Molecular Dynamics Insights into Mechanical Stability, Elastic Properties, and Fracture Behavior of PHOTH-Graphene
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Optimization of the Potential Function
3.2. Size Effects
3.3. Mechanical Properties
3.3.1. Mechanical Properties in Different Orientations
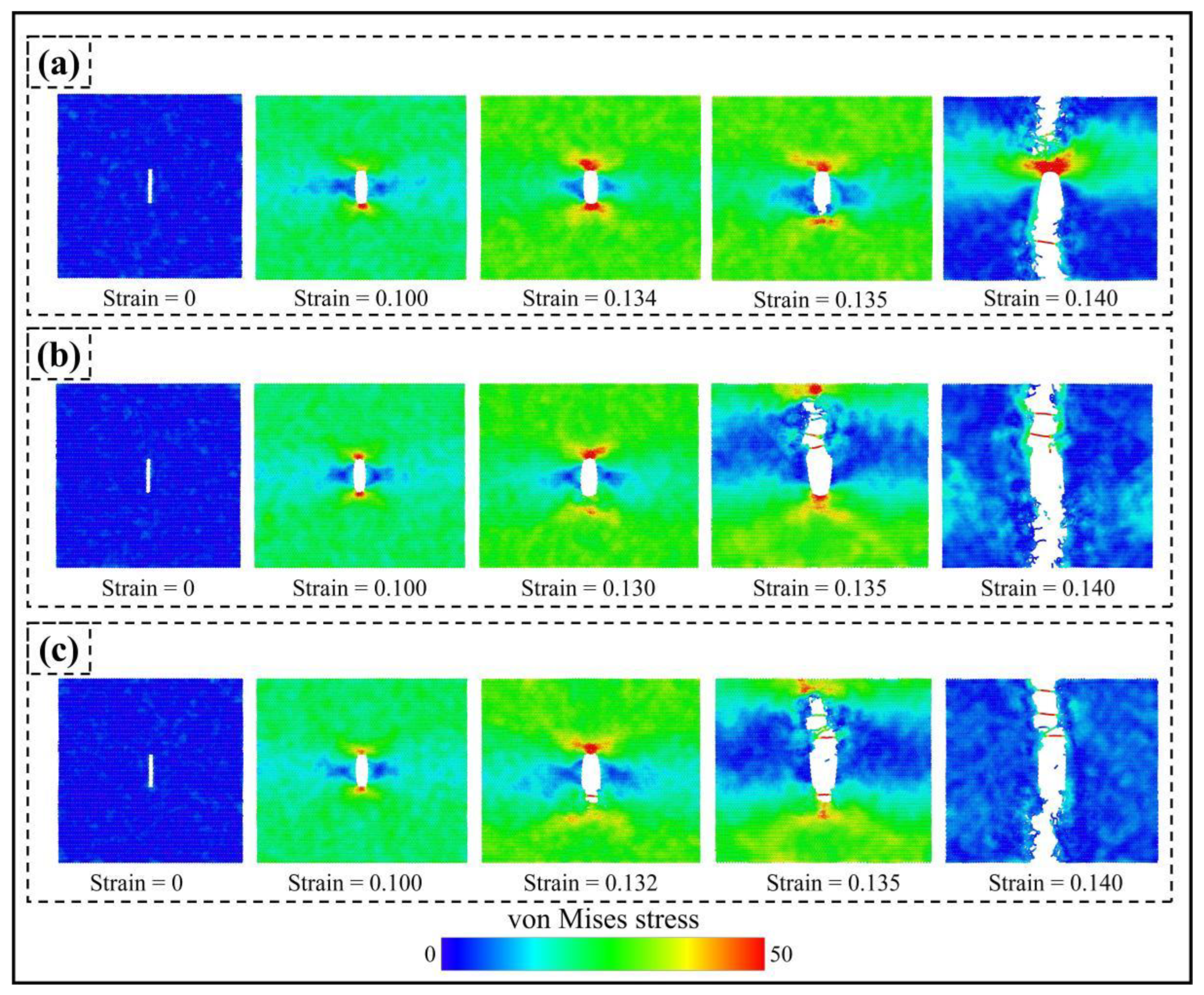
3.3.2. Forms of Failure and Fracture
3.3.3. Mechanical Properties at Different Temperatures
3.4. Effect of Defects
3.4.1. Random Vacancy Defects
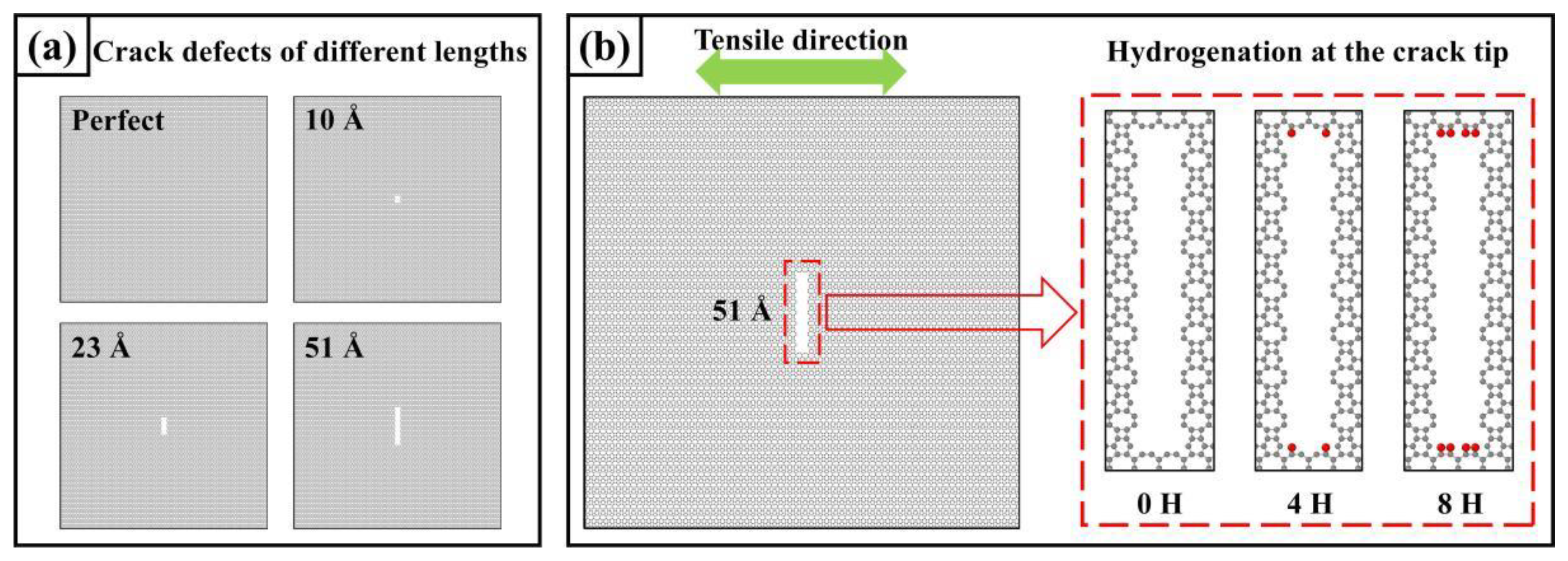
3.4.2. Type I Cracks and Crack Tip Hydrogen Absorption
4. Conclusions
- (1)
- PHOTH-graphene exhibits excellent mechanical properties in both the armchair and zigzag directions, but there are also significant differences. The material has stronger ultimate stress, ultimate strain, and toughness in the armchair direction and greater Young’s modulus in the zigzag direction.
- (2)
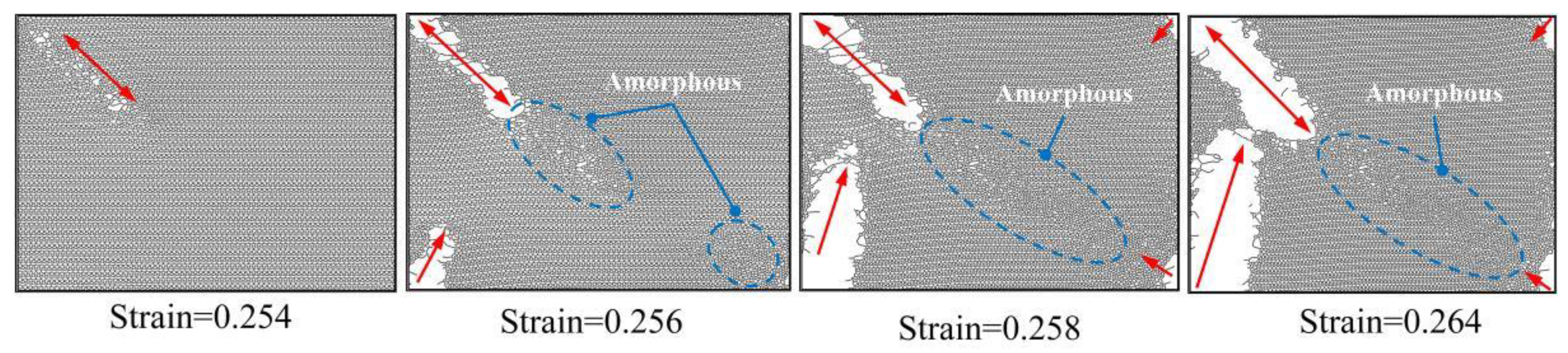
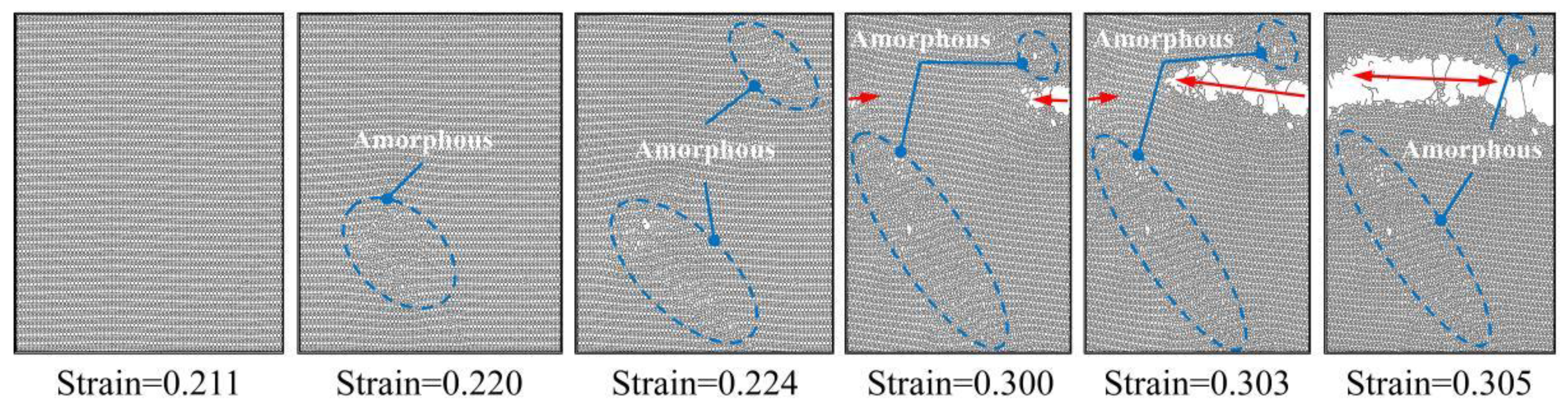
- The failure fracture patterns of PHOTH-graphene differ between the armchair and zigzag directions. Microcracks are first observed when the material is stretched uniaxially in the armchair direction, and regions of the amorphous structure appear in the direction of crack tip expansion. Finally, brittle fracture occurs as the crack expands. The region containing the amorphous structure is observed first when the material is stretched uniaxially in the zigzag direction; then, microcracks appear in the neighborhood of the amorphous region; and the material fails due to fracture as the crack expands.
- (3)
- Increasing the temperature weakens the strength of PHOTH-graphene. An increase in temperature by 1000 K (from 200 K to 1200 K) results in varying temperature sensitivities of the material’s mechanical properties in both directions. Young’s modulus is less sensitive to temperature increases than toughness, ultimate stress, and strain.
- (4)
- The introduction of random vacancy defects significantly weakened the strength of PHOTH-graphene. The values of ultimate stress, Young’s modulus, and toughness all decreased monotonically with increasing concentrations of random vacancy defects. Notably, increasing the concentration of random vacancy defects can enhance the ultimate strain of the material. Particularly, after increasing the concentration of vacancy defects by 3% in the zigzag direction, the ultimate strain of the material is higher than that of the material with no vacancy defects.
- (5)
- The stress concentration area during uniaxial stretching appears at the tip of a type I crack, significantly weakening the mechanical properties of PHOTH-graphene. The adsorption of hydrogen atoms at the crack tip induces the crack to expand under more minor strains, further weakening the material’s strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torkaman-Asadi, M.A.; Kouchakzadeh, M.A. Atomistic simulations of mechanical properties and fracture of graphene: A review. Comput. Mater. Sci. 2022, 210, 111457. [Google Scholar] [CrossRef]
- Gounzari, M.; Amallal, F.; Bouzelmad, M.; Belkassmi, Y.; Kotri, A. Exploring the potential of nanoporous amorphous graphene as a reverse osmosis membrane: Insights from molecular dynamics simulations. Chem. Phys. Lett. 2024, 848, 141393. [Google Scholar] [CrossRef]
- Tao, Y.; Sui, Z.-Y.; Han, B.-H. Advanced porous graphene materials: From in-plane pore generation to energy storage applications. J. Mater. Chem. A 2020, 8, 6125–6143. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; Grossman, J.C. Water Desalination across Nanoporous Graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Chamberland, J.P.; Sellathurai, A.J.; Scott Parent, J.; Barz, D.P.J. Optimized hydrogel electrodes for supercapacitors from high-concentration aqueous graphene nanoplatelet dispersions. J. Power Sources 2024, 605, 234435. [Google Scholar] [CrossRef]
- Jin, X.; Feng, C.; Ponnamma, D.; Yi, Z.; Parameswaranpillai, J.; Thomas, S.; Salim, N.V. Review on exploration of graphene in the design and engineering of smart sensors, actuators and soft robotics. Chem. Eng. J. Adv. 2020, 4, 100034. [Google Scholar] [CrossRef]
- Tan, Y.; Tao, X.; Ouyang, Y.; Peng, Q. Stable and 7.7 wt% hydrogen storage capacity of Ti decorated Irida-Graphene from first-principles calculations. Int. J. Hydrogen Energy 2024, 50, 738–748. [Google Scholar] [CrossRef]
- Varillas, J.; Lukeš, J.; Manikas, A.; Maňák, J.; Dluhoš, J.; Melníková, Z.; Kalbáč, M.; Galiotis, C.; Frank, O. Mechanical response of monolayer graphene via a multi-probe approach. Int. J. Mech. Sci. 2024, 273, 109208. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Liu, X.; Yu, P.; Zheng, B.; Oterkus, E.; He, X.; Lu, C. Prediction of graphene’s mechanical and fracture properties via peridynamics. Int. J. Mech. Sci. 2024, 266, 108914. [Google Scholar] [CrossRef]
- Cao, K.; Feng, S.; Han, Y.; Gao, L.; Hue Ly, T.; Xu, Z.; Lu, Y. Elastic straining of free-standing monolayer graphene. Nat. Commun. 2020, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.W.; Warner, J.H. Atomic resolution imaging of graphene by transmission electron microscopy. Nanoscale 2013, 5, 4079–4093. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Aluru, N.R. Temperature and strain-rate dependent fracture strength of graphene. J. Appl. Phys. 2010, 108, 064321. [Google Scholar] [CrossRef]
- Polewczyk, F.; Leyssale, J.-M.; Lafourcade, P. Temperature-dependent elasticity of single crystalline graphite. Comput. Mater. Sci. 2023, 220, 112045. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Pei, Q.-X.; Mai, Y.-W.; Gu, Y.-T. Temperature and strain-rate dependent fracture strength of graphynes. J. Phys. D Appl. Phys. 2014, 47, 425301. [Google Scholar] [CrossRef]
- Gamboa-Suárez, A.; Seuret-Hernández, H.Y.; Leyssale, J.-M. Mechanical properties of pristine and nanocrystalline graphene up to ultra-high temperatures. Carbon Trends 2022, 9, 100197. [Google Scholar] [CrossRef]
- Grantab, R.; Shenoy, V.B.; Ruoff, R.S. Anomalous Strength Characteristics of Tilt Grain Boundaries in Graphene. Science 2010, 330, 946–948. [Google Scholar] [CrossRef]
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural Defects in Graphene. ACS Nano 2011, 5, 26–41. [Google Scholar] [CrossRef]
- Rajasekaran, G.; Narayanan, P.; Parashar, A. Effect of Point and Line Defects on Mechanical and Thermal Properties of Graphene: A Review. Crit. Rev. Solid State Mater. Sci. 2016, 41, 47–71. [Google Scholar] [CrossRef]
- Zandiatashbar, A.; Lee, G.-H.; An, S.J.; Lee, S.; Mathew, N.; Terrones, M.; Hayashi, T.; Picu, C.R.; Hone, J.; Koratkar, N. Effect of defects on the intrinsic strength and stiffness of graphene. Nat. Commun. 2014, 5, 3186. [Google Scholar] [CrossRef]
- Gavallas, P.; Savvas, D.; Stefanou, G. Mechanical properties of graphene nanoplatelets containing random structural defects. Mech. Mater. 2023, 180, 104611. [Google Scholar] [CrossRef]
- Bedi, D.; Sharma, S.; Tiwari, S.K. Effect of chirality and defects on tensile behavior of carbon nanotubes and graphene: Insights from molecular dynamics. Diam. Relat. Mater. 2022, 121, 108769. [Google Scholar] [CrossRef]
- Zheng, B.; Gu, G.X. Tuning the graphene mechanical anisotropy via defect engineering. Carbon 2019, 155, 697–705. [Google Scholar] [CrossRef]
- He, L.; Guo, S.; Lei, J.; Sha, Z.; Liu, Z. The effect of Stone–Thrower–Wales defects on mechanical properties of graphene sheets—A molecular dynamics study. Carbon 2014, 75, 124–132. [Google Scholar] [CrossRef]
- Ajori, S.; Eftekharfar, A.R. Mechanical properties and fracture analysis of defective penta-graphene under temperature variation: Insight from molecular dynamics. Diam. Relat. Mater. 2022, 124, 108956. [Google Scholar] [CrossRef]
- Chen, M.; Han, X.; Tang, K. Topological regulations of Stone-Wales graphene. Carbon 2024, 226, 119163. [Google Scholar] [CrossRef]
- Peng, Q.; Liang, C.; Ji, W.; De, S. A theoretical analysis of the effect of the hydrogenation of graphene to graphane on its mechanical properties. Phys. Chem. Chem. Phys. 2013, 15, 2003–2011. [Google Scholar] [CrossRef]
- Tozzini, V.; Pellegrini, V. Prospects for hydrogen storage in graphene. Phys. Chem. Chem. Phys. 2013, 15, 80–89. [Google Scholar] [CrossRef]
- Pei, Q.X.; Zhang, Y.W.; Shenoy, V.B. A molecular dynamics study of the mechanical properties of hydrogen functionalized graphene. Carbon 2010, 48, 898–904. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Katsnelson, M.I.; Lichtenstein, A.I. Hydrogen on graphene: Electronic structure, total energy, structural distortions and magnetism from first-principles calculations. Phys. Rev. B 2008, 77, 035427. [Google Scholar] [CrossRef]
- Balog, R.; Jørgensen, B.; Nilsson, L.; Andersen, M.; Rienks, E.; Bianchi, M.; Fanetti, M.; Lægsgaard, E.; Baraldi, A.; Lizzit, S.; et al. Bandgap opening in graphene induced by patterned hydrogen adsorption. Nat. Mater. 2010, 9, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Montazeri, A.; Rafii-Tabar, H. Molecular dynamics study of a new mechanism for ripple formation on graphene nanoribbons at very low temperatures based on H2 physisorption. Solid State Commun. 2013, 159, 84–87. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, L.; Xu, J.; Liu, W. Effective regulation of the electronic properties of a biphenylene network by hydrogenation and halogenation. RSC Adv. 2022, 12, 20088–20095. [Google Scholar] [CrossRef] [PubMed]
- Haberer, D.; Petaccia, L.; Wang, Y.; Quian, H.; Farjam, M.; Jafari, S.A.; Sachdev, H.; Federov, A.V.; Usachov, D.; Vyalikh, D.V.; et al. Electronic properties of hydrogenated quasi-free-standing graphene. Phys. Status Solidi (B) 2011, 248, 2639–2643. [Google Scholar] [CrossRef]
- Li, N.-N.; Sha, Z.-D.; Pei, Q.-X.; Zhang, Y.-W. Hydrogenated Grain Boundaries Control the Strength and Ductility of Polycrystalline Graphene. J. Phys. Chem. C 2014, 118, 13769–13774. [Google Scholar] [CrossRef]
- Tabarraei, A.; Wang, X.; Jia, D. Effects of hydrogen adsorption on the fracture properties of graphene. Comput. Mater. Sci. 2016, 121, 151–158. [Google Scholar] [CrossRef]
- Ebrahimi, S. Effect of hydrogen coverage on the buckling of penta-graphene by molecular dynamics simulation. Mol. Simul. 2016, 42, 1485–1489. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Jana, D. TPO12-graphene: A new two-dimensional metallic carbon with 4–5 ring for Lithium ion battery. Appl. Surf. Sci. 2024, 669, 160495. [Google Scholar] [CrossRef]
- Laranjeira, J.A.S.; Martins, N.F.; Denis, P.A.; Sambrano, J.R. Graphenyldiene: A new sp2-graphene-like nanosheet. Carbon Trends 2024, 14, 100321. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Jana, D. TPDH-graphene: A new two dimensional metallic carbon with NDR behaviour of its one dimensional derivatives. Phys. E Low-Dimens. Syst. Nanostructures 2021, 127, 114569. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Majumdar, A.; Chowdhury, S.; Ahuja, R.; Jana, D. 8-16-4 graphyne: Square-lattice two-dimensional nodal line semimetal with a nontrivial topological Zak index. Phys. Rev. B 2021, 103, 075137. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Jana, D. Worm-graphene: A two-dimensional orthorhombic carbon semimetal with massless Dirac fermion. Appl. Surf. Sci. 2022, 585, 152457. [Google Scholar] [CrossRef]
- Chen, H.-T.; Dhanthala Chittibabu, D.K. A new carbon allotrope: Biphenylene as promising anode materials for Li-ion and LiO2 batteries. Solid State Ion. 2023, 395, 116214. [Google Scholar] [CrossRef]
- Peng, Q.; Huang, Z.; Chen, G.; Zhang, Y.; Zhang, X.; Chen, X.-J.; Hu, Z. Effect of Strain Rate, Temperature, Vacancy, and Microcracks on Mechanical Properties of 8-16-4 Graphyne. Nanomaterials 2024, 14, 556. [Google Scholar] [CrossRef] [PubMed]
- Farzin, A.; Etemadi, M.; Mehran, S.; Rouhi, S. Investigating the mechanical properties of perfect and defective Ψ-graphene: A molecular dynamics simulation. Mater. Today Commun. 2023, 37, 106908. [Google Scholar] [CrossRef]
- Alves, R.A.F.; Giozza, W.F.; Ribeiro, L.A.; Pereira, M.L. Exploring the thermal and mechanical properties of PAI-Graphene monolayers and nanotubes: Insights from molecular dynamics simulations. Mater. Today Commun. 2024, 40, 109591. [Google Scholar] [CrossRef]
- Pereira, M.L.; De Sousa, J.M.; Brandão, W.H.S.; Galvão, D.S.; Fonseca, A.F.; Ribeiro, L.A. Exploring the elastic properties and fracture patterns of Me-graphene monolayers and nanotubes through reactive molecular dynamics simulations. Chem. Phys. Lett. 2023, 830, 140812. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Guo, Z.; Jiang, J.-W.; Chang, T. Thermal stability of twin graphene: A Reaxff molecular dynamics study. Appl. Surf. Sci. 2023, 623, 157038. [Google Scholar] [CrossRef]
- Santos, E.A.J.; Lima, K.A.L.; Mendonça, F.L.L.; Silva, D.A.d.; Giozza, W.F.; Junior, L.A.R. PHOTH-graphene: A new 2D carbon allotrope with low barriers for Li-ion mobility. Sci. Rep. 2024, 14, 9526. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Yedla, N.; Gupta, P.; Ng, T.Y.; Geethalakshmi, K.R. Effect of loading direction and defects on the strength and fracture behavior of biphenylene based graphene monolayer. Mater. Chem. Phys. 2017, 202, 127–135. [Google Scholar] [CrossRef]
- Wang, X.; Che, J.; Huang, W.; Linghu, J.; Hou, Z. Mechanism of crack propagation in penta-graphene. Vacuum 2023, 207, 111582. [Google Scholar] [CrossRef]
- Mortazavi, B.; Ahzi, S. Thermal conductivity and tensile response of defective graphene: A molecular dynamics study. Carbon 2013, 63, 460–470. [Google Scholar] [CrossRef]
- Griffith, A.A. The Phenomena of Rupture and Flow in Solids. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1920, A221, 163–198. [Google Scholar]
- Donald, W.B.; Olga, A.S.; Judith, A.H.; Steven, J.S.; Boris, N.; Susan, B.S. A second-generation reactive empirical bond order (REBO) potential energy expression for hydrocarbons. J. Phys. Condens. Matter 2002, 14, 783. [Google Scholar] [CrossRef]
- Li, M.; Li, M.; Zheng, F. New three-dimensional orthorhombic graphene structures and their elastic properties. Phys. B Condens. Matter 2024, 678, 415753. [Google Scholar] [CrossRef]
- Niu, M.; Miao, L.; Xu, Z.; Zhao, Y.; Hao, W.; Li, J.; Li, J.; Sui, C.; Wang, C. Tunable tensile mechanical properties of bilayer graphene through inter-layer rotation. Diam. Relat. Mater. 2022, 130, 109522. [Google Scholar] [CrossRef]
- Zhu, C.; Ji, J.; Zhang, Z.; Dong, S.; Wei, N.; Zhao, J. Huge stretchability and reversibility of helical graphenes using molecular dynamics simulations and simplified theoretical models. Mech. Mater. 2021, 153, 103683. [Google Scholar] [CrossRef]
- Dewapriya, M.A.N.; Rajapakse, R.K.N.D. Molecular Dynamics Simulations and Continuum Modeling of Temperature and Strain Rate Dependent Fracture Strength of Graphene with Vacancy Defects. J. Appl. Mech. 2014, 81, 081010. [Google Scholar] [CrossRef]
- Cao, A.; Qu, J. Atomistic simulation study of brittle failure in nanocrystalline graphene under uniaxial tension. Appl. Phys. Lett. 2013, 102, 071902. [Google Scholar] [CrossRef]
- Zhang, B.; Mei, L.; Xiao, H. Nanofracture in graphene under complex mechanical stresses. Appl. Phys. Lett. 2012, 101, 121915. [Google Scholar] [CrossRef]
- Dewapriya, M.A.N.; Meguid, S.A.; Rajapakse, R.K.N.D. Atomistic modelling of crack-inclusion interaction in graphene. Eng. Fract. Mech. 2018, 195, 92–103. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Gao, H. Designing graphene structures with controlled distributions of topological defects: A case study of toughness enhancement in graphene ruga. Extrem. Mech. Lett. 2014, 1, 3–8. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, J.; Yin, H.; Shi, X.; Yang, R.; Dresselhaus, M. The nature of strength enhancement and weakening by pentagon–heptagon defects in graphene. Nat. Mater. 2012, 11, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Sunnardianto, G.K.; Bokas, G.; Hussein, A.; Walters, C.; Moultos, O.A.; Dey, P. Efficient hydrogen storage in defective graphene and its mechanical stability: A combined density functional theory and molecular dynamics simulation study. Int. J. Hydrogen Energy 2021, 46, 5485–5494. [Google Scholar] [CrossRef]
- Li, Y.; Datta, D.; Li, Z.; Shenoy, V.B. Mechanical properties of hydrogen functionalized graphene allotropes. Comput. Mater. Sci. 2014, 83, 212–216. [Google Scholar] [CrossRef]
- Budarapu, P.R.; Javvaji, B.; Sutrakar, V.K.; Mahapatra, D.R.; Paggi, M.; Zi, G.; Rabczuk, T. Lattice orientation and crack size effect on the mechanical properties of Graphene. Int. J. Fract. 2017, 203, 81–98. [Google Scholar] [CrossRef]
- Rajasekaran, G.; Kumar, R.; Parashar, A. Tersoff potential with improved accuracy for simulating graphene in molecular dynamics environment. Mater. Res. Express 2016, 3, 035011. [Google Scholar] [CrossRef]
- Zhurkov, S.N. Kinetic concept of the strength of solids. Int. J. Fract. 1984, 26, 295–307. [Google Scholar] [CrossRef]
- Halsey, G.; White, H.J.; Eyring, H. Mechanical Properties of Textiles, I. Text. Res. J. 1945, 15, 295–311. [Google Scholar] [CrossRef]
- Elapolu, M.S.R.; Tabarraei, A. Mechanical and Fracture Properties of Polycrystalline Graphene with Hydrogenated Grain Boundaries. J. Phys. Chem. C 2021, 125, 11147–11158. [Google Scholar] [CrossRef]
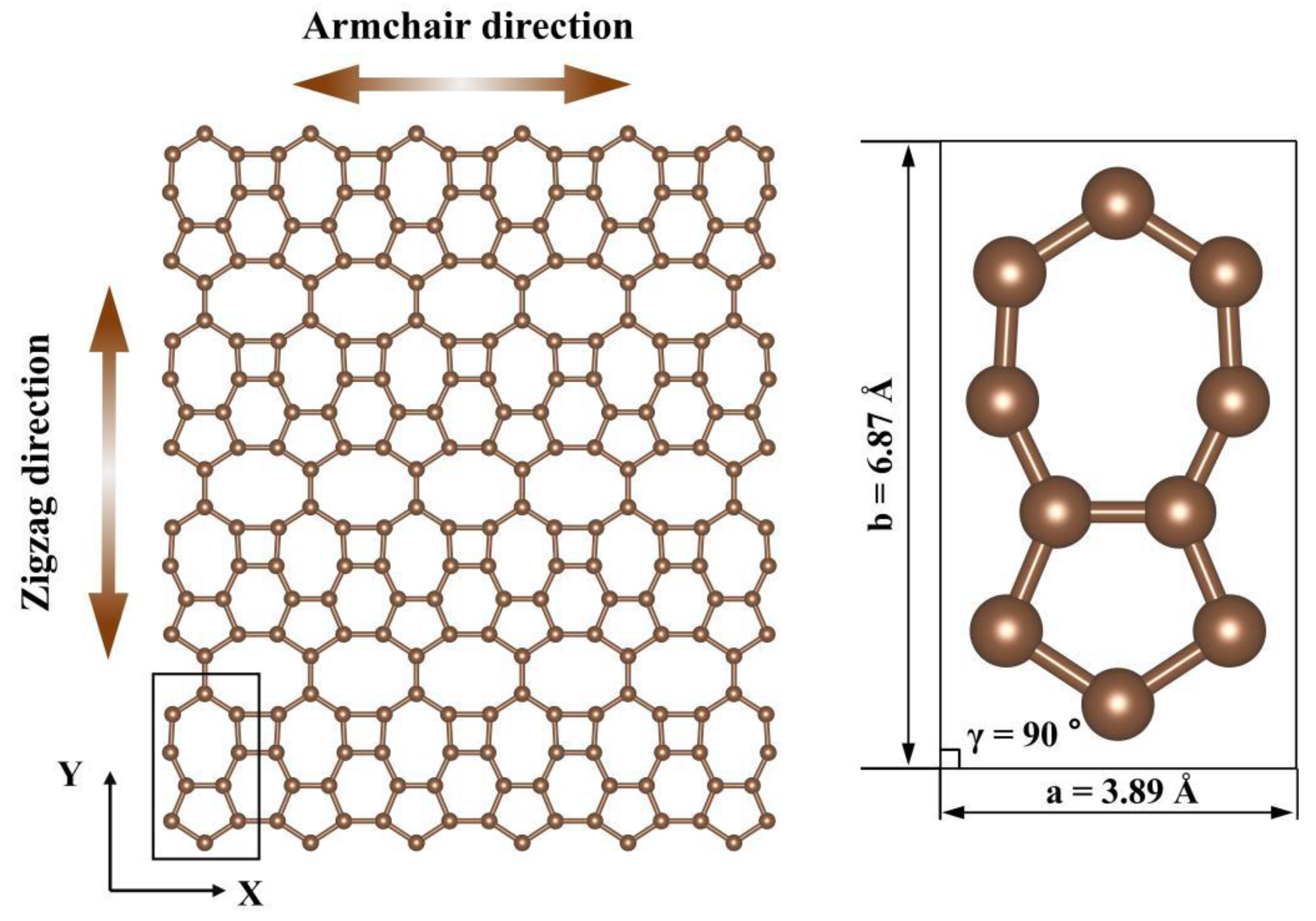
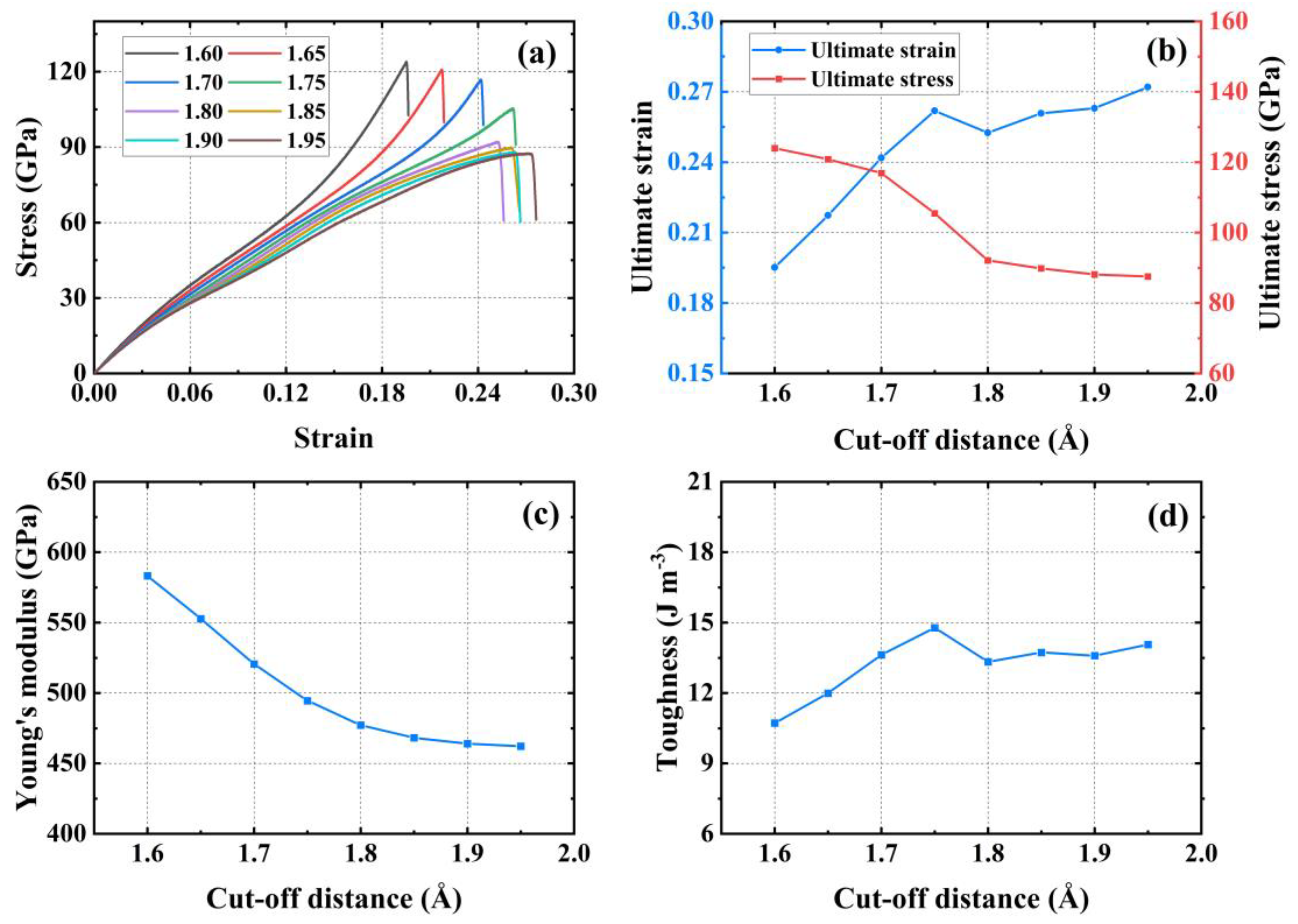
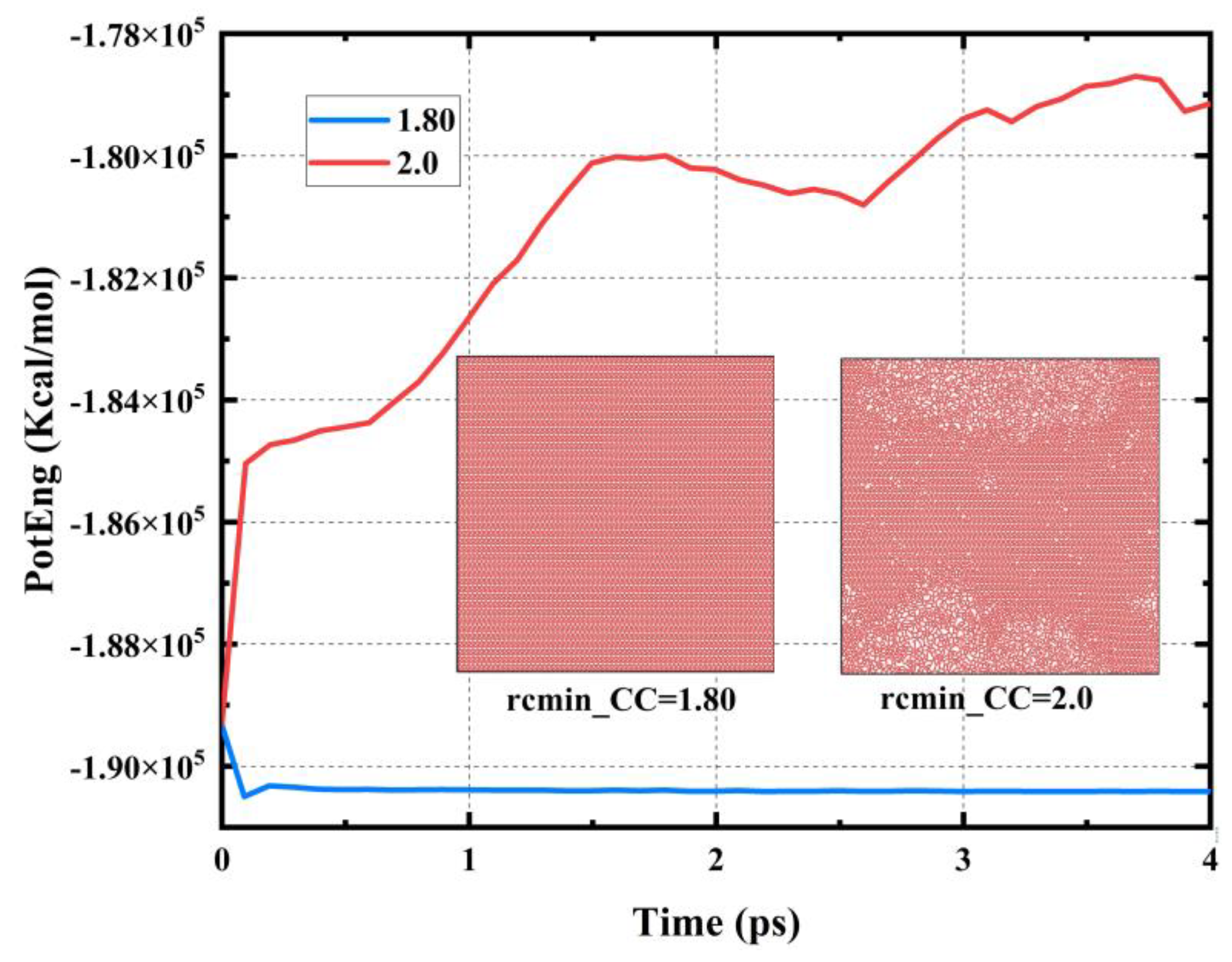

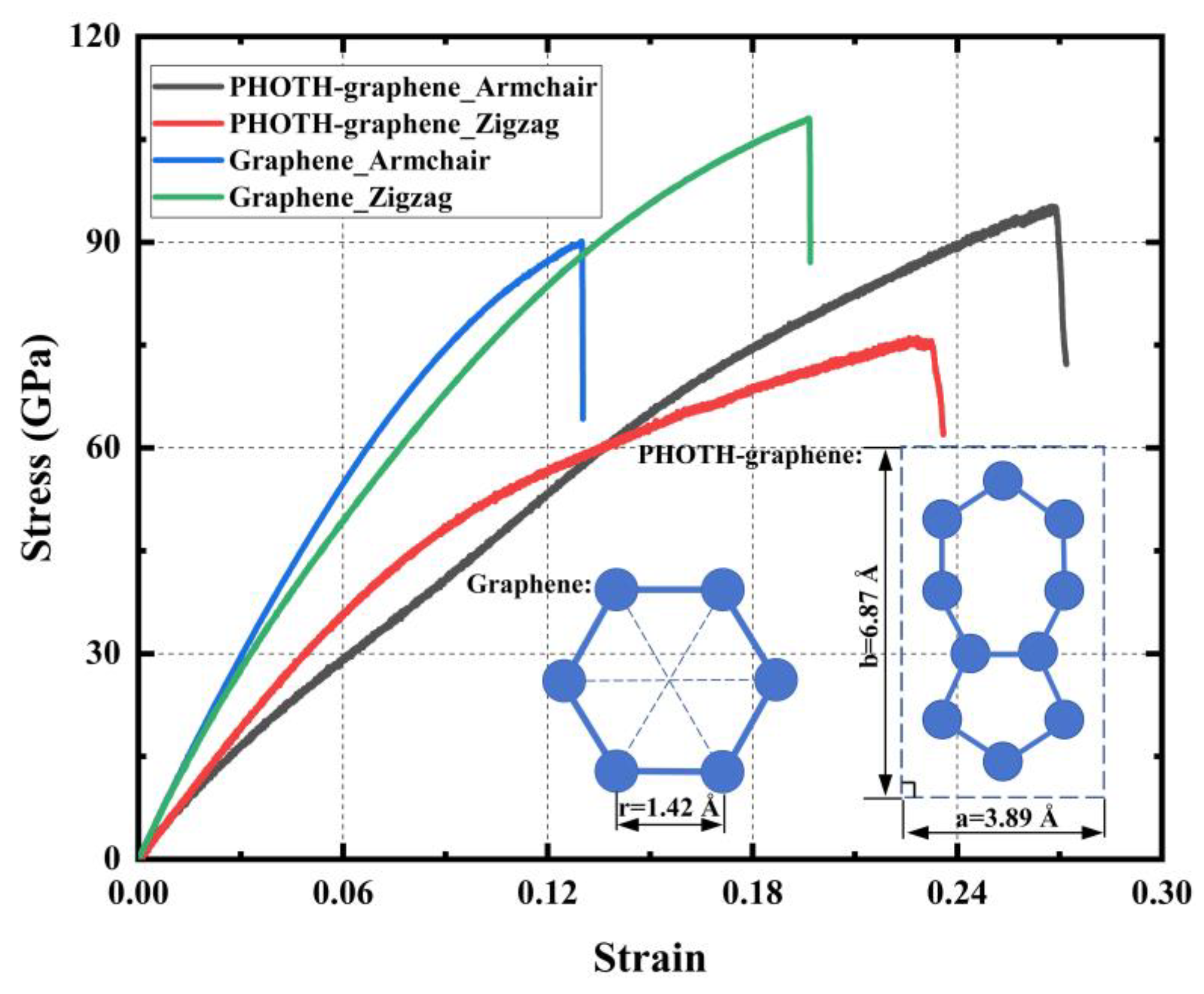


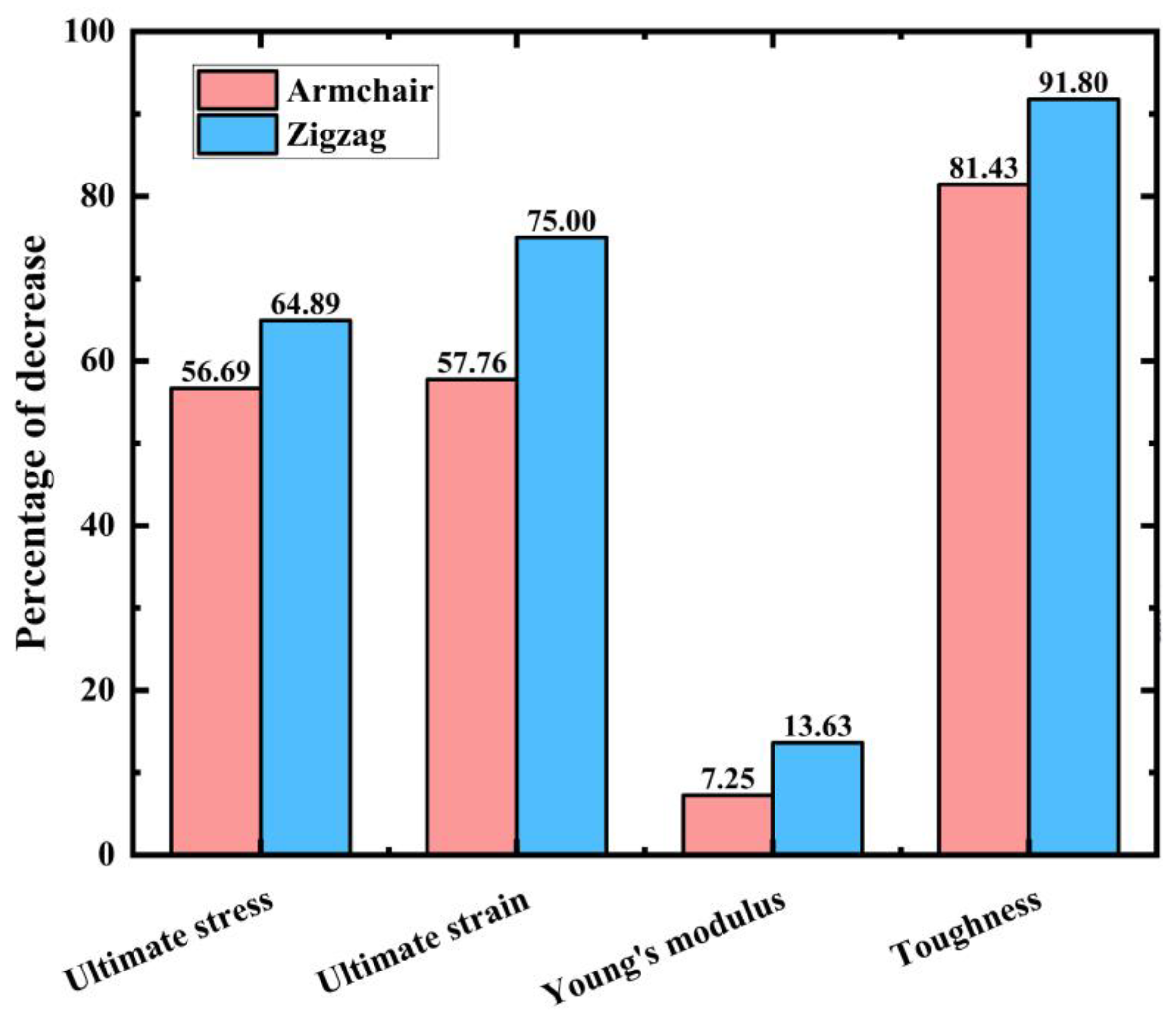



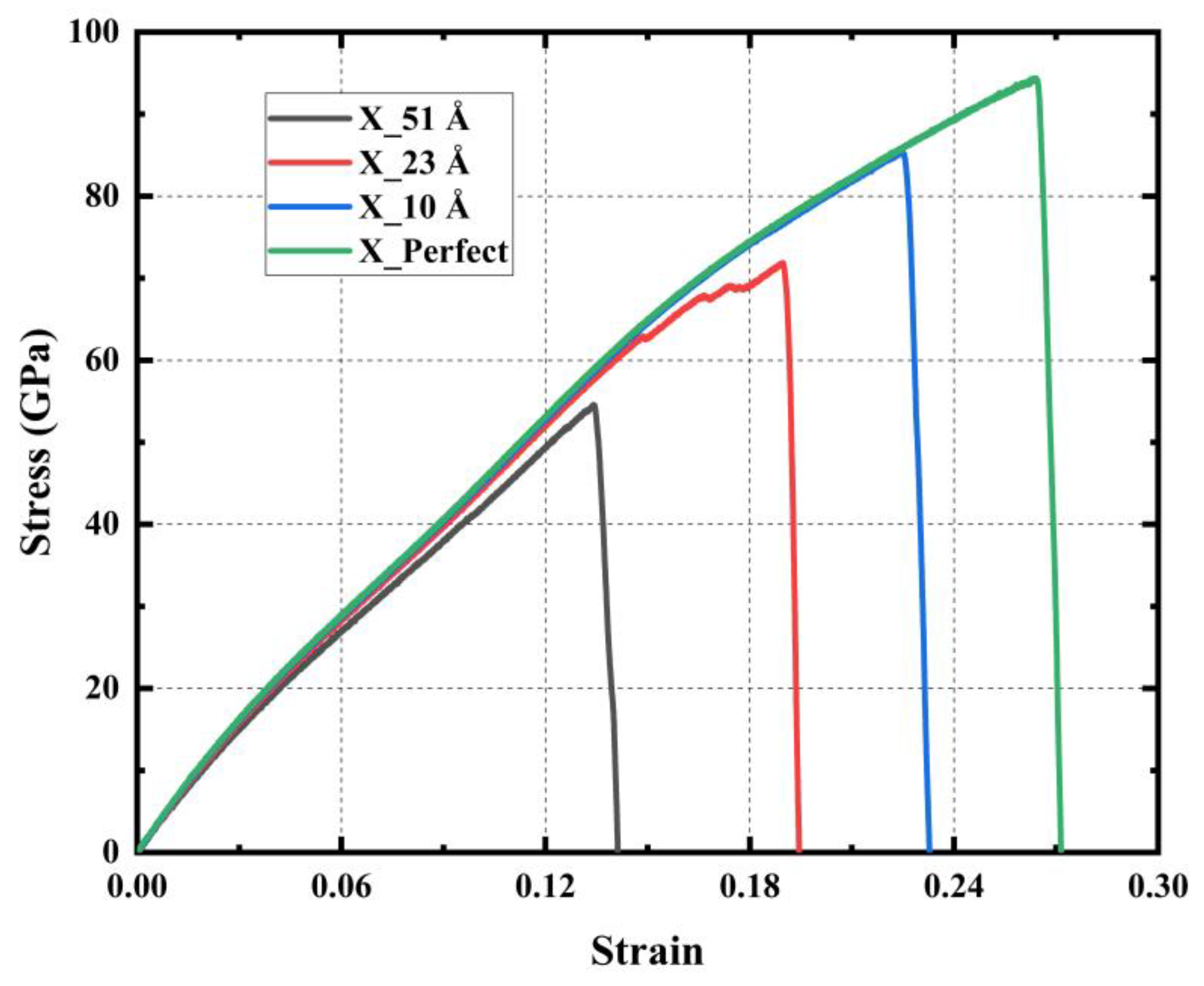

| Tensile Direction | Ultimate Stress (GPa) | Ultimate Strain | Young’s Modulus (GPa) | Toughness (J m−3) |
|---|---|---|---|---|
| Along zigzag | 73.14 | 0.211 | 602.16 | 10.56 |
| Along armchair | 94.38 | 0.264 | 477.38 | 14.41 |
| Type of Crack | Ultimate Stress (GPa) | Ultimate Strain | Young’s Modulus (GPa) | Toughness (J m−3) |
|---|---|---|---|---|
| Perfect | 94.38 | 0.264 | 477.38 | 14.41 |
| 10 Å | 85.43 | 0.225 | 476.65 | 10.79 |
| 23 Å | 71.88 | 0.189 | 470.39 | 7.72 |
| 51 Å | 54.59 | 0.134 | 461.88 | 3.88 |
| 51 Å_4H | 50.95 | 0.130 | 460.87 | 3.64 |
| 51 Å_8H | 48.88 | 0.132 | 460.50 | 3.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Q.; Chen, G.; Huang, Z.; Chen, X.; Li, A.; Cai, X.; Zhang, Y.; Chen, X.-J.; Hu, Z. Molecular Dynamics Insights into Mechanical Stability, Elastic Properties, and Fracture Behavior of PHOTH-Graphene. Materials 2024, 17, 4740. https://doi.org/10.3390/ma17194740
Peng Q, Chen G, Huang Z, Chen X, Li A, Cai X, Zhang Y, Chen X-J, Hu Z. Molecular Dynamics Insights into Mechanical Stability, Elastic Properties, and Fracture Behavior of PHOTH-Graphene. Materials. 2024; 17(19):4740. https://doi.org/10.3390/ma17194740
Chicago/Turabian StylePeng, Qing, Gen Chen, Zeyu Huang, Xue Chen, Ao Li, Xintian Cai, Yuqiang Zhang, Xiao-Jia Chen, and Zhongwei Hu. 2024. "Molecular Dynamics Insights into Mechanical Stability, Elastic Properties, and Fracture Behavior of PHOTH-Graphene" Materials 17, no. 19: 4740. https://doi.org/10.3390/ma17194740







