation and electrical properties of high-k/SiO2 interface according to the density of SiO2 interfacial layer. Curr. Appl. Phys. 2022, 37, 45–51. [Google Scholar] [CrossRef]
- Yang, J.-H.; Jang, G.-P.; Kim, S.-Y.; Chae, Y.-B.; Lee, K.-H.; Moon, D.-G.; Kim, C.-K. Highly Efficient All-Solution-Processed Quantum Dot Light-Emitting Diodes Using MoOx Nanoparticle Hole Injection Layer. Nanomaterials 2023, 13, 2324. [Google Scholar] [CrossRef]
- Kröger, M.; Hamwi, S.; Meyer, J.; Riedl, T.; Kowalsky, W.; Kahn, A. Role of the deep-lying electronic states of MoO3 in the enhancement of hole-injection in organic thin films. Appl. Phys. Lett. 2009, 95, 123301. [Google Scholar] [CrossRef]
- Hildebrandt, E.; Kurian, J.; Müller, M.M.; Schroeder, T.; Kleebe, H.-J.; Alff, L. Controlled oxygen vacancy induced p-type conductivity in HfO2−x thin films. Appl. Phys. Lett. 2011, 99, 112902. [Google Scholar] [CrossRef]
- Ha, H.J.; Kim, M.G.; Ma, J.H.; Jeong, J.H.; Park, M.H.; Kang, S.J.; Kim, W.; Park, S.; Kang, S.J. Enhanced performance of flexible quantum dot light-emitting diodes using a low-temperature processed PTAA hole transport layer. Sci. Rep. 2023, 13, 3780. [Google Scholar] [CrossRef]
- Haneef, H.F.; Zeidell, A.M.; Jurchescu, O.D. Charge carrier traps in organic semiconductors: A review on the underlying physics and impact on electronic devices. J. Mater. Chem. C 2020, 8, 759–787. [Google Scholar] [CrossRef]
- Padmasali, A.N.; Kini, S.G. A lifetime performance analysis of LED luminaires under real-operation profiles. IEEE Trans. Electron Devices 2019, 67, 146–153. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, J.M.; Park, M.H.; Kim, Y.B.; Choi, M.J.; Kim, S.; Yi, Y.; Park, S.; Kang, S.J. Improvement of the Stability of Quantum-Dot Light Emitting Diodes Using Inorganic HfOx Hole Transport Layer. Materials 2024, 17, 4739. https://doi.org/10.3390/ma17194739
Yun JM, Park MH, Kim YB, Choi MJ, Kim S, Yi Y, Park S, Kang SJ. Improvement of the Stability of Quantum-Dot Light Emitting Diodes Using Inorganic HfOx Hole Transport Layer. Materials. 2024; 17(19):4739. https://doi.org/10.3390/ma17194739
Chicago/Turabian StyleYun, Jung Min, Min Ho Park, Yu Bin Kim, Min Jung Choi, Seunghwan Kim, Yeonjin Yi, Soohyung Park, and Seong Jun Kang. 2024. "Improvement of the Stability of Quantum-Dot Light Emitting Diodes Using Inorganic HfOx Hole Transport Layer" Materials 17, no. 19: 4739. https://doi.org/10.3390/ma17194739







