Pt3(CoNi) Ternary Intermetallic Nanoparticles Immobilized on N-Doped Carbon Derived from Zeolitic Imidazolate Frameworks for Oxygen Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Co-ZIFs Precursors and CoNi-NC Supports
2.2. Synthesis of Pt/nCoNi-NC and Ternary IMCs Catalysts
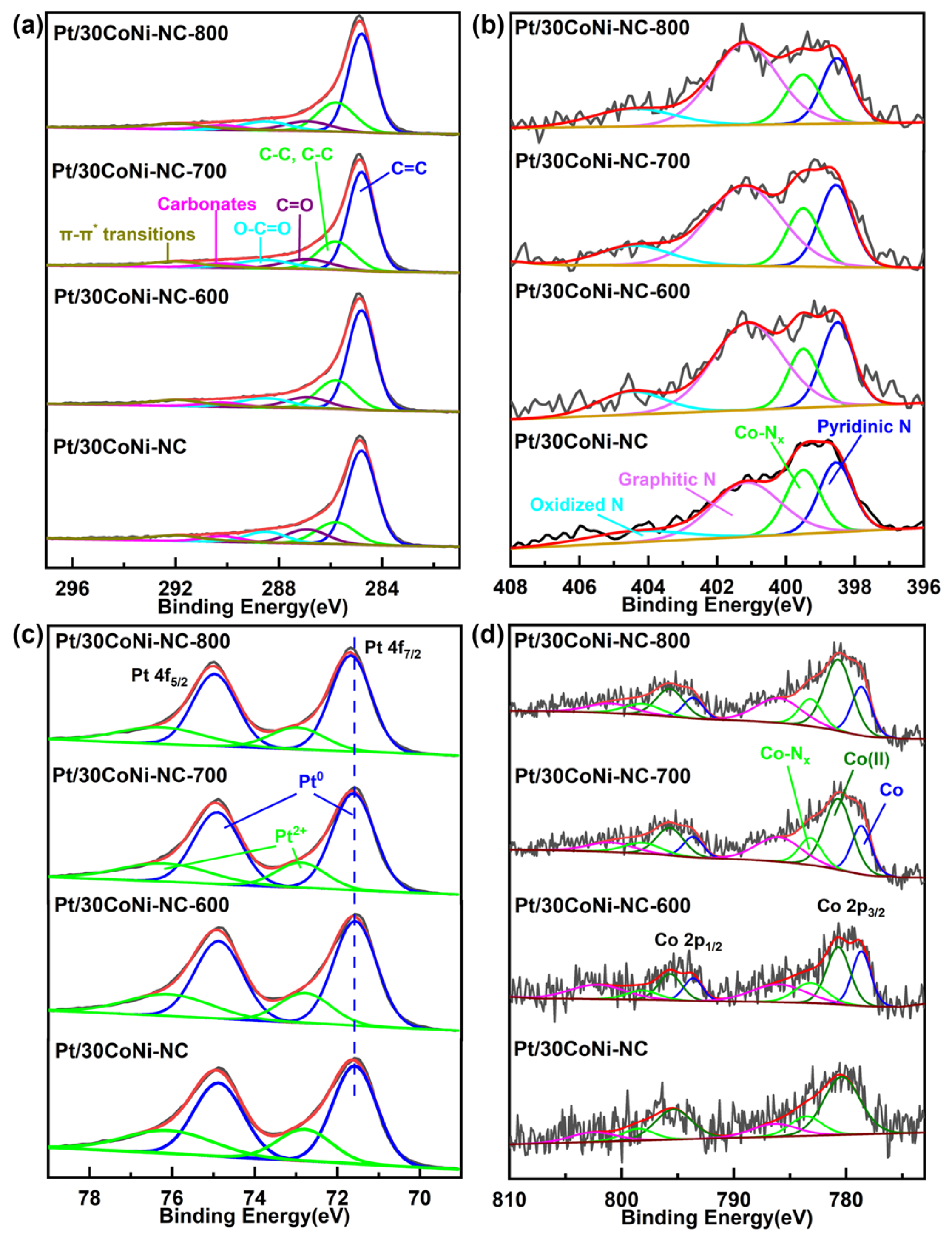
2.3. Material Characterization
2.4. Electrocatalytic Measurements
3. Results
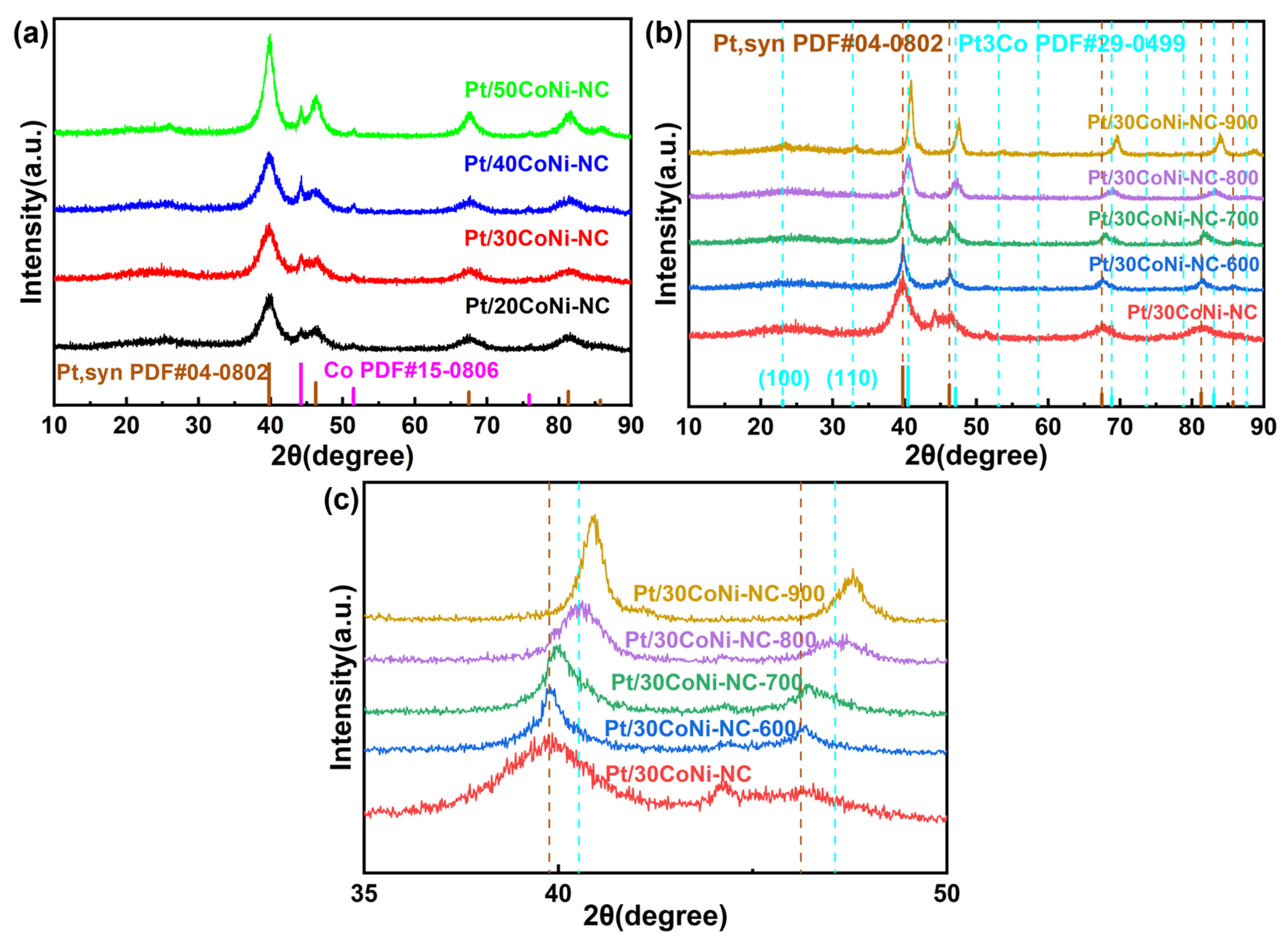
3.1. Phase Transformation from Pt to Pt3(CoNi) during the Preparation Process
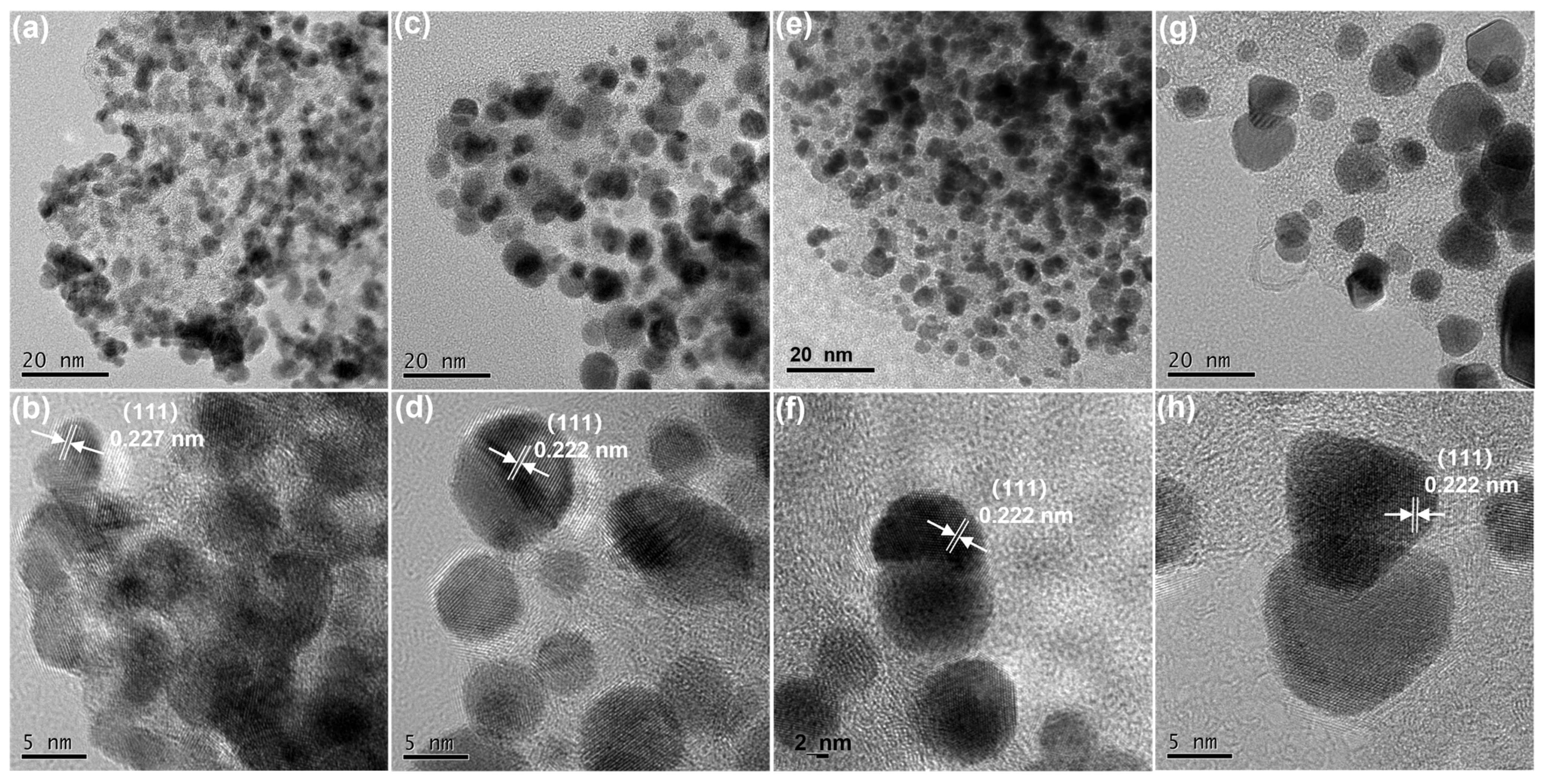
3.2. Characterization of Microstructure of the Pt and Pt3(CoNi) Nanoparticles
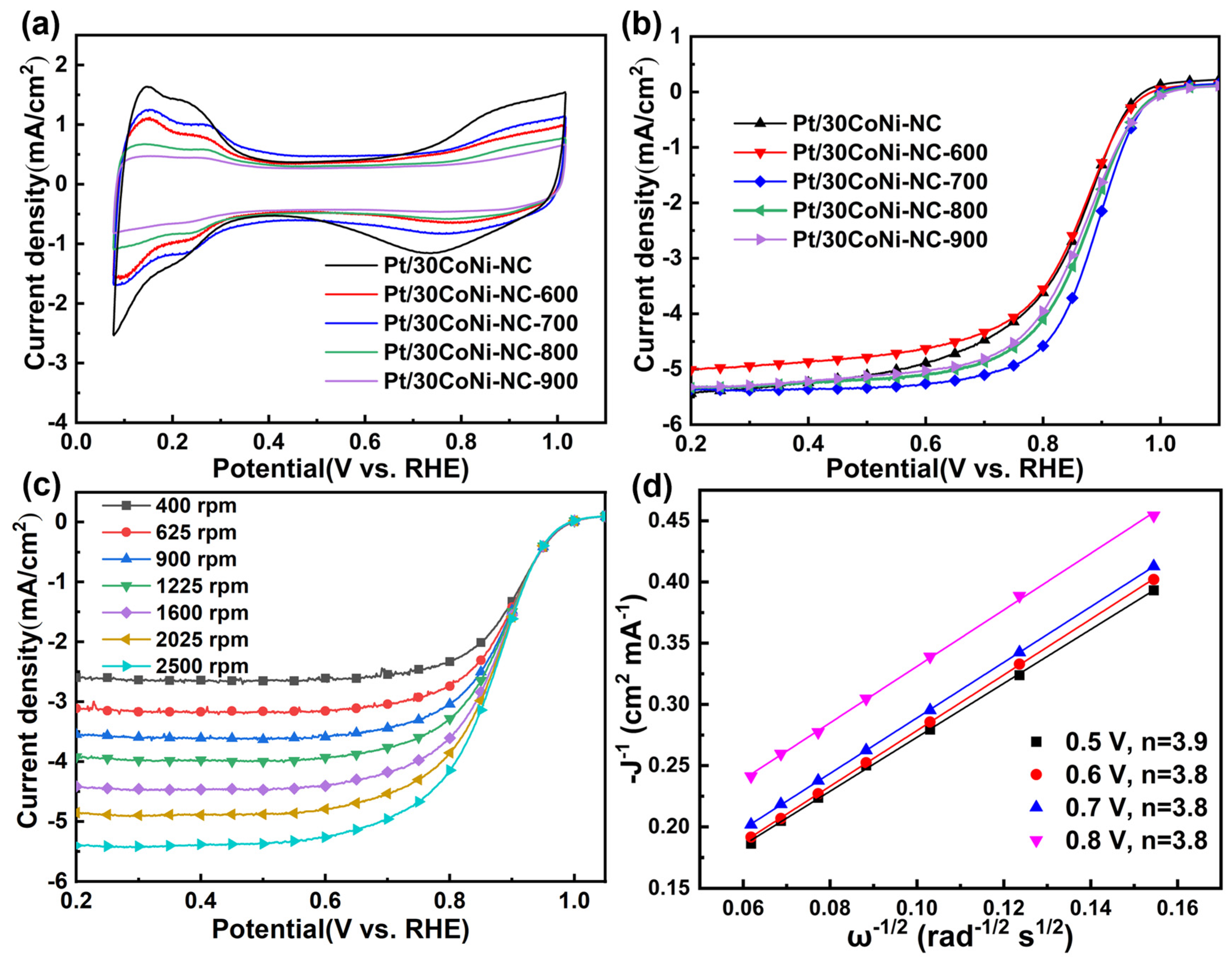
3.3. Electrochemical Measurement Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, L.; Tu, F.; Shang, Z.; Ma, M.; Xia, Y.; Zhao, Z.; Zhao, L.; Wang, Z.; Shao, G. Surfactant-assisted synthesis of platinum nanoparticle catalysts for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2022, 47, 15001–15011. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, J.; Zheng, Y.; Xiao, M.; Gao, R.; Zhang, Z.; Wen, G.; Dou, H.; Deng, Y.-P.; Yu, A.; et al. Materials Engineering toward Durable Electrocatalysts for Proton Exchange Membrane Fuel Cells. Adv. Energy Mater. 2022, 12, 2102665. [Google Scholar] [CrossRef]
- Yi, S.; Jiang, H.; Bao, X.; Zou, S.; Liao, J.; Zhang, Z. Recent progress of Pt-based catalysts for oxygen reduction reaction in preparation strategies and catalytic mechanism. J. Electroanal. Chem. 2019, 848, 113279. [Google Scholar] [CrossRef]
- Wang, X.X.; Prabhakaran, V.; He, Y.; Shao, Y.; Wu, G. Iron-Free Cathode Catalysts for Proton-Exchange-Membrane Fuel Cells: Cobalt Catalysts and the Peroxide Mitigation Approach. Adv. Mater. 2019, 31, 1805126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Seo, B.; Wang, B.; Zamel, N.; Jiao, K.; Adroher, X.C. Fundamentals, materials, and machine learning of polymer electrolyte membrane fuel cell technology. Energy AI 2020, 1, 100014. [Google Scholar] [CrossRef]
- Janssen, M.; Weber, P.; Oezaslan, M. Recent advances of various Pt-based catalysts for oxygen reduction reaction (ORR) in polymer electrolyte membrane fuel cells (PEMFCs). Curr. Opin. Electrochem. 2023, 40, 101337. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Z.; Zhang, A.; Yan, X.; Xue, W.; Peng, B.; Xin, H.L.; Pan, X.; Duan, X.; Huang, Y. Graphene-nanopocket-encaged PtCo nanocatalysts for highly durable fuel cell operation under demanding ultralow-Pt-loading conditions. Nat. Nanotechnol. 2022, 17, 968–975. [Google Scholar] [CrossRef]
- Mukherjee, P.; Kakade, B.; Swami, A. Current Trends in Platinum-Based Ternary Alloys as Promising Electrocatalysts for the Oxygen Reduction Reaction: A Mini Review. Energy Fuels 2022, 36, 2306–2322. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, G.; Li, Y.; Liang, L.; Grundish, N.S.; Tang, Y.; Goodenough, J.B.; Cui, Z. General Strategy for Synthesis of Ordered Pt3M Intermetallics with Ultrasmall Particle Size. Angew. Chem. Int. Ed. 2020, 59, 7857–7863. [Google Scholar] [CrossRef]
- Bai, J.; Yang, L.; Jin, Z.; Ge, J.; Xing, W. Advanced Pt-based intermetallic nanocrystals for the oxygen reduction reaction. Chin. J. Catal. 2022, 43, 1444–1458. [Google Scholar] [CrossRef]
- Cheng, H.; Gui, R.; Yu, H.; Wang, C.; Liu, S.; Liu, H.; Zhou, T.; Zhang, N.; Zheng, X.; Chu, W.; et al. Subsize Pt-based intermetallic compound enables long-term cyclic mass activity for fuel-cell oxygen reduction. Proc. Natl. Acad. Sci. USA 2021, 118, e2104026118. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-L.; Wang, L.-N.; Yin, P.; Liu, J.; Chen, M.-X.; Yan, Q.-Q.; Wang, Z.-S.; Xu, S.-L.; Chu, S.-Q.; Cui, C.; et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 2021, 374, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, J.; Huo, J.; Li, C.; Du, L.; Cui, Z.; Liao, S. Ultrathin Co-N-C Layer Modified Pt–Co Intermetallic Nanoparticles Leading to a High-Performance Electrocatalyst toward Oxygen Reduction and Methanol Oxidation. Small 2023, 19, 2301337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.Y.; Ruan, Y.; Zhu, M.Z.; Gao, X.P.; Guo, W.X.; Liu, X.K.; Wang, W.Y.; Chen, M.; Wu, G.; Yao, T.; et al. Coupling Single-Atom Sites and Ordered Intermetallic PtM Nanoparticles for Efficient Catalysis in Fuel Cells. Small 2023, 19, 2302328. [Google Scholar] [CrossRef]
- Wang, X.X.; Swihart, M.T.; Wu, G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat. Catal. 2019, 2, 578–589. [Google Scholar] [CrossRef]
- Du, N.; Wang, C.; Long, R.; Xiong, Y. N-doped carbon-stabilized PtCo nanoparticles derived from Pt@ZIF-67: Highly active and durable catalysts for oxygen reduction reaction. Nano Res. 2017, 10, 3228–3237. [Google Scholar] [CrossRef]
- Mai, Y.-L.; Xie, X.-S.; Wang, Z.-D.; Yan, C.-F.; Liu, G.-H. Effect of heat treatment temperature on the Pt3Co binary metal catalysts for oxygen reduced reaction and DFT calculations. J. Fuel Chem. Technol. 2022, 50, 114–121. [Google Scholar] [CrossRef]
- Yang, W.; Zou, L.; Huang, Q.; Zou, Z.; Hu, Y.; Yang, H. Lattice Contracted Ordered Intermetallic Core-Shell PtCo@Pt Nanoparticles: Synthesis, Structure and Origin for Enhanced Oxygen Reduction Reaction. J. Electrochem. Soc. 2017, 164, H331–H337. [Google Scholar] [CrossRef]
- Zeng, W.-J.; Wang, C.; Yin, P.; Tong, L.; Yan, Q.-Q.; Chen, M.-X.; Xu, S.-L.; Liang, H.-W. Alloying Matters for Ordering: Synthesis of Highly Ordered PtCo Intermetallic Catalysts for Fuel Cells. Inorg. Chem. 2023, 62, 5262–5269. [Google Scholar] [CrossRef]
- Yamazaki, S.-I.; Asahi, M.; Ioroi, T. Promotion of oxygen reduction on a porphyrazine-modified Pt catalyst surface. Electrochim. Acta 2019, 297, 725–734. [Google Scholar] [CrossRef]
- Wang, X.X.; Hwang, S.; Pan, Y.-T.; Chen, K.; He, Y.; Karakalos, S.; Zhang, H.; Spendelow, J.S.; Su, D.; Wu, G. Ordered Pt3Co Intermetallic Nanoparticles Derived from Metal–Organic Frameworks for Oxygen Reduction. Nano Lett. 2018, 18, 4163–4171. [Google Scholar] [CrossRef] [PubMed]
- Mardle, P.; Thirunavukkarasu, G.; Guan, S.; Chiu, Y.-L.; Du, S. Comparative Study of PtNi Nanowire Array Electrodes toward Oxygen Reduction Reaction by Half-Cell Measurement and PEMFC Test. ACS Appl. Mater. Interfaces 2020, 12, 42832–42841. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xiong, Y.; Liu, C.; Zhang, H.; Zhao, M.; Chen, W.; Chen, W.; Huang, W. Electron-rich isolated Pt active sites in ultrafine PtFe3 intermetallic catalyst for efficient alkene hydrosilylation. J. Catal. 2021, 396, 351–359. [Google Scholar] [CrossRef]
- Wegener, E.C.; Bukowski, B.C.; Yang, D.; Wu, Z.; Kropf, A.J.; Delgass, W.N.; Greeley, J.; Zhang, G.; Miller, J.T. Intermetallic Compounds as an Alternative to Single-atom Alloy Catalysts: Geometric and Electronic Structures from Advanced X-ray Spectroscopies and Computational Studies. Chemcatchem 2020, 12, 1325–1333. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, X.; Pan, Y.; Peng, Z. A review of Pt-based electrocatalysts for oxygen reduction reaction. Front. Energy 2017, 11, 268–285. [Google Scholar] [CrossRef]
- Asano, M.; Kawamura, R.; Sasakawa, R.; Todoroki, N.; Wadayama, T. Oxygen Reduction Reaction Activity for Strain-Controlled Pt-Based Model Alloy Catalysts: Surface Strains and Direct Electronic Effects Induced by Alloying Elements. ACS Catal. 2016, 6, 5285–5289. [Google Scholar] [CrossRef]
- Kim, J.; Hong, Y.; Lee, K.; Kim, J.Y. Highly Stable Pt-Based Ternary Systems for Oxygen Reduction Reaction in Acidic Electrolytes. Adv. Energy Mater. 2020, 10, 2002049. [Google Scholar] [CrossRef]
- Liang, J.; Li, N.; Zhao, Z.; Ma, L.; Wang, X.; Li, S.; Liu, X.; Wang, T.; Du, Y.; Lu, G.; et al. Tungsten-Doped L10-PtCo Ultrasmall Nanoparticles as a High-Performance Fuel Cell Cathode. Angew. Chem. Int. Ed. 2019, 58, 15471–15477. [Google Scholar] [CrossRef]
- Wang, T.; Liang, J.; Zhao, Z.; Li, S.; Lu, G.; Xia, Z.; Wang, C.; Luo, J.; Han, J.; Ma, C.; et al. Sub-6 nm Fully Ordered L10-Pt–Ni–Co Nanoparticles Enhance Oxygen Reduction via Co Doping Induced Ferromagnetism Enhancement and Optimized Surface Strain. Adv. Energy Mater. 2019, 9, 1803771. [Google Scholar] [CrossRef]
- Zhao, W.; Chi, B.; Liang, L.; Yang, P.; Zhang, W.; Ge, X.; Wang, L.; Cui, Z.; Liao, S. Optimizing the Electronic Structure of Ordered Pt–Co–Ti Ternary Intermetallic Catalyst to Boost Acidic Oxygen Reduction. ACS Catal. 2022, 12, 7571–7578. [Google Scholar] [CrossRef]
- Rethinasabapathy, M.; Kang, S.-M.; Haldorai, Y.; Jonna, N.; Jankiraman, M.; Lee, G.-W.; Jang, S.-C.; Natesan, B.; Roh, C.; Huh, Y.S. Quaternary PtRuFeCo nanoparticles supported N-doped graphene as an efficient bifunctional electrocatalyst for low-temperature fuel cells. J. Ind. Eng. Chem. 2019, 69, 285–294. [Google Scholar] [CrossRef]
- Sravani, B.; Raghavendra, P.; Chandrasekhar, Y.; Veera Manohara Reddy, Y.; Sivasubramanian, R.; Venkateswarlu, K.; Madhavi, G.; Subramanyam Sarma, L. Immobilization of platinum-cobalt and platinum-nickel bimetallic nanoparticles on pomegranate peel extract-treated reduced graphene oxide as electrocatalysts for oxygen reduction reaction. Int. J. Hydrog. Energy 2020, 45, 7680–7690. [Google Scholar] [CrossRef]
- Wang, X.X.; Sokolowski, J.; Liu, H.; Wu, G. Pt alloy oxygen-reduction electrocatalysts: Synthesis, structure, and property. Chin. J. Catal. 2020, 41, 739–755. [Google Scholar] [CrossRef]
- Di Noto, V.; Negro, E.; Nale, A.; Kulesza, P.J.; Rutkowska, I.A.; Vezzù, K.; Pagot, G. Correlation between Precursor Properties and Performance in the Oxygen Reduction Reaction of Pt and Co “Core-shell” Carbon Nitride-Based Electrocatalysts. Electrocatalysis 2020, 11, 143–159. [Google Scholar] [CrossRef]
- Sun, L.; Qin, Y.; Yin, Y. ZIF derived PtCo alloys-based nitrogen-doped Graphene as cathode catalyst for proton exchange membrane fuel cell. J. Power Sources 2023, 562, 232758. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Jang, M.-S.; Kwon, H.-J.; Ahn, W.-S. Zeolitic Imidazolate Frameworks: Synthesis, Functionalization, and Catalytic/Adsorption Applications. Catal. Surv. Asia 2014, 18, 101–127. [Google Scholar] [CrossRef]
- Xiao, Y.; Hong, A.N.; Hu, D.; Wang, Y.; Bu, X.; Feng, P. Solvent-Free Synthesis of Zeolitic Imidazolate Frameworks and the Catalytic Properties of Their Carbon Materials. Chem.—A Eur. J. 2019, 25, 16358–16365. [Google Scholar] [CrossRef] [PubMed]
- Hanif, S.; Shi, X.; Iqbal, N.; Noor, T.; Anwar, R.; Kannan, A.M. ZIF derived PtNiCo/NC cathode catalyst for proton exchange membrane fuel cell. Appl. Catal. B Environ. 2019, 258, 117947. [Google Scholar] [CrossRef]
- Mo, R.; Zhang, X.; Chen, Z.; Huang, S.; Li, Y.; Liang, L.; Tian, Z.Q.; Shen, P.K. Highly efficient PtCo nanoparticles on Co–N–C nanorods with hierarchical pore structure for oxygen reduction reaction. Int. J. Hydrog. Energy 2021, 46, 15991–16002. [Google Scholar] [CrossRef]
- Chong, L.; Wen, J.; Kubal, J.; Sen, F.G.; Zou, J.; Greeley, J.; Chan, M.; Barkholtz, H.; Ding, W.; Liu, D.J. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 2018, 362, 1276–1281. [Google Scholar] [CrossRef]
- Zhu, Z.; Yin, H.; Wang, Y.; Chuang, C.-H.; Xing, L.; Dong, M.; Lu, Y.-R.; Casillas-Garcia, G.; Zheng, Y.; Chen, S.; et al. Coexisting Single-Atomic Fe and Ni Sites on Hierarchically Ordered Porous Carbon as a Highly Efficient ORR Electrocatalyst. Adv. Mater. 2020, 32, 2004670. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, Y.-G.; Chen, W.; Xu, R.; Zheng, L.; Zhang, J.; Luo, J.; Shen, R.-A.; Zhu, Y.; Cheong, W.-C.; et al. Hollow N-Doped Carbon Spheres with Isolated Cobalt Single Atomic Sites: Superior Electrocatalysts for Oxygen Reduction. J. Am. Chem. Soc. 2017, 139, 17269–17272. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Xia, Y.; Liu, B.; Ma, M.; Shen, L.; Dai, Y.; Zhang, Z.; Zhao, Z.; Zhang, Y.; Zhao, L.; et al. Low-Loading Sub-3 nm PtCo Nanoparticles Supported on Co–N–C with Dual Effect for Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. ACS Appl. Mater. Interfaces 2022, 14, 53819–53827. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yamada, I.; Yagi, S. ZIF-Derived Co9–xNixS8 Nanoparticles Immobilized on N-Doped Carbons as Efficient Catalysts for High-Performance Zinc–Air Batteries. ACS Appl. Mater. Interfaces 2020, 12, 5847–5856. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shan, W.; Zachman, M.J.; Wang, M.; Hwang, S.; Tabassum, H.; Yang, J.; Yang, X.; Karakalos, S.; Feng, Z.; et al. Atomically Dispersed Dual-Metal Site Catalysts for Enhanced CO2 Reduction: Mechanistic Insight into Active Site Structures. Angew. Chem. Int. Ed. 2022, 61, e202205632. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Tian, C.; Zhao, W.; Li, P.; Liu, W.; Song, L.; Liu, Y.; Wang, C.-A.; Xie, Z. MOF-Derived CoNi Nanoalloy Particles Encapsulated in Nitrogen-Doped Carbon as Superdurable Bifunctional Oxygen Electrocatalyst. Nanomaterials 2023, 13, 715. [Google Scholar] [CrossRef]
- Koh, S.; Toney, M.F.; Strasser, P. Activity–stability relationships of ordered and disordered alloy phases of Pt3Co electrocatalysts for the oxygen reduction reaction (ORR). Electrochim. Acta 2007, 52, 2765–2774. [Google Scholar] [CrossRef]
- Zeng, Y.; Liang, J.; Li, C.; Qiao, Z.; Li, B.; Hwang, S.; Kariuki, N.N.; Chang, C.-W.; Wang, M.; Lyons, M.; et al. Regulating Catalytic Properties and Thermal Stability of Pt and PtCo Intermetallic Fuel-Cell Catalysts via Strong Coupling Effects between Single-Metal Site-Rich Carbon and Pt. J. Am. Chem. Soc. 2023, 145, 17643–17655. [Google Scholar] [CrossRef]
- Mohamud, M.A.; Yurtcan, A.B. Zeolotic imidazolate frameworks (ZIFs) derived porous carbon: A review from crystal growth & green synthesis to oxygen reduction reaction activity. Int. J. Hydrog. Energy 2021, 46, 33782–33800. [Google Scholar]
- Ahmadi, R.; Amini, M.K.; Bennett, J.C. Pt–Co alloy nanoparticles synthesized on sulfur-modified carbon nanotubes as electrocatalysts for methanol electrooxidation reaction. J. Catal. 2012, 292, 81–89. [Google Scholar] [CrossRef]
- Cai, Y.; Gao, P.; Wang, F.; Zhu, H. Carbon supported chemically ordered nanoparicles with stable Pt shell and their superior catalysis toward the oxygen reduction reaction. Electrochim. Acta 2017, 245, 924–933. [Google Scholar] [CrossRef]
- Xia, B.Y.; Wu, H.B.; Li, N.; Yan, Y.; Lou, X.W.; Wang, X. One-Pot Synthesis of Pt–Co Alloy Nanowire Assemblies with Tunable Composition and Enhanced Electrocatalytic Properties. Angew. Chem. Int. Ed. 2015, 54, 3797–3801. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Jishkariani, D.; Zhao, Y.; Najmr, S.; Rosen, D.; Kikkawa, J.M.; Stach, E.A.; Murray, C.B. Tuning the Electrocatalytic Oxygen Reduction Reaction Activity of Pt-Co Nanocrystals by Cobalt Concentration with Atomic-Scale Understanding. ACS Appl. Mater. Interfaces 2019, 11, 26789–26797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ye, Y.; Jiang, W.; Li, J.; Tang, H.; Hu, J.; Du, L.; Cui, Z.; Liao, S. Mesoporous carbon confined intermetallic nanoparticles as highly durable electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2020, 8, 15822–15828. [Google Scholar] [CrossRef]
- Dai, S.; You, Y.; Zhang, S.; Cai, W.; Xu, M.; Xie, L.; Wu, R.; Graham, G.W.; Pan, X. In situ atomic-scale observation of oxygen-driven core-shell formation in Pt3Co nanoparticles. Nat. Commun. 2017, 8, 204. [Google Scholar] [CrossRef]
- Tang, X.; Fang, D.; Qu, L.; Xu, D.; Qin, X.; Qin, B.; Song, W.; Shao, Z.; Yi, B. Carbon-supported ultrafine Pt nanoparticles modified with trace amounts of cobalt as enhanced oxygen reduction reaction catalysts for proton exchange membrane fuel cells. Chin. J. Catal. 2019, 40, 504–514. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Chen, D.; Cui, P.; Ye, F.; Yang, J. Uniformly dispersed platinum-cobalt alloy nanoparticles with stable compositions on carbon substrates for methanol oxidation reaction. Sci. Rep. 2017, 7, 11421. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yin, L.; Yang, T.; Huang, Q.; He, M.; Zhao, H.; Zhang, D.; Wang, M.; Tong, Z. One-Pot Synthesis of Concave Platinum–Cobalt Nanocrystals and Their Superior Catalytic Performances for Methanol Electrochemical Oxidation and Oxygen Electrochemical Reduction. ACS Appl. Mater. Interfaces 2017, 9, 36164–36172. [Google Scholar] [CrossRef]
- Xu, W.C.; Zhang, Z.M.; Yang, C.H.; Zhao, K.M.; Wang, Y.; Tian, N.; Zhou, Z.-Y.; Sun, S.-G. Promotion mechanism of PtCo intermetallic ordered alloys in oxygen reduction reaction and its application in fuel cells. Electrochem. Commun. 2023, 152, 107516. [Google Scholar] [CrossRef]
- Nie, Y.; Deng, J.; Jin, W.; Guo, W.; Wu, G.; Deng, M.; Zhou, J.; Yang, R.; Zhang, S.; Wei, Z. Engineering multi-hollow PtCo nanoparticles for oxygen reduction reaction via a NaCl-sealed annealing strategy. J. Alloys Compd. 2021, 884, 161063. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, C.; Li, C.; Zeng, Y.; Hwang, S.; Li, B.; Karakalos, S.; Park, J.; Kropf, A.J.; Wegener, E.C.; et al. Atomically dispersed single iron sites for promoting Pt and Pt3Co fuel cell catalysts: Performance and durability improvements. Energy Environ. Sci. 2021, 14, 4948–4960. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Hu, J.; Wang, C.; Luo, M.; Wang, X.; Zhai, F.; Zheng, J. Pt3(CoNi) Ternary Intermetallic Nanoparticles Immobilized on N-Doped Carbon Derived from Zeolitic Imidazolate Frameworks for Oxygen Reduction. Materials 2024, 17, 4775. https://doi.org/10.3390/ma17194775
Song S, Hu J, Wang C, Luo M, Wang X, Zhai F, Zheng J. Pt3(CoNi) Ternary Intermetallic Nanoparticles Immobilized on N-Doped Carbon Derived from Zeolitic Imidazolate Frameworks for Oxygen Reduction. Materials. 2024; 17(19):4775. https://doi.org/10.3390/ma17194775
Chicago/Turabian StyleSong, Shiqi, Junhua Hu, Chupeng Wang, Mingsheng Luo, Xiaoxia Wang, Fengxia Zhai, and Jianyong Zheng. 2024. "Pt3(CoNi) Ternary Intermetallic Nanoparticles Immobilized on N-Doped Carbon Derived from Zeolitic Imidazolate Frameworks for Oxygen Reduction" Materials 17, no. 19: 4775. https://doi.org/10.3390/ma17194775






