The Performance of Partial Least Squares Methods in Virtual Nanosensor Array—Multiple Metal Ions Sensing Based on Multispectral Fluorescence of Quantum Dots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Qualitative Analysis Experiments
2.3. Quantitative Analysis Experiments
2.4. EEM Fluorescence Measurements
2.5. Data Pretreatment and Chemometric Analysis
2.6. Software
3. Results
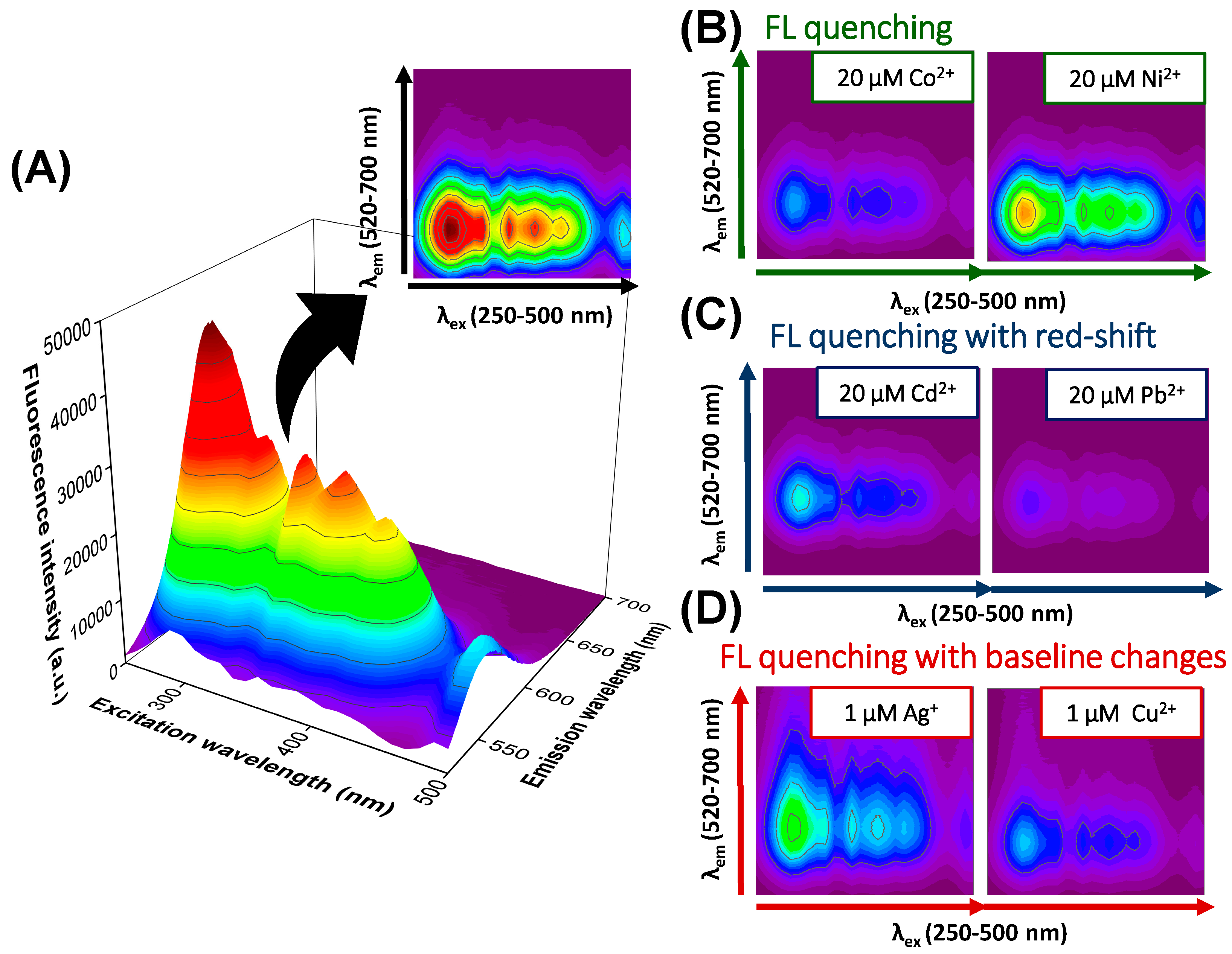
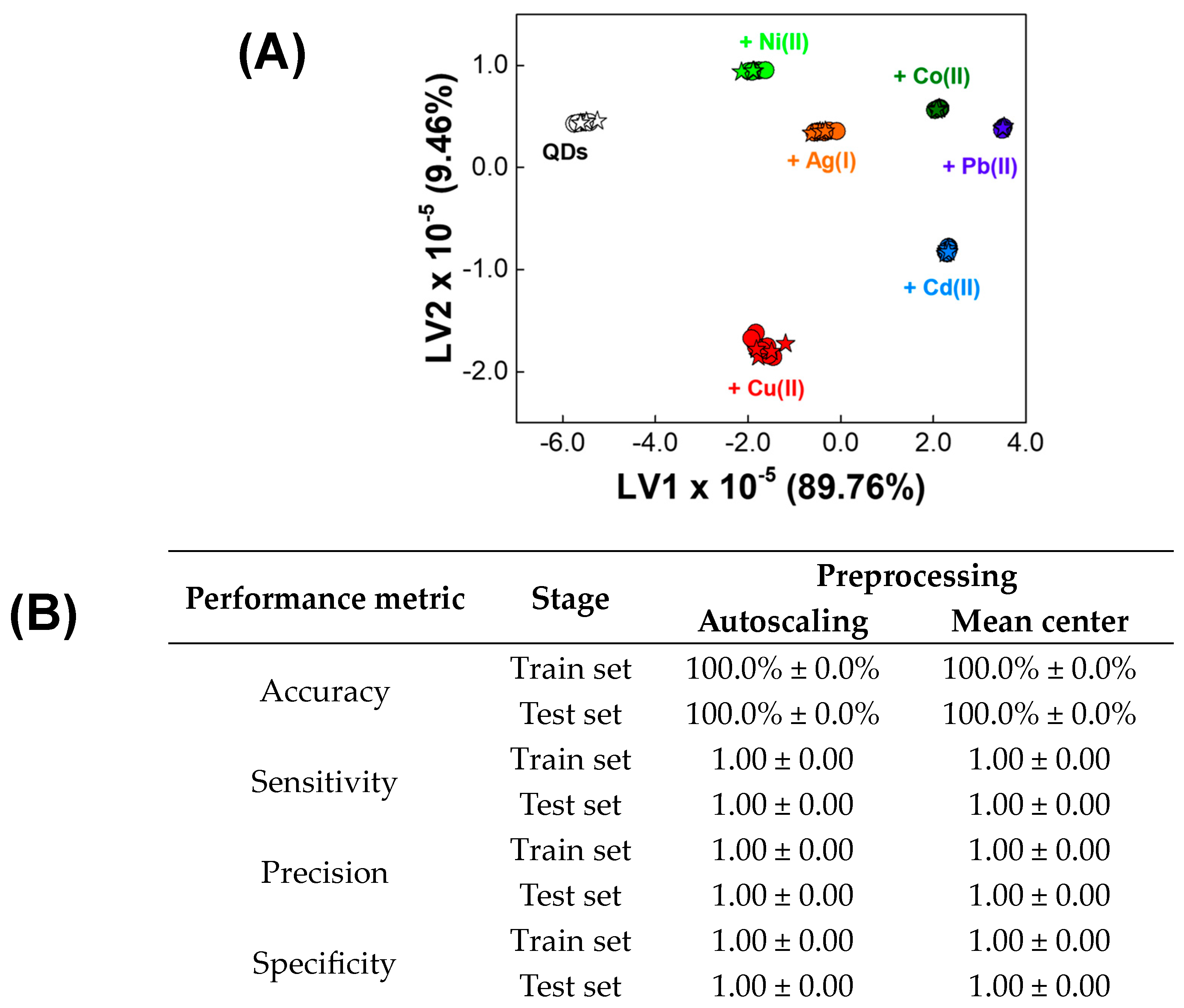
3.1. Qualitative Analysis of Metal Ions
3.2. Quantitative Analysis of Metal Ions
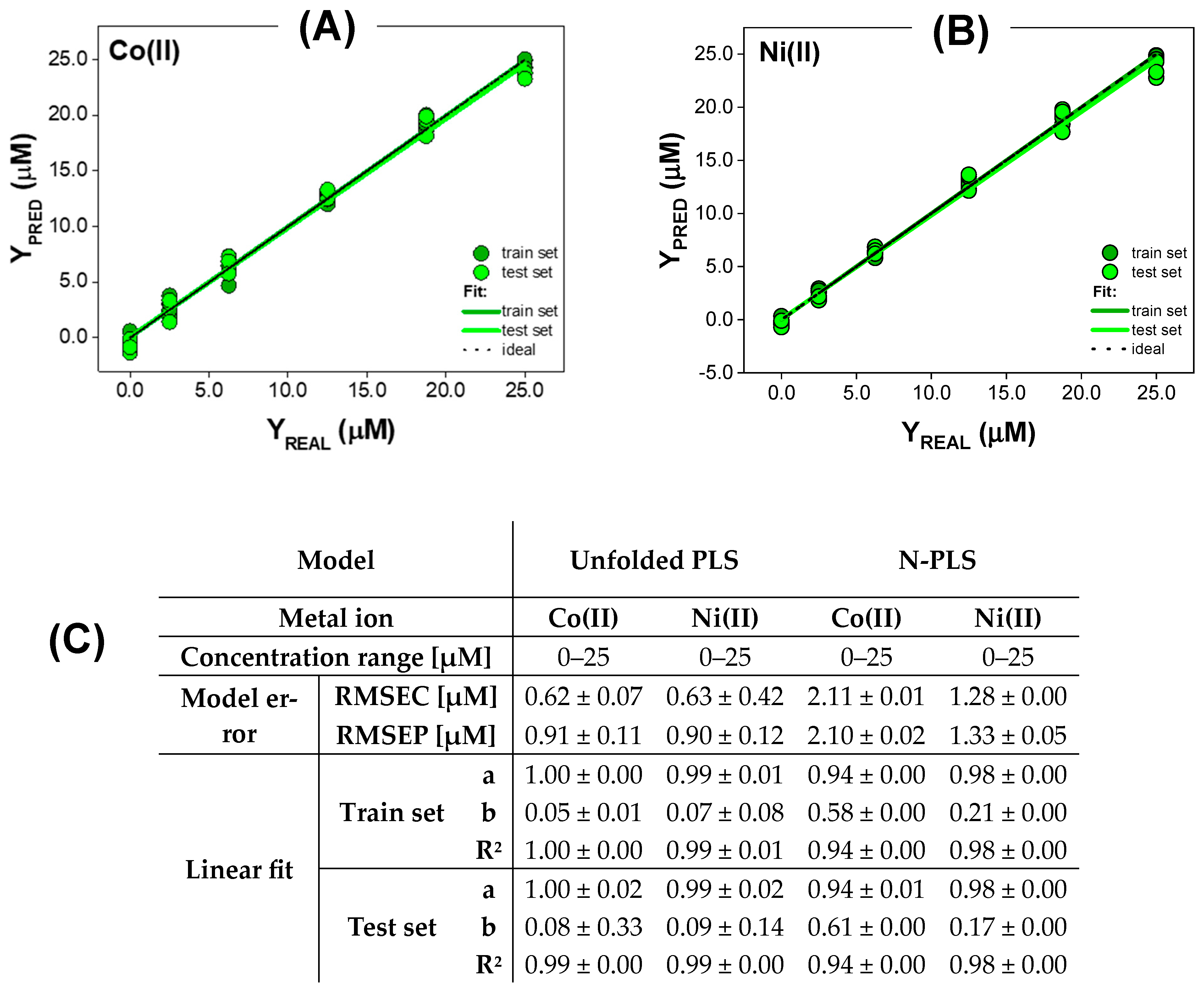
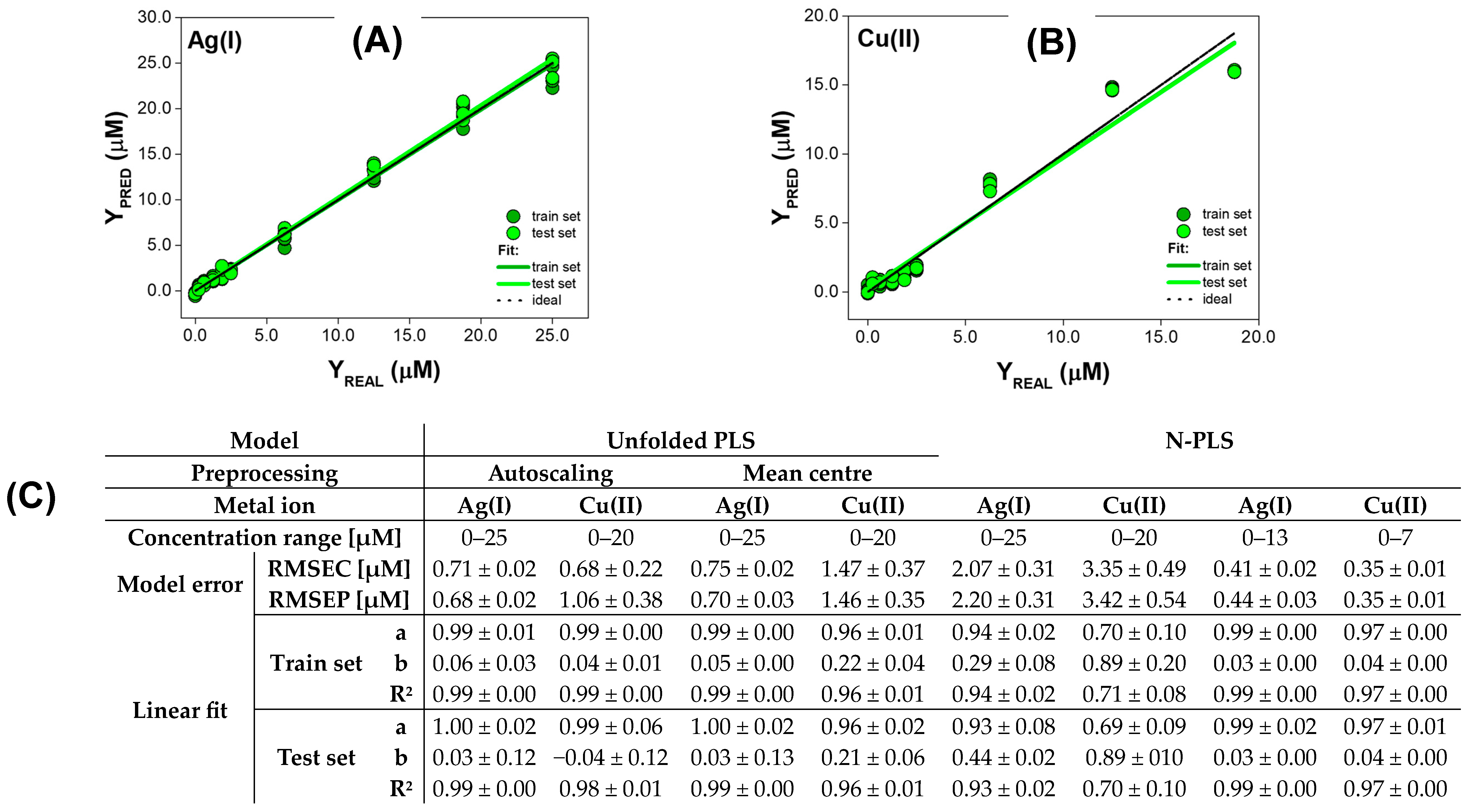
3.2.1. Quantitative Analysis for Metal Ions Only Quenching QDs Fluorescence
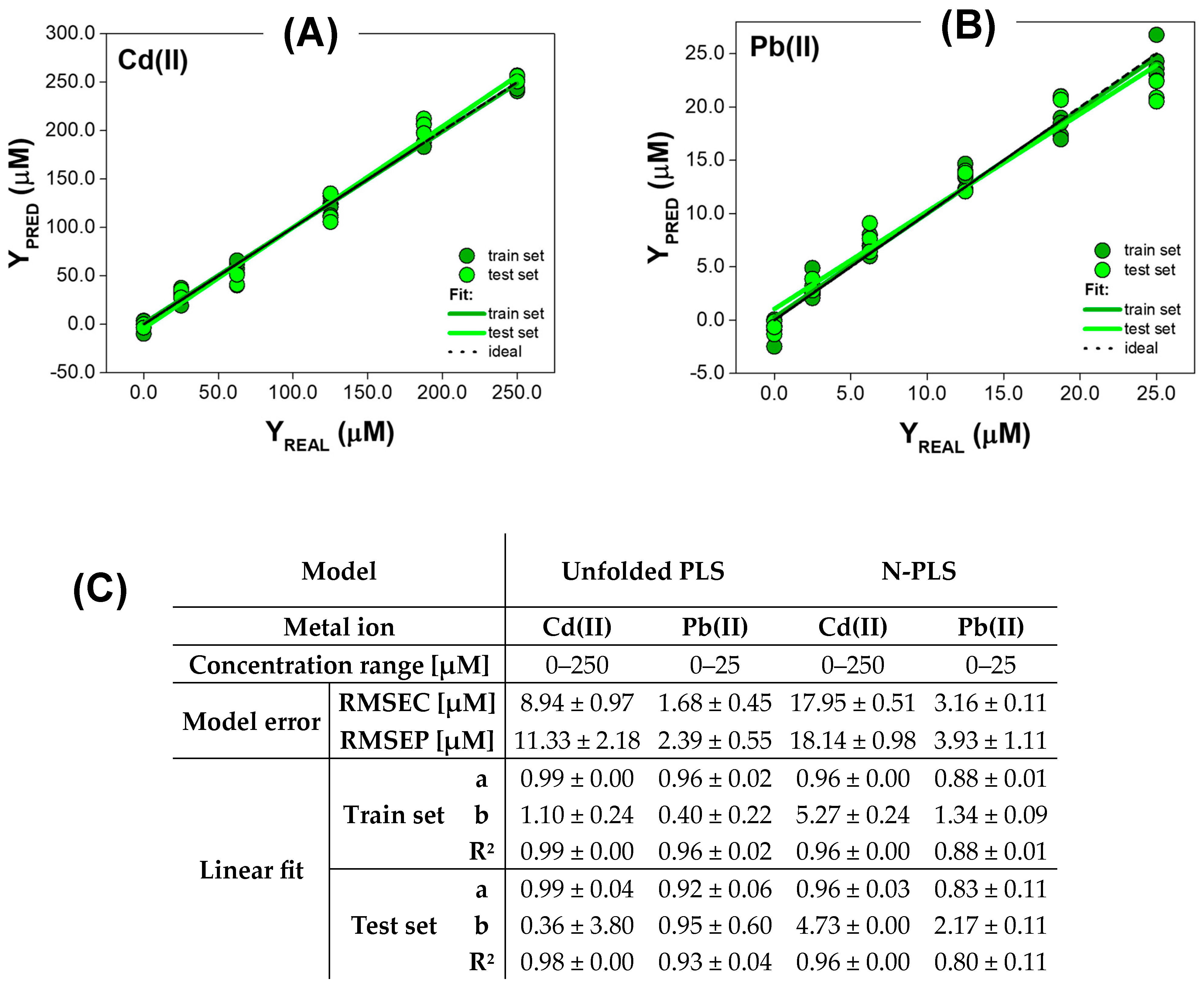
3.2.2. Quantitative Analysis for Metal Ions Quenching QDs Fluorescence with Red-Shift
3.2.3. Quantitative Analysis for Metal Ions Quenching QDs Fluorescence with Baseline Changes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petryayeva, E.; Algar, W.R.; Medintz, I.L. Quantum dots in bioanalysis: A review of applications across various platforms for fluorescence spectroscopy and imaging. Appl. Spectrosc. 2013, 67, 215–252. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Zhao, Y.; Chen, J.; Zhu, J.J. Metal ions optical sensing by semiconductor quantum dots. J. Mater. Chem. C 2014, 2, 595–613. [Google Scholar] [CrossRef]
- Algar, W.R.; Susumu, K.; Delehanty, J.B.; Medintz, I.L. Semiconductor quantum dots in bioanalysis: Crossing the valley of death. Anal. Chem. 2011, 83, 8826–8837. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.N.; Nagaraju, D.H.; Yhobu, Z.; Shivakumar, P.; Kumara, K.S.M.; Budagumpi, S.; Praveen, B.M. Recent advances in on-site monitoring of heavy metal ions in the environment. Microchem. J. 2022, 182, 107894. [Google Scholar] [CrossRef]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, W.; Ji, C.; Zhang, J.; Yin, M. Detection of metal ions in biological systems: A review. Rev. Anal. Chem. 2020, 39, 231–246. [Google Scholar] [CrossRef]
- Yoo, D.; Park, Y.; Cheon, B.; Park, M. Carbon Dots as an Effective Fluorescent Sensing Platform for Metal Ion Detection. Nanoscale Res. Lett. 2019, 14, 272. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, B.; Xiao, Y.; Tian, X.; Wang, Y. Fluorescent Quantum Dots and Its Composites for Highly Sensitive Detection of Heavy Metal Ions and Pesticide Residues: A Review. Chemosensors 2023, 11, 405. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Carrillo-Carrion, C. Analytical strategies based on quantum dots for heavy metal ions detection. J. Biomed. Opt. 2014, 19, 101503. [Google Scholar] [CrossRef]
- Yin, H.; Truskewycz, A.; Cole, I.S. Quantum dot (QD)-based probes for multiplexed determination of heavy metal ions. Microchim. Acta 2020, 187, 336. [Google Scholar] [CrossRef]
- Fargher, H.A.; d’Oelsnitz; Diaz, D.J.S.; Anslyn, E.V. Pushing Differential Sensing Further: The Next Steps in Design and Analysis of Bio-Inspired Cross-Reactive Arrays. Anal. Sens. 2023, 3, 2629–2742. [Google Scholar] [CrossRef]
- Umali, A.P.; Anslyn, E.V. A general approach to differential sensing using synthetic molecular receptors. Curr. Opin. Chem. Biol. 2010, 14, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Bittar, D.B.; Ribeiro, D.S.; Páscoa, R.N.; Soares, J.X.; Rodrigues, S.S.M.; Castro, R.C.; Pezza, L.; Pezza, H.R.; Santos, J.L. Multiplexed analysis combining distinctly-sized CdTe-MPA quantum dots and chemometrics for multiple mutually interfering analyte determination. Talanta 2017, 174, 572–580. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Fan, Q.-X.; Han, X.-Y.; Shi, G.; Zhang, M. Design of smart chemical ‘tongue’ sensor arrays for pattern-recognition-based biochemical sensing applications. TrAC Trends Anal. Chem. 2020, 124, 115794. [Google Scholar] [CrossRef]
- Jiang, Z.; Le, N.D.B.; Gupta, A.; Rotello, V.M. Cell surface-based sensing with metallic nanoparticles. Chem. Soc. Rev. 2015, 44, 4264. [Google Scholar] [CrossRef]
- Zabadaj, M.; Ciosek-Skibińska, P. Quantum dots—Assisted 2D fluorescence for pattern based sensing of amino acids, oligopeptides and neurotransmitters. Sensors 2019, 19, 3655. [Google Scholar] [CrossRef]
- Głowacz, K.; Drozd, M.; Ciosek-Skibińska, P. Excitation-emission fluorescence matrix acquired from glutathione capped CdSeS/ZnS quantum dots in combination with chemometric tools for pattern-based sensing of neurotransmitters. Microchim. Acta 2021, 188, 343. [Google Scholar] [CrossRef]
- Askim, J.R.; Mahmoudiab, M.; Suslick, K.S. Optical sensor arrays for chemical sensing: The optoelectronic nose. Chem. Soc. Rev. 2013, 42, 8649–8682. [Google Scholar] [CrossRef]
- Głowacz, K.; Wawrzyniak, U.E.; Ciosek-Skibińska, P. Comparison of various data analysis techniques applied for the classification of oligopeptides and amino acids by voltammetric electronic tongue. Sens. Actuators B 2021, 331, 129354. [Google Scholar] [CrossRef]
- Kitamura, M.; Shabbir, S.H.; Anslyn, E.V. Guidelines for pattern recognition using differential receptors and indicator displacement assays. J. Org. Chem. 2009, 74, 4479–4489. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.C.; Pascoa, R.N.M.J.; Saraiva, L.M.F.S.; Santos, J.L.M.; Ribeiro, D.S.M. Chemometric models for data processing in quantum dots-based photoluminescence methodologies. Coord. Chem. Rev. 2024, 502, 21560. [Google Scholar] [CrossRef]
- Jin, Y.; Duan, W.; Wo, F.; Wu, J. Two-Dimensional Fluorescent Strategy Based on Porous Silicon Quantum Dots for Metal-Ion Detection and Recognition. ACS Appl. Nano Mater. 2019, 2, 6110–6115. [Google Scholar] [CrossRef]
- Kolanowski, J.L.; Liu, F.; New, E.J. Fluorescent probes for the simultaneous detection of multiple analytes in biology. Chem. Soc. Rev. 2018, 47, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Miki, T.; Citterio, D.; Kubota, T.; Shindo, Y.; Kitamura, Y.; Oka, K.; Suzuki, K. Single Molecular Multianalyte (Ca2+, Mg2+) Fluorescent Probe and Applications to Bioimaging. J. Am. Chem. Soc. 2005, 127, 10798–10799. [Google Scholar] [CrossRef]
- Guo, J.; Fang, B.; Bai, H.; Wang, L.; Peng, B.; Qin, X.; Fu, L.; Yao, C.; Li, L.; Huang, W. Dual/Multi-responsive fluorogenic probes for multiple analytes in mitochondria: From design to applications. TrAC Trends Anal. Chem. 2022, 155, 116697. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Yan, X.-P. CdTe Quantum Dots (QDs) Based Kinetic Discrimination of Fe2+ and Fe3+, and CdTe QDs-Fenton Hybrid System for Sensitive Photoluminescent Detection of Fe2+. Anal. Chem. 2009, 81, 6252–6257. [Google Scholar] [CrossRef]
- Ma, Q.; Ha, E.; Yang, F.; Su, X. Synchronous determination of mercury (II) and copper (II) based on quantum dots-multilayer film. Anal. Chim. Acta 2011, 701, 60–65. [Google Scholar] [CrossRef]
- Bro, R. Multiway calibration. Multilinear PLS. J. Chemom. 1996, 10, 47–61. [Google Scholar] [CrossRef]
- Ballabio, D.; Consonni, V. Classification tools in chemistry. Part 1: Linear models. PLS-DA. Anal. Methods 2013, 5, 3790–3798. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Centering and scaling in component analysis. J. Chemom. 2003, 17, 16–33. [Google Scholar] [CrossRef]
- Ballabio, D.; Grisoni, F.; Todeschini, R. Multivariate comparison of classification performance measures. Chemom. Intell. Lab. Syst. 2018, 174, 33–44. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Vinzi, V.E.; Chin, W.W.; Henseler, J.; Wang, H. (Eds.) Handbook of Partial Least Squares: Concepts, Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Tenenhaus, M.; Naes, T. Special issue—PLS methods, Chemom. Intell. Lab. Syst. 2001, 58, 75. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głowacz, K.; Cieślak, M.; Ciosek-Skibińska, P. The Performance of Partial Least Squares Methods in Virtual Nanosensor Array—Multiple Metal Ions Sensing Based on Multispectral Fluorescence of Quantum Dots. Materials 2024, 17, 4766. https://doi.org/10.3390/ma17194766
Głowacz K, Cieślak M, Ciosek-Skibińska P. The Performance of Partial Least Squares Methods in Virtual Nanosensor Array—Multiple Metal Ions Sensing Based on Multispectral Fluorescence of Quantum Dots. Materials. 2024; 17(19):4766. https://doi.org/10.3390/ma17194766
Chicago/Turabian StyleGłowacz, Klaudia, Mikołaj Cieślak, and Patrycja Ciosek-Skibińska. 2024. "The Performance of Partial Least Squares Methods in Virtual Nanosensor Array—Multiple Metal Ions Sensing Based on Multispectral Fluorescence of Quantum Dots" Materials 17, no. 19: 4766. https://doi.org/10.3390/ma17194766





