The Influence of Selected Titanium Alloy Micro-Texture Parameters on Bacterial Adhesion
Abstract
:1. Introduction
2. Materials and Methods
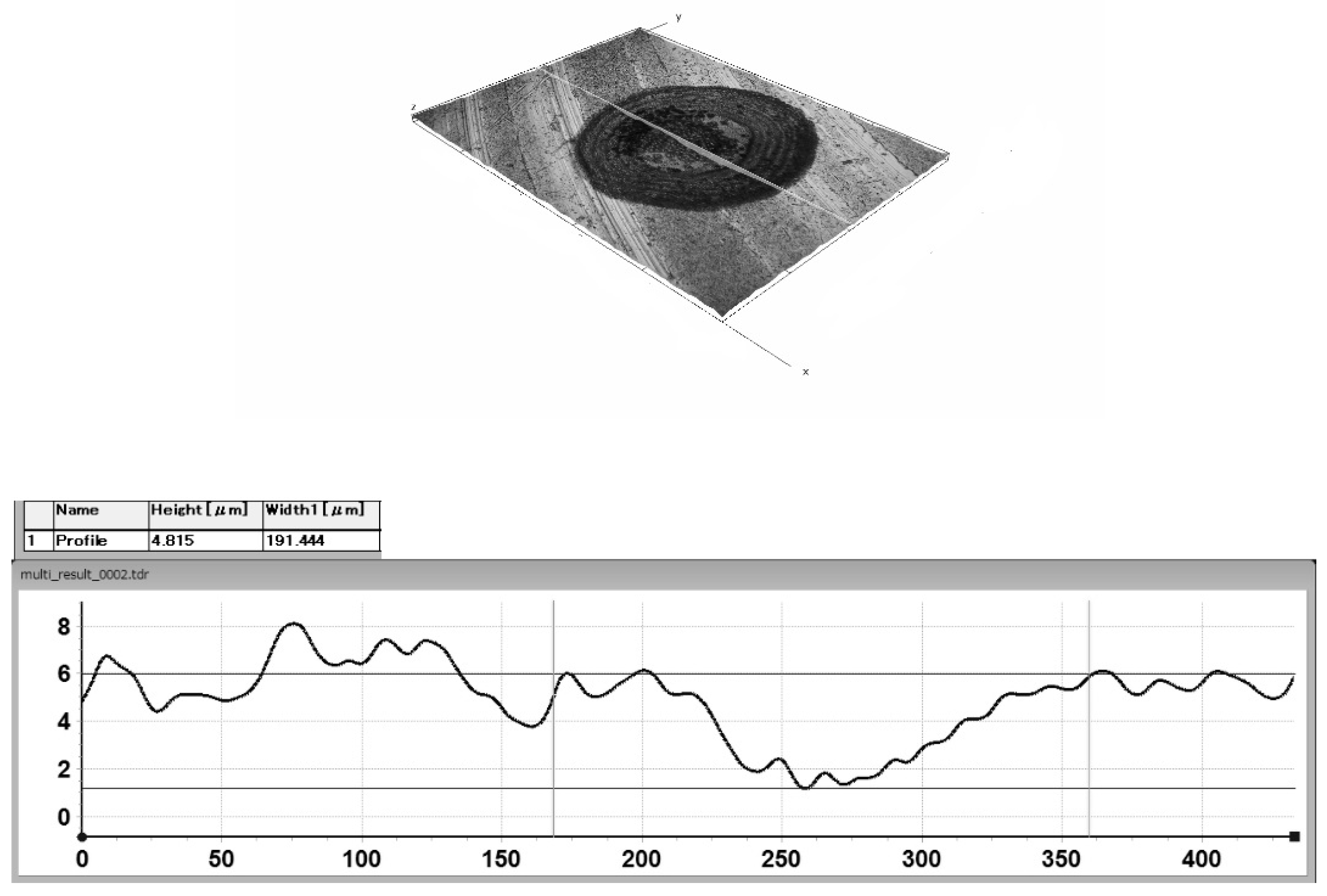
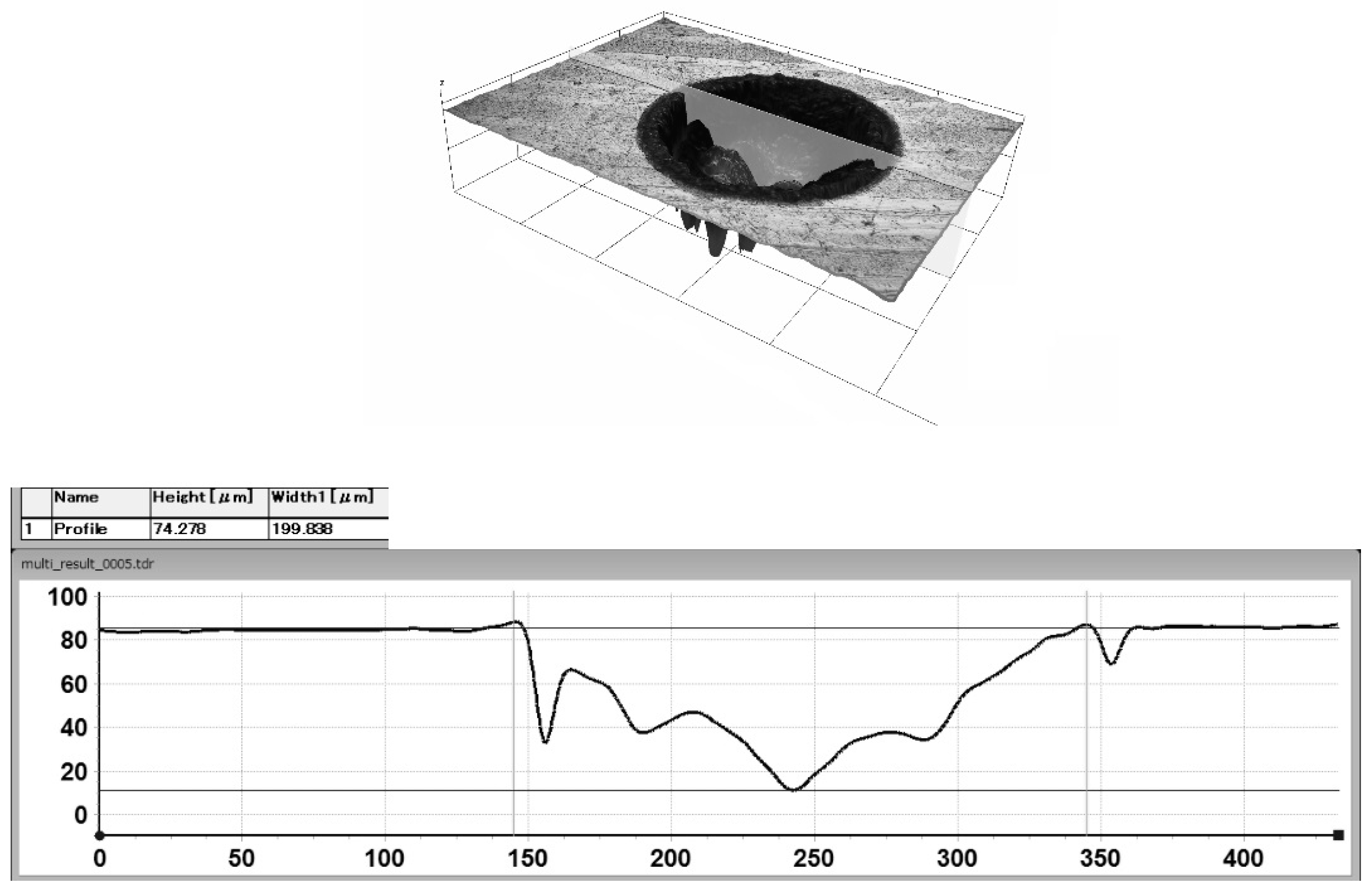
2.1. Laser Surface Texture Preparation
2.2. Fungal and Bacterial Cell Culture and Assessment of Different Species of Oral Microbiota Colonization and Biofilm Formation
Preparation of Microorganisms for Analysis
2.3. Statistical Analysis
3. Results and Discussion
3.1. The Microbial Reference Strains Representing Oral Microbiota Colonization and Biofilm Formation
3.2. The Dependence of the Number of Adhering Microorganisms on Texture Geometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szpak, P.; Szymańska, J. The survival of dental implants with different implant-abutment connection systems. Curr. Issues Pharm. Med. Sci. 2016, 29, 11–13. [Google Scholar] [CrossRef]
- Larsen, T.; Fiehn, N.E. Dental biofilm infections-an Update. APMIS 2017, 125, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Meto, A.; Colombari, B.; Castagnoli, A.; Sarti, M.; Denti, L.; Blasi, E. Efficacy of a Copper–Calcium–Hydroxide Solution in Reducing Microbial Plaque on Orthodontic Clear Aligners: A Case Report. Eur. J. Dent. 2019, 13, 478–484. [Google Scholar] [CrossRef]
- Lasserre, J.F.; Brecx, M.C.; Toma, S. Oral microbes, biofilms and their role in periodontal and peri-implant diseases. Materials 2018, 11, 1802. [Google Scholar] [CrossRef]
- Shrivastava, D.; Srivastava, K.C.; Ganji, K.K.; Alam, M.K.; Al Zoubi, I.; Sghairee, M.G. Quantitative assessment of gingival inflammation in patients undergoing nonsurgical periodontal therapy using photometric CIELab Analysis. BioMed. Res. Int. 2021, 30, 6615603. [Google Scholar] [CrossRef]
- Shrivastava, D.; Srivastava, K.C.; Dayakara, J.K.; Sghaireen, M.G.; Gudipaneni, R.K.; Al-Johani, K.; Baig, M.N.; Khurshid, Z. Bactericidal Activity of Crevicular Polymorphonuclear Neutrophils in Chronic Periodontitis Patients and Healthy Subjects under the Influence of Areca Nut Extract: An In Vitro Study. Appl. Sci. 2020, 10, 5008. [Google Scholar] [CrossRef]
- Dhir, S. Biofilm and dental implant: The microbial link. J. Indian Soc. Periodontol. 2013, 17, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Charalampakis, G.; Belibasakis, G.N. Microbiome of peri-implant infections: Lessons from conventional, molecular and metagenomic analyses. Virulence 2015, 6, 183–187. [Google Scholar] [CrossRef]
- Sahrmann, P.; Gilli, F.; Wiedemeier, D.B.; Attin, T.; Schmidlin, P.R.; Karygianni, L. The microbiome of peri-implantitis: A systematic review and meta-analysis. Microorganisms 2020, 8, 661. [Google Scholar] [CrossRef]
- Siddiqui, D.A.; Fidai, A.B.; Natarajan, S.G.; Rodrigues, D.C. Succession of oral bacterial colonizers on dental implant materials: An in vitro biofilm model. Dent. Mater. 2022, 38, 384–396. [Google Scholar] [CrossRef]
- Mombelli, A.; Lang, N.P. Microbial aspects of implant dentistry. Periodontol. 2000 1994, 4, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Korsch, M.; Marten, S.M.; Stoll, D.; Prechtl, C.; Dötsch, A. Microbiological findings in early and late implant loss: An observational clinical case-controlled study. BMC Oral Health 2021, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, X.; Gao, X.; Chen, F.; Liang, X.; Li, D. Effects of surface properties of polymer-based restorative materials on early adhesion of Streptococcus mutans in vitro. J. Dent. 2016, 54, 33–40. [Google Scholar] [CrossRef]
- Nakanishi, E.Y.; Palacios, J.H.; Godbout, S.; Fournel, S. Interaction between Biofilm Formation, Surface Material and Cleanability Considering Different Materials Used in Pig Facilities—An Overview. Sustainability 2021, 13, 5836. [Google Scholar] [CrossRef]
- Ferraris, S.; Cochis, S.A.; Scalia, A.C.; Tori, A.; Rimondini, L.; Spriano, S. Laser surface texturing of Ti-cp and Ti6Al4V alloy for the improvement of fibroblast adhesion and alignment and the reduction of bacterial adhesion. J. Mater. Res. Technol. 2024, 29, 5464–5472. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Xu, Y.; Zhang, X.; Chen, C.; Geng, P.; Ma, N. Effect of nanosecond pulsed laser parameters on texturing formation of metallic surface: Experiment and modelling. J. Mater. Res. Technol. 2023, 26, 7775–7788. [Google Scholar] [CrossRef]
- Costa, R.C.; Nagay, B.E.; Bertolini, M.; Costa-Oliveira, B.E.; Sampaio, A.A.; Retamal-Valdes, B.; Shibli, J.A.; Feres, M.; Barão, V.A.R.; Souza, J.G.S. Fitting pieces into the puzzle: The impact of titanium-based dental implant surface modifications on bacterial accumulation and polymicrobial infections. Adv. Colloid Interface Sci. 2021, 298, 102551. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Krzywicka, M.; Szymańska, J.; Tofil, S.; Malm, A.; Grzegorczyk, A. Surface Properties of Ti6Al7Nb Alloy: Surface Free Energy and Bacteria Adhesion. J. Funct. Biomater. 2022, 13, 26. [Google Scholar] [CrossRef]
- Corsaro, C.; Orlando, G.; Costa, G.; Latino, M.; Barreca, F.; Mezzasalma, A.M.; Neri, F.; Fazio, E. Wetting Behavior Driven by Surface Morphology Changes Induced by Picosecond Laser Texturing. Materials 2024, 17, 1719. [Google Scholar] [CrossRef]
- Voisey, K.T.; Scotchford, C.A.; Martin, L.; Gill, H.S. Effect of Q-switched Laser Surface Texturing of Titanium on Osteoblast Cell Response. Phys. Procedia 2014, 56, 1126–1135. [Google Scholar] [CrossRef]
- Microbe Notes. Available online: https://microbenotes.com (accessed on 24 September 2024).
- Wood, B.J.B.; Holzapfel, W.H. Genera of Lactic Acid Bacteria; Blackie Academic and Professional: London, UK, 1995; p. 420. ISBN 075140215X. [Google Scholar]
- Singh, S.M.; George, A.; Tiwari, J.; Ramkumar, K. Balani Influence of laser surface texturing on the wettability and antibacterial properties of metallic, ceramic, and polymeric surfaces. J. Mater. Res. 2021, 36, 398–3999. [Google Scholar] [CrossRef]
- Esfahanizadeh, N.; Mirmalek, N.; Bahador, A.; Daneshparvar, H.; Akhoundi, N.; Pourhajibagher, M. Formation of biofilm on various implant abutment materials. Gen. Dent. 2018, 65, 39–44. [Google Scholar]
- Uhlmann, E.; Schweitzer, L.; Kieburg, H.; Spielvogel, A.; Huth-Herms, K. The effects of laser microtexturing of biomedical Grade 5 Ti-6Al-4V dental implants (Abutment) on biofilm formation. Procedia CIRP 2018, 68, 184–189. [Google Scholar] [CrossRef]
- Grössner-Schreiber, B.; Griepentrog, M.; Haustein, I.; Müller, W.-D.; Briedigkeit, H.; Göbel, U.B.; Lange, K.-P. Plaque formation on surface modified dental implants. Clin. Oral Implant. Res. 2001, 12, 543–551. [Google Scholar] [CrossRef]
- Hauser-Gerspach, I.; Mauth, C.; Waltimo, T.; Meyer, J.; Stübinger, S. Effects of Er:YAG laser on bacteria associated with titanium surfaces and cellular response in vitro. Laser Med. Sci. 2014, 29, 1329–1337. [Google Scholar] [CrossRef]
- Du, C.; Wang, C.; Zhang, T.; Zheng, L. Antibacterial performance of Zr-BMG, stainless steel, and titanium alloy with laser-induced periodic surface structures ACS. Appl. Bio. Mater. 2022, 5, 272–284. [Google Scholar] [CrossRef]
- Parmar, V.; Kumar, A.; Mani Sankar, M.; Datta, S.; Vijaya Prakash, G.; Mohanty, S.; Kalyanasundaram, D. Oxidation facilitated antimicrobial ability of laser micro-textured titanium alloy against gram-positive Staphylococcus aureus for biomedical applications. J. Laser Appl. 2018, 30, 032001. [Google Scholar] [CrossRef]
- Shiju, V.P.; Abhijith, N.V.; Sudeep, U. Experimental study on the influence of hydrophilicity on bacterial adhesion in bioimplants. J. Phys: Conf. Ser. 2019, 1355, 012028. [Google Scholar] [CrossRef]
- Doll, K.; Fadeeva, E.; Stumpp, N.S.; Grade, S.; Chichkov, B.N.; Stiesch, M. Reduced bacterial adhesion on titanium surfaces micro-structured by ultra-short pulsed laser ablation. BioNanoMaterials 2016, 17, 53–57. [Google Scholar] [CrossRef]
- Eghbali, N.; Naffakh-Moosavy, H.; Sadeghi Mohammadi, S.; Naderi-Manesh, H. The influence of laser frequency and groove distance on cell adhesion, cell viability, and antibacterial characteristics of Ti-6Al-4V dental implants treated by modern fiber engraving laser. Dent. Mater. 2021, 37, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Chik, N.; Wan, W.S.; Zain, M.d.; Mohamad, A.J.; Sidek, M.Z.; Wan, W.H.; Reif, A.; Rakebrandt, J.H.; Pfleging, W.; Liu, X. Bacterial Adhesion on the Titanium and Stainless-Steel Surfaces Undergone Two Different Treatment Methods: Polishing and Ultrafast Laser Treatment. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012034. [Google Scholar] [CrossRef]
- Zwahr, C.; Helbig, R.; Werner, C.; Lasagni, A.F. Fabrication of multifunctional titanium surfaces by producing hierarchical surface patterns using laser based ablation methods. Sci. Rep. 2019, 9, 6721. [Google Scholar] [CrossRef]
- Yao, W.L.; Lin, J.C.Y.; Salamanca, E.; Pan, Y.-H.; Tsai, P.-Y.; Leu, S.-J.; Yang, K.-C.; Huang, H.-M.; Huang, H.-Y.; Chang, W.-J. Er,Cr:YSGG Laser Performance Improves Biological Response on Titanium Surfaces. Materials 2020, 13, 756. [Google Scholar] [CrossRef] [PubMed]
- Orazi, L.; Pogorielov, M.; Deineka, V.; Husak, V.; Korniienko, O.; Mishchenko, O.; Reggiani, B. Osteoblast cell response to LIPSS-modified Ti-implants. Key Eng. Mater. 2019, 813, 322–327. [Google Scholar] [CrossRef]
- Meinshausen, A.K.; Herbster, M.; Zwahr, C.; Soldera, M.; Müller, A.; Halle, T.; Lasagni, A.F.; Bertrand, J. Aspect ratio of nano/microstructures determines Staphylococcus aureus adhesion on PET and titanium surfaces. J. Appl. Microbiol. 2021, 131, 1498–1514. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yao, S.; Zhang, H.; Cai, M.; Liu, W.; Pan, R.; Chen, C.; Wang, X.; Wang, L.; Zhong, M. Biocompatible nano-ripples structured surfaces induced by femtosecond laser to rebel bacterial colonization and biofilm formation. Opt. Laser Technol. 2020, 124, 105973. [Google Scholar] [CrossRef]
- Donaghy, C.L.; McFadden, R.; Smith, G.C.; Kelaini, S.; Carson, L.; Malinov, S.; Margariti, A.; Chan, C.-W. Fibre Laser Treatment of Beta TNZT Titanium Alloys for Load-Bearing Implant Applications: Effects of Surface Physical and Chemical Features on Mesenchymal Stem Cell Response and Staphylococcus aureus Bacterial Attachment. Coatings 2019, 9, 186. [Google Scholar] [CrossRef]
| Fe | O | N | C | H | Al | Nb | Ta | Ti |
|---|---|---|---|---|---|---|---|---|
| max. 0.25% | max. 0.2% | max. 0.05% | max. 0.08% | max. 0.009% | 5.5–6.5% | 6.5–7.5% | max. 0.5% | rest |
| Microbial Species | Sample | Biofilm Size | |
|---|---|---|---|
| CFU/mL | log CFU/mL | ||
| C. albicans ATCC 10231 | Model | 0.75 × 104 ± 0.13 × 104 | 3.88 ± 3.11 |
| 10%, 5 μm | 0.8 × 104 ± 0.15 × 104 | 3.90 ± 3.17 | |
| 10%, 78 μm | 2.0 × 104 ± 0.36 × 104 | 4.30 ± 3.55 | |
| 50%, 5 μm | 1.7 × 104 ± 0.53 × 104 | 4.23 ± 3.72 | |
| S. mutans ATCC 25175 | Model | 0.61 × 104 ± 0.23 × 104 | 3.78 ± 3.36 |
| 10%, 5 μm | 2.50 × 104 ± 0.36 × 104 | 4.39 ± 3.55 | |
| 10%, 78 μm | 4.36 × 104 ± 1.7 × 104 | 4.63 ± 4.23 | |
| 50%, 5 μm | 3.05 × 104 ± 1.9 × 104 | 4.48 ± 4.28 | |
| L. rhamnosus ATCC 53103 | Model | 2.13 × 107 ± 1.09 × 107 | 7.32 ± 7.03 |
| 10%, 5 μm | 4.47 × 107 ± 2.4 × 107 | 7.65 ± 7.38 | |
| 10%, 78 μm | 3.36 × 107 ± 1.25 × 107 | 7.53 ± 7.10 | |
| 50%, 5 μm | 2.77 × 107 ± 1.5 × 107 | 7.44 ± 7.17 | |
| F. nucleatum ATCC 25586 | Model | 0.27 × 106 ± 0.10 × 106 | 5.43± 5.00 |
| 10%, 5 μm | 0.48 × 106 ± 0.19 × 106 | 5.68 ± 5.28 | |
| 10%, 78 μm | 0.56 × 106 ± 0.19 ×106 | 5.74 ± 5.28 | |
| 50%, 5 μm | 2.96 × 106 ± 0.94 × 106 | 6.47 ± 5.97 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska, J.; Krzywicka, M.; Kobus, Z.; Malm, A.; Grzegorczyk, A. The Influence of Selected Titanium Alloy Micro-Texture Parameters on Bacterial Adhesion. Materials 2024, 17, 4765. https://doi.org/10.3390/ma17194765
Szymańska J, Krzywicka M, Kobus Z, Malm A, Grzegorczyk A. The Influence of Selected Titanium Alloy Micro-Texture Parameters on Bacterial Adhesion. Materials. 2024; 17(19):4765. https://doi.org/10.3390/ma17194765
Chicago/Turabian StyleSzymańska, Jolanta, Monika Krzywicka, Zbigniew Kobus, Anna Malm, and Agnieszka Grzegorczyk. 2024. "The Influence of Selected Titanium Alloy Micro-Texture Parameters on Bacterial Adhesion" Materials 17, no. 19: 4765. https://doi.org/10.3390/ma17194765









