Advancing Food Packaging: Exploring Cyto-Toxicity of Shape Memory Polyurethanes
Abstract
:1. Introduction
2. Materials and Methods
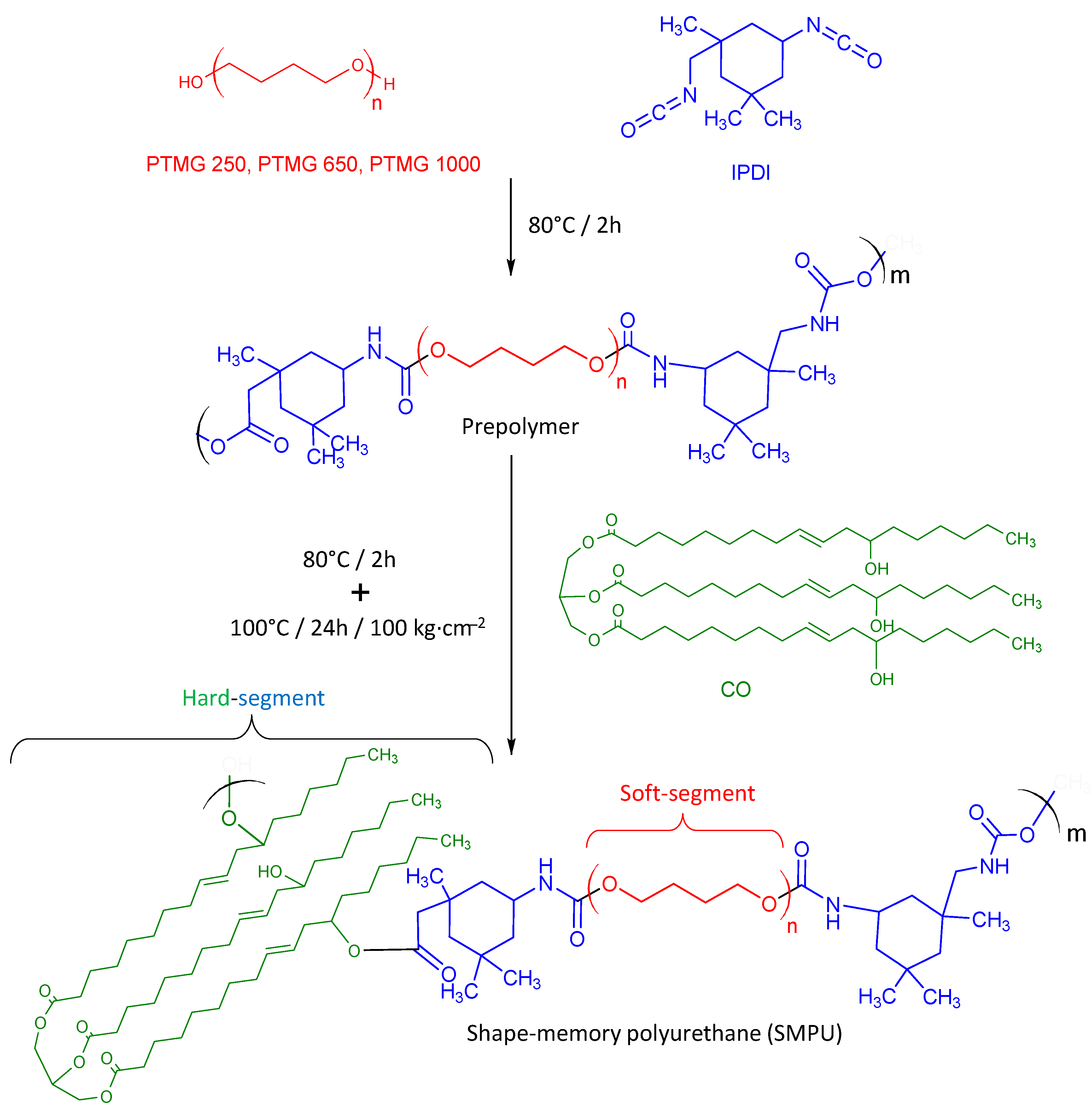
2.1. Materials
Synthesis
2.2. Characterization Methods
2.2.1. Crystal Violet Assay for Determining Adhesion of Cultured Cells (Cytotoxicity)
2.2.2. Environmental Degradation Test
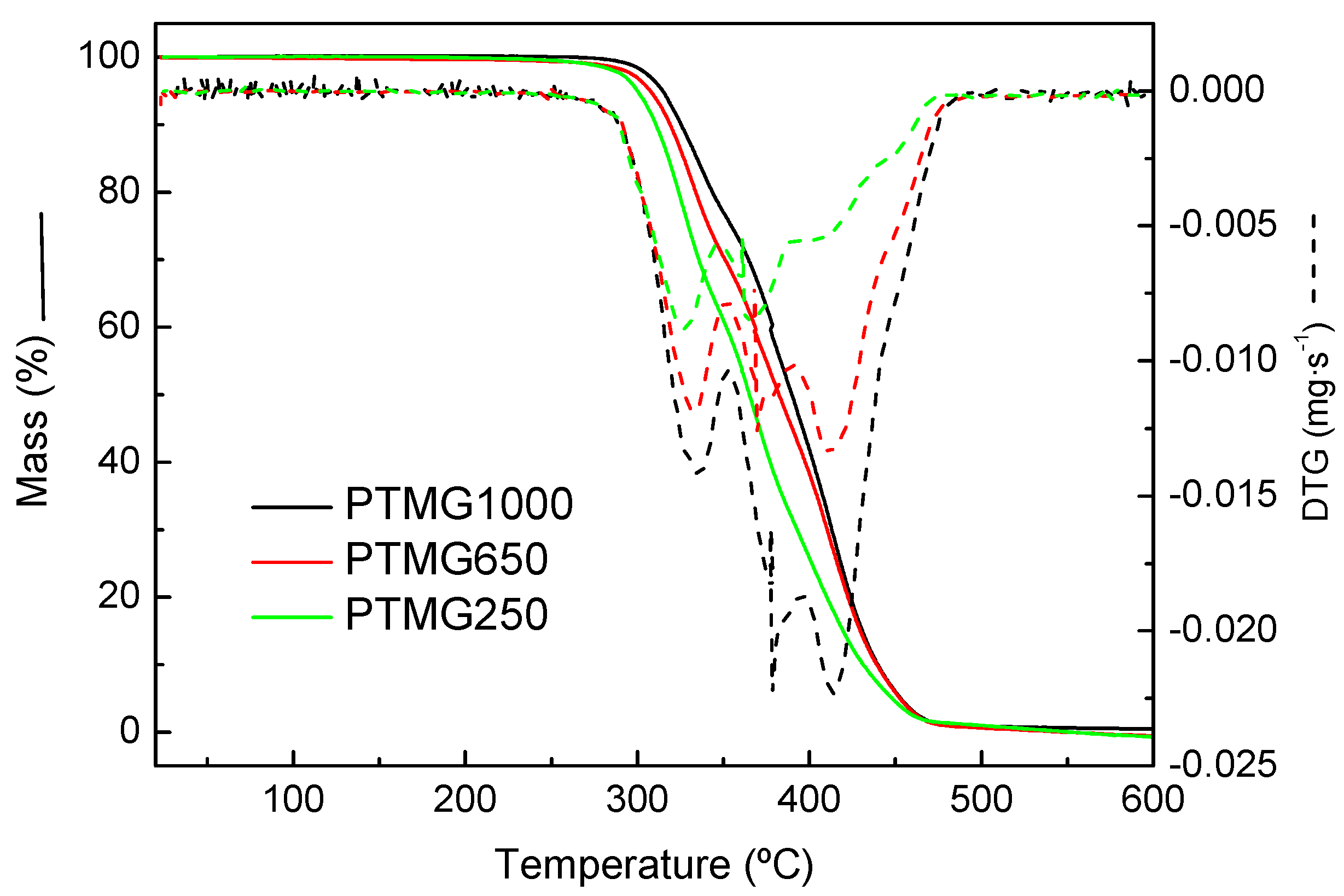
2.2.3. Thermogravimetric Analysis (TGA)
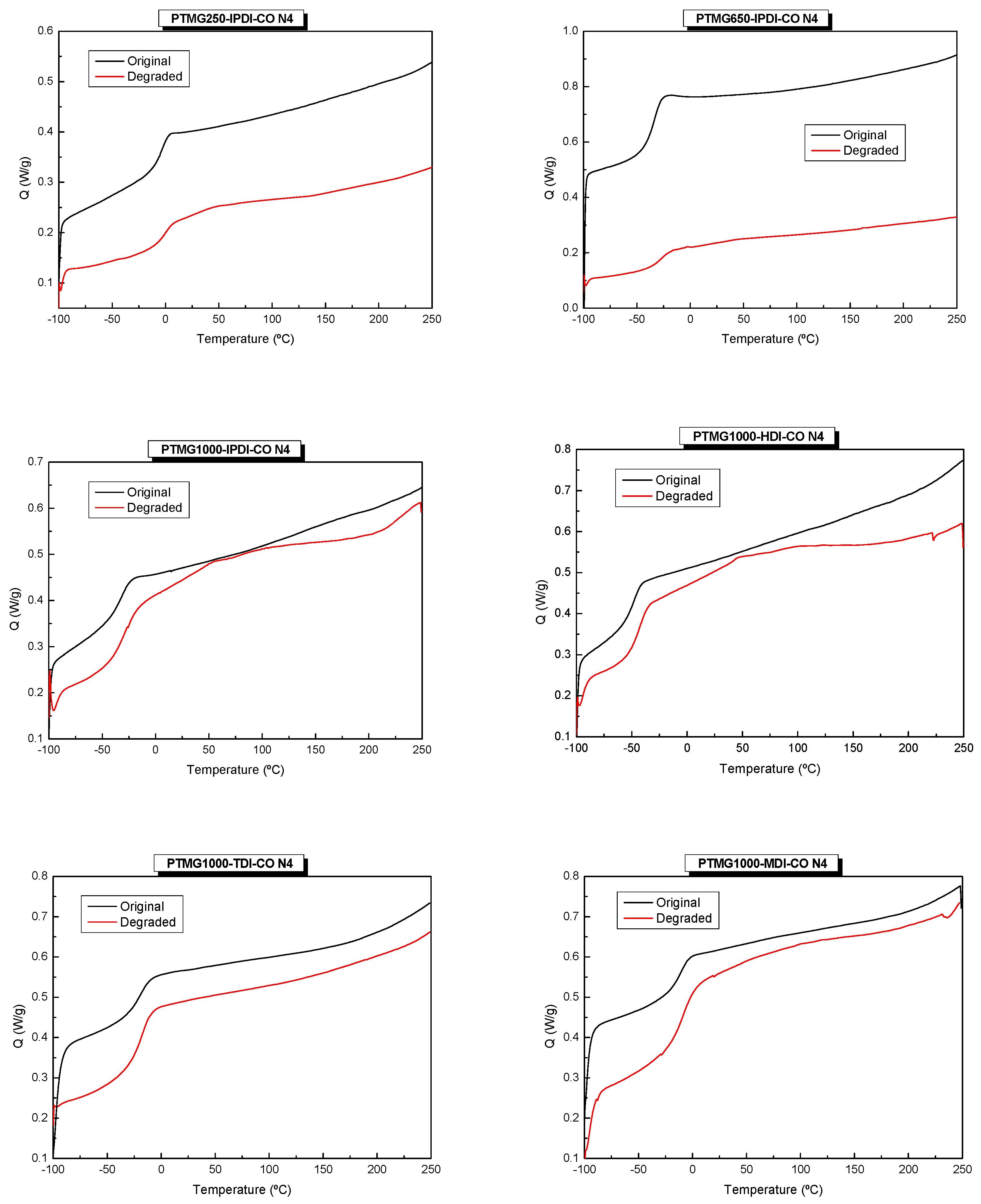
2.2.4. Differential Scanning Calorimetry (DSC)
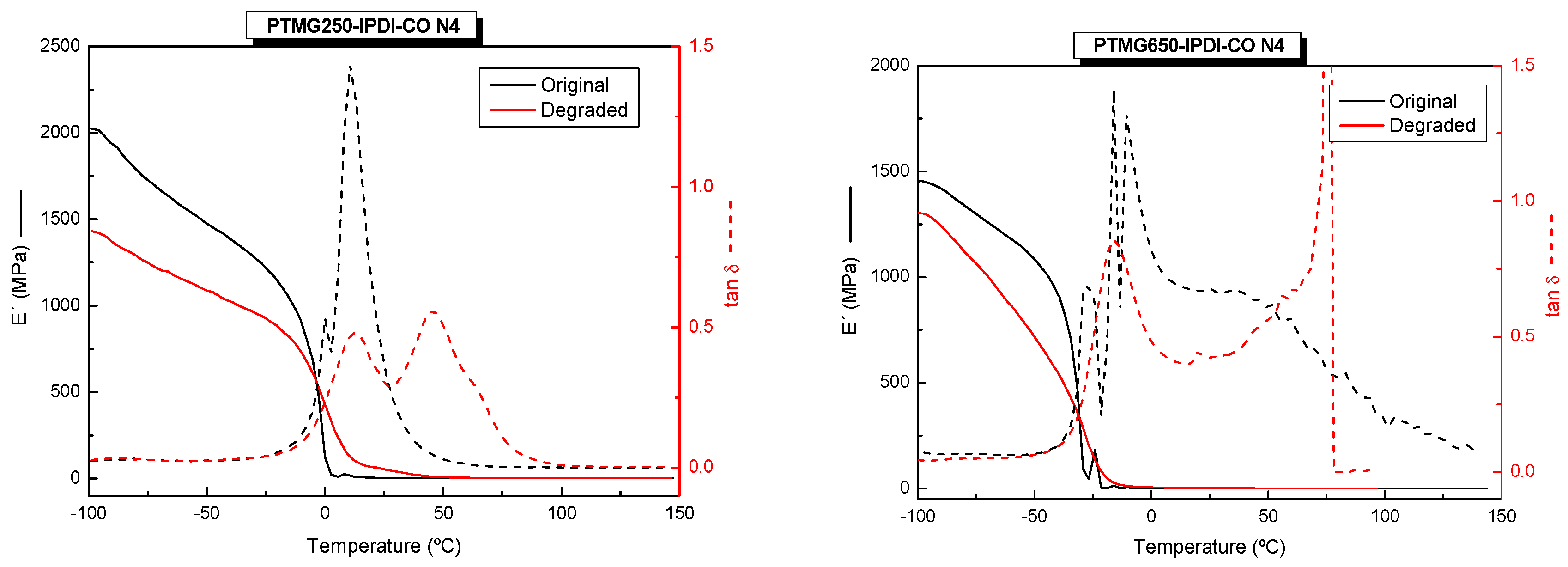
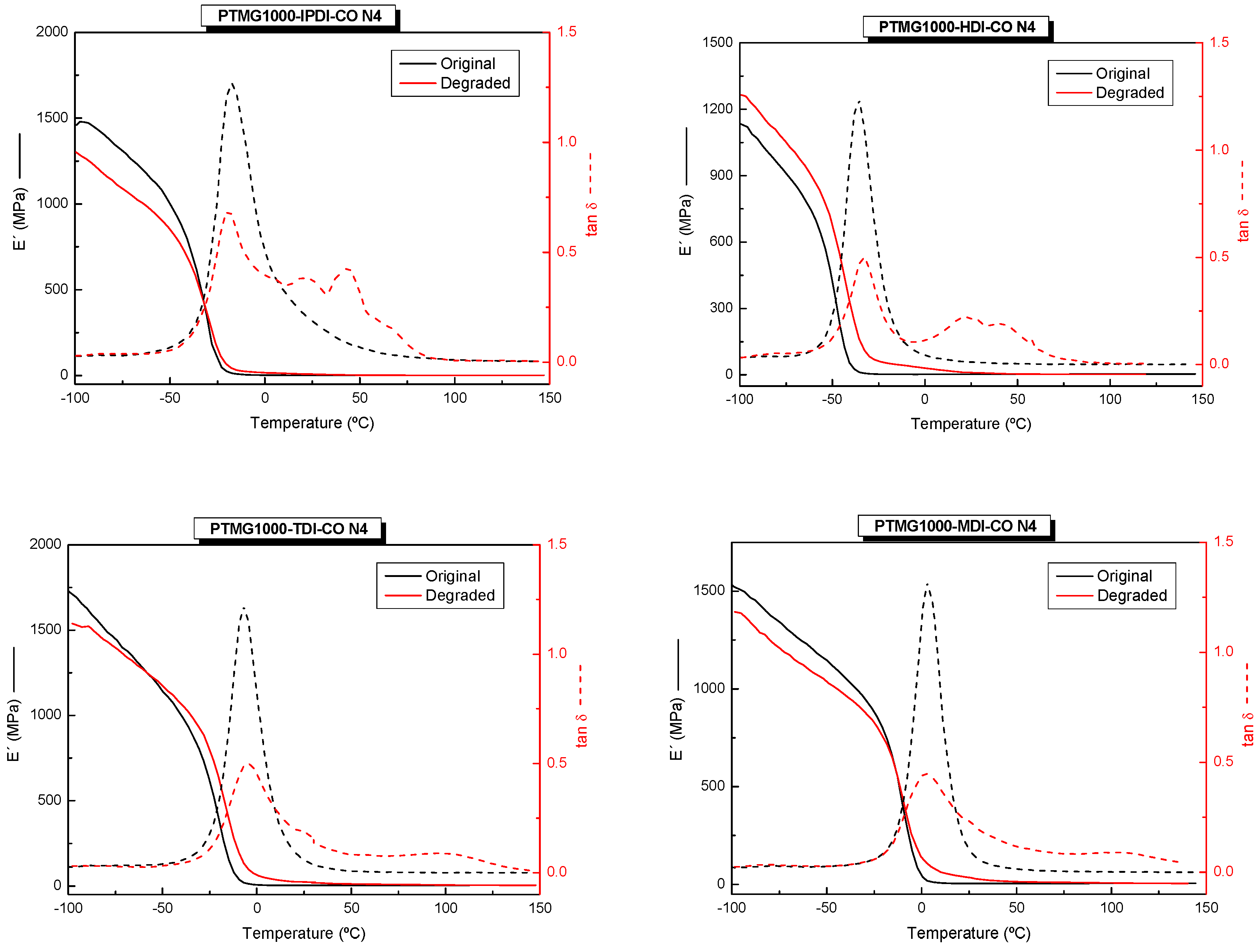
2.2.5. Dynamic Mechanical Thermal Analysis (DMA)
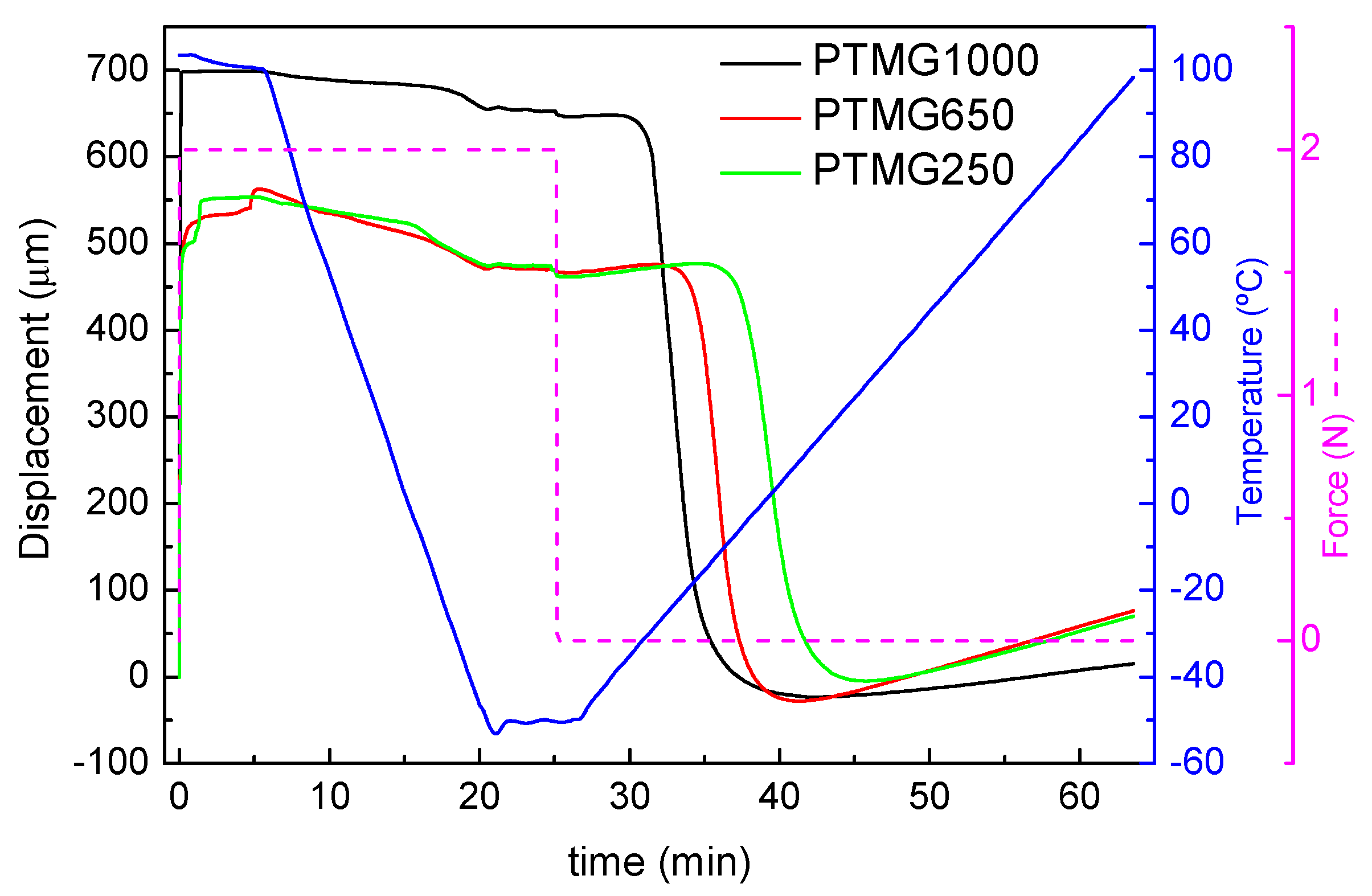
2.2.6. Thermomechanical Analysis (TMA)
3. Results and Discussion
3.1. Effect of the Isocyanate in Cytotoxicity
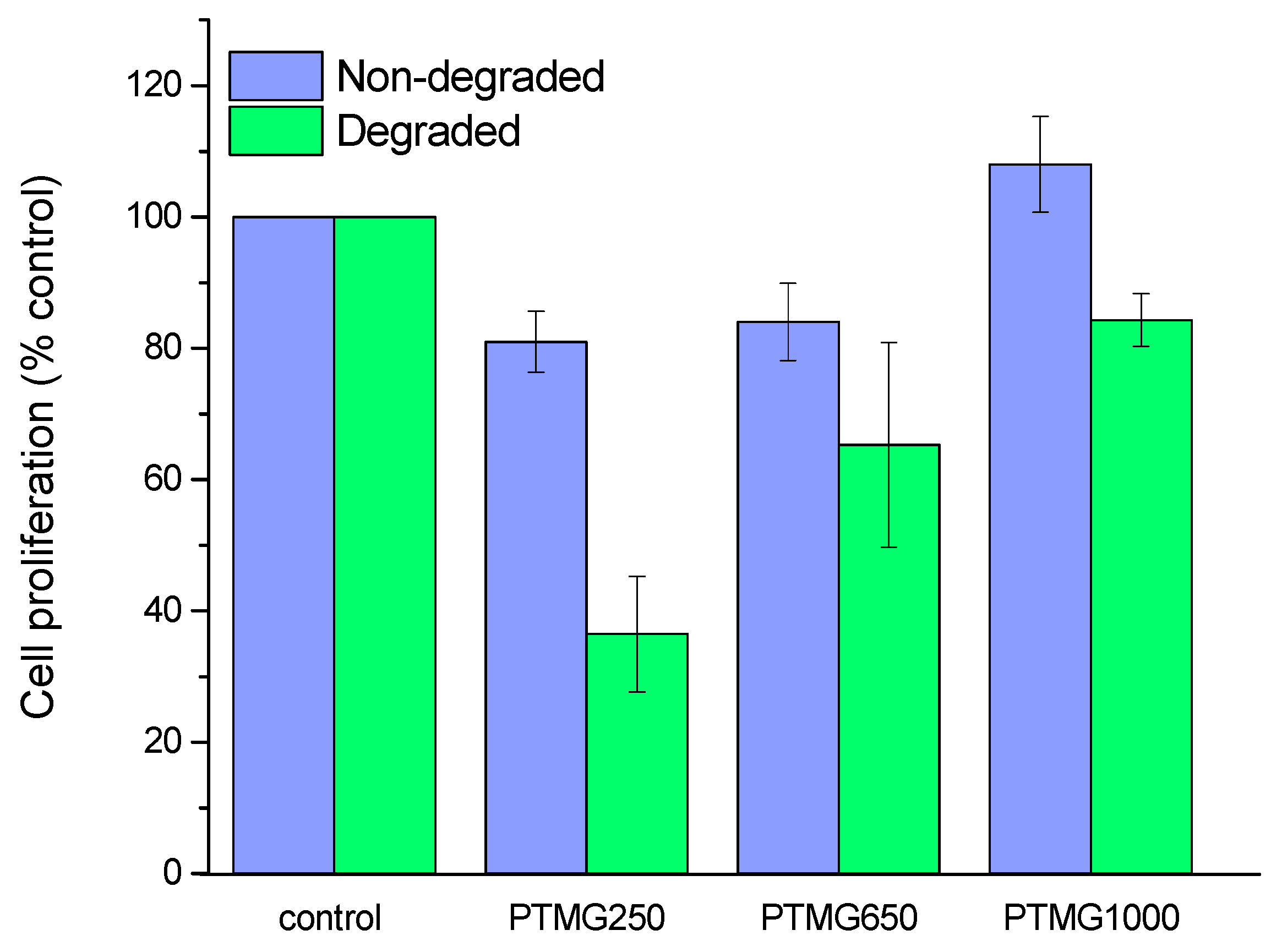
3.2. Effect of the Polyol Chain Length in Cytotoxicity
3.3. Thermomechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Xiao, R.; Huang, W.M. Heating/Solvent Responsive Shape-memory Polymers for Implant Biomedical Devices in Minimally Invasive Surgery: Current Status and Challenge. Macromol. Biosci. 2020, 20, 2000108. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.; Wyatt, M.; Bryers, J.; Ratner, B. Biocompatibility Evolves: Phenomenology to Toxicology to Regeneration. Adv. Healthc. Mater. 2021, 10, 2002153. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of Polymer-Based Biomaterials and Medical Devices–Regulations, in Vitro Screening and Risk-Management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Ojanguren, A.; Niederberger, M.; Lizundia, E. Degradation Behavior, Biocompatibility, Electrochemical Performance, and Circularity Potential of Transient Batteries. Adv. Sci. 2021, 8, 2004814. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Liu, Y.; Leng, J. Shape Memory Polymer Fibers: Materials, Structures, and Applications. Adv. Fiber Mater. 2022, 4, 5–23. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Rehman, H.U.; Zheng, X.; Li, H.; Liu, H.; Hedenqvist, M.S. Shape-Memory Polymeric Artificial Muscles: Mechanisms, Applications and Challenges. Molecules 2020, 25, 4246. [Google Scholar] [CrossRef]
- Yan, M.R.; Hsieh, S.; Ricacho, N. Innovative Food Packaging, Food Quality and Safety, and Consumer Perspectives. Processes 2022, 10, 747. [Google Scholar] [CrossRef]
- Sáenz-Pérez, M.; Lizundia, E.; Laza, J.M.; García-Barrasa, J.; Vilas, J.L.; León, L.M. Methylene Diphenyl Diisocyanate (MDI) and Toluene Diisocyanate (TDI) Based Polyurethanes: Thermal, Shape-Memory and Mechanical Behavior. RSC Adv. 2016, 6, 69094–69102. [Google Scholar] [CrossRef]
- Veloso-Fernández, A.; Laza, J.M.; Ruiz-Rubio, L.; Martín, A.; Taguado, M.; Benito-Vicente, A.; Martín, C.; Vilas, J.L. Towards a New Generation of Non-Cytotoxic Shape Memory Thermoplastic Polyurethanes for Biomedical Applications. Mater. Today Commun. 2022, 33, 104730. [Google Scholar] [CrossRef]
- Sáenz-Pérez, M.; Bashir, T.; Laza, J.M.; García-Barrasa, J.; Vilas, J.L.; Skrifvars, M.; León, L.M. Novel Shape-Memory Polyurethane Fibers for Textile Applications. Text. Res. J. 2019, 89, 1027–1037. [Google Scholar] [CrossRef]
- Gupta, R.K.; Hashmi, S.A.R.; Verma, S.; Naik, A.; Nair, P. Recovery Stress and Storage Modulus of Microwave-Induced Graphene-Reinforced Thermoresponsive Shape Memory Polyurethane Nanocomposites. J. Mater. Eng. Perform. 2020, 29, 205–214. [Google Scholar] [CrossRef]
- Laza, J.M.; Veloso-Fernandez, A.; Sanchez-Bodon, J.; Martín, A.; Goitandia, A.M.; Monteserin, C.; Mendibil, X.; Vidal, K.; Lambarri, J.; Aranzabe, E. Analysis of the Influence of Microencapsulated Phase Change Materials on the Behavior of a New Generation of Thermo-Regulating Shape Memory Polyurethane Fibers. Polym. Test. 2022, 116, 107807. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Yang, L.; Wei, Y.; Wang, X.; Wang, Z.; Tao, L. Cytotoxicity Study of Polyethylene Glycol Derivatives. RSC Adv. 2017, 7, 18252–18259. [Google Scholar] [CrossRef]
- Marcos, A.; Abraham, G.A.; Valentına, J.L.; San Roman, J. Synthesis and Characterization of Biodegradable Non-Toxic Poly (Ester-Urethane-Urea) s Based on Poly (ɛ-Caprolactone) and Amino Acid Derivatives. Polymer 2006, 47, 785–798. [Google Scholar] [CrossRef]
- Vermette, P.; Griesser, H.J.; Laroche, G.; Guidoin, R. Biomedical Applications of Polyurethanes; Landes Bioscience: Georgetown, TX, USA, 2001; Volume 6, ISBN 1587060825. [Google Scholar]
- Golling, F.E.; Pires, R.; Hecking, A.; Weikard, J.; Richter, F.; Danielmeier, K.; Dijkstra, D. Polyurethanes for Coatings and Adhesives–Chemistry and Applications. Polym. Int. 2019, 68, 848–855. [Google Scholar] [CrossRef]
- Mudri, N.H.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Awang Biak, D.R.; Rayung, M. Comparative Study of Aromatic and Cycloaliphatic Isocyanate Effects on Physico-Chemical Properties of Bio-Based Polyurethane Acrylate Coatings. Polymers 2020, 12, 1494. [Google Scholar] [CrossRef]
- Król, P.; Uram, Ł.; Król, B.; Pielichowska, K.; Sochacka-Piętal, M.; Walczak, M. Synthesis and Property of Polyurethane Elastomer for Biomedical Applications Based on Nonaromatic Isocyanates, Polyesters, and Ethylene Glycol. Colloid Polym. Sci. 2020, 298, 1077–1093. [Google Scholar] [CrossRef]
- Corcuera, M.A.; Rueda, L.; d’Arlas, B.F.; Arbelaiz, A.; Marieta, C.; Mondragon, I.; Eceiza, A. Microstructure and Properties of Polyurethanes Derived from Castor Oil. Polym. Degrad. Stab. 2010, 95, 2175–2184. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, H.; Liang, D.; Lin, Z.; Chen, Q.; Feng, P.; Wang, Q. Renewable Castor-oil-based Waterborne Polyurethane Networks: Simultaneously Showing High Strength, Self-healing, Processability and Tunable Multishape Memory. Angew. Chemie Int. Ed. 2021, 60, 4289–4299. [Google Scholar] [CrossRef]
- Gurunathan, T.; Arukula, R. High Performance Polyurethane Dispersion Synthesized from Plant Oil Renewable Resources: A Challenge in the Green Materials. Polym. Degrad. Stab. 2018, 150, 122–132. [Google Scholar] [CrossRef]
- Laza, J.M.; Veloso, A.; Vilas, J.L. Tailoring New Bisphenol a Ethoxylated Shape Memory Polyurethanes. J. Appl. Polym. Sci. 2021, 138, 49660. [Google Scholar] [CrossRef]
- Wu, X.L.; Huang, W.M.; Lu, H.B.; Wang, C.C.; Cui, H.P. Characterization of Polymeric Shape Memory Materials. J. Polym. Eng. 2017, 37, 1–20. [Google Scholar] [CrossRef]
- Wang, T.-L.; Hsieh, T.-H. Effect of Polyol Structure and Molecular Weight on the Thermal Stability of Segmented Poly (Urethaneureas). Polym. Degrad. Stab. 1997, 55, 95–102. [Google Scholar] [CrossRef]
- Cardy, R.H. Carcinogenicity and Chronic Toxicity of 2, 4-Toluenediamine in F344 Rats. J. Natl. Cancer Inst. 1979, 62, 1107–1116. [Google Scholar]
- Rabek, J.F. Photodegradation and Photo-Oxidative Degradation of Heterochain Polymers. Polym. Photodegradation 1995, 255–352. [Google Scholar]
- Wang, L.; Liang, G.; Dang, G.; Wang, F.; Fan, X.; Fu, W. Photochemical Degradation Study of Polyurethanes as Relic Protection Materials by FTIR-ATR. Chin. J. Chem. 2005, 23, 1257–1263. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Ren, B.; Hu, C.; Wang, H. Light-driven Self-healing Castor Oil Based Polyurethane Film with Enhanced Mechanical Properties. J. Appl. Polym. Sci. 2022, 139, e52958. [Google Scholar] [CrossRef]
- Park, C.K.; Lee, J.H.; Kim, I.S.; Kim, S.H. Castor Oil-based Polyols with Gradually Increasing Functionalities for Biopolyurethane Synthesis. J. Appl. Polym. Sci. 2020, 137, 48304. [Google Scholar] [CrossRef]
- Cakić, S.M.; Ristić, I.S.; Milena, M.; Stojiljković, D.T.; Jaroslava, B. Preparation and Characterization of Waterborne Polyurethane/Silica Hybrid Dispersions from Castor Oil Polyols Obtained by Glycolysis Poly (Ethylene Terephthalate) Waste. Int. J. Adhes. Adhes. 2016, 70, 329–341. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, M. Addition of Anti-Flaming Agents in Castor Oil Based Rigid Polyurethane Foams: Studies on Mechanical and Flammable Behaviour. Mater. Res. Express 2020, 7, 15333. [Google Scholar] [CrossRef]
- Sultan, M.; Jamal, Z.; Jubeen, F.; Farooq, A.; Bibi, I.; Uroos, M.; Chaudhry, H.; Alissa, S.A.; Iqbal, M. Green Synthesis of Biodegradable Polyurethane and Castor Oil-Based Composite for Benign Transformation of Methylene Blue. Arab. J. Chem. 2021, 14, 103417. [Google Scholar] [CrossRef]
- Huang, H.; Pang, H.; Huang, J.; Yu, P.; Li, J.; Lu, M.; Liao, B. Influence of Hard Segment Content and Soft Segment Length on the Microphase Structure and Mechanical Performance of Polyurethane-Based Polymer Concrete. Constr. Build. Mater. 2021, 284, 122388. [Google Scholar] [CrossRef]
- Pourmohammadi-Mahunaki, M.; Haddadi-Asl, V.; Roghani-Mamaqani, H.; Koosha, M.; Yazdi, M. Effect of Chain Extender Length and Molecular Architecture on Phase Separation and Rheological Properties of Ether-Based Polyurethanes. Polym. Bull. 2022, 79, 8653–8668. [Google Scholar] [CrossRef]


| Sample Code | Thermal Stability | |||
|---|---|---|---|---|
| Polyol/Isocyanate/CO | N | Ti (°C) | Tdmax1 (°C) | Tdmax2 (°C) |
| 4 | 290.6 | 330.5 | 407.5 | |
| PTMG1000/IPDI/CO | 5 | 294.8 | 335.9 | 414.2 |
| 6 | 282.0 | 332.9 | 413.8 | |
| 4 | 301.0 | 340.0 | 422.0 | |
| PTMG650/IPDI/CO | 5 | 293.0 | 333.3 | 412.6 |
| 6 | 299.9 | 333.2 | 418.2 | |
| 4 | 285.8 | 321.1 | 365.4 | |
| PTMG250/IPDI/CO | 5 | 301.0 | 340.0 | 423.0 |
| 6 | 290.6 | 327.5 | 375.6 | |
| Sample Code | DSC | DMA | ||
|---|---|---|---|---|
| Polyol/Isocyanate/CO | N | Tg onset (°C) | Tg endset (°C) | Tg-peak (°C) |
| 4 | −39.0 | −24.6 | −17.4 | |
| PTMG1000/IPDI/CO | 5 | −36.7 | −26.2 | −16.3 |
| 6 | −38.7 | −20.8 | −6.5 | |
| 4 | −41.5 | −27.9 | −10.6 | |
| PTMG650/IPDI/CO | 5 | −30.8 | −14.3 | −7.8 |
| 6 | −28.8 | −13.8 | −3.1 | |
| 4 | −11.2 | 0.8 | 17.2 | |
| PTMG250/IPDI/CO | 5 | −17.9 | −1.1 | 18.1 |
| 6 | −15.2 | 2.1 | 19.9 | |
| Sample Code | TMA | |||
|---|---|---|---|---|
| Polyol/Isocyanate/CE | N | Deformation (Rd%) | Fixing (Rf%) | Recovery (Rr%) |
| 4 | 11.2 | 93.0 | 89.6 | |
| PTMG1000/IPDI/CO | 5 | 13.9 | 92.5 | 98.1 |
| 6 | 9.9 | 90.9 | 97.4 | |
| 4 | 9.2 | 93.0 | 89.6 | |
| PTMG650/IPDI/CO | 5 | 11.0 | 82.3 | 88.3 |
| 6 | 9.3 | 70.5 | 89.3 | |
| 4 | 7.7 | 77.6 | 89.7 | |
| PTMG250/IPDI/CO | 5 | 10.9 | 86.8 | 87.6 |
| 6 | 8.8 | 81.5 | 98.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veloso-Fernández, A.; Laza, J.M.; Ruiz-Rubio, L.; Martín, A.; Benito-Vicente, A.; Martín, C.; Vilas-Vilela, J.L. Advancing Food Packaging: Exploring Cyto-Toxicity of Shape Memory Polyurethanes. Materials 2024, 17, 4770. https://doi.org/10.3390/ma17194770
Veloso-Fernández A, Laza JM, Ruiz-Rubio L, Martín A, Benito-Vicente A, Martín C, Vilas-Vilela JL. Advancing Food Packaging: Exploring Cyto-Toxicity of Shape Memory Polyurethanes. Materials. 2024; 17(19):4770. https://doi.org/10.3390/ma17194770
Chicago/Turabian StyleVeloso-Fernández, Antonio, José Manuel Laza, Leire Ruiz-Rubio, Ane Martín, Asier Benito-Vicente, Cesar Martín, and José Luis Vilas-Vilela. 2024. "Advancing Food Packaging: Exploring Cyto-Toxicity of Shape Memory Polyurethanes" Materials 17, no. 19: 4770. https://doi.org/10.3390/ma17194770







