An Up-to-Date Review of Materials Science Advances in Bone Grafting for Oral and Maxillofacial Pathology
Abstract
:1. Introduction
2. Autografts, Allografts, and Xenografts and Current Challenges
3. Recent Advancements in Materials Science Applied to Bone Grafting
3.1. Growth Factors and Biologics
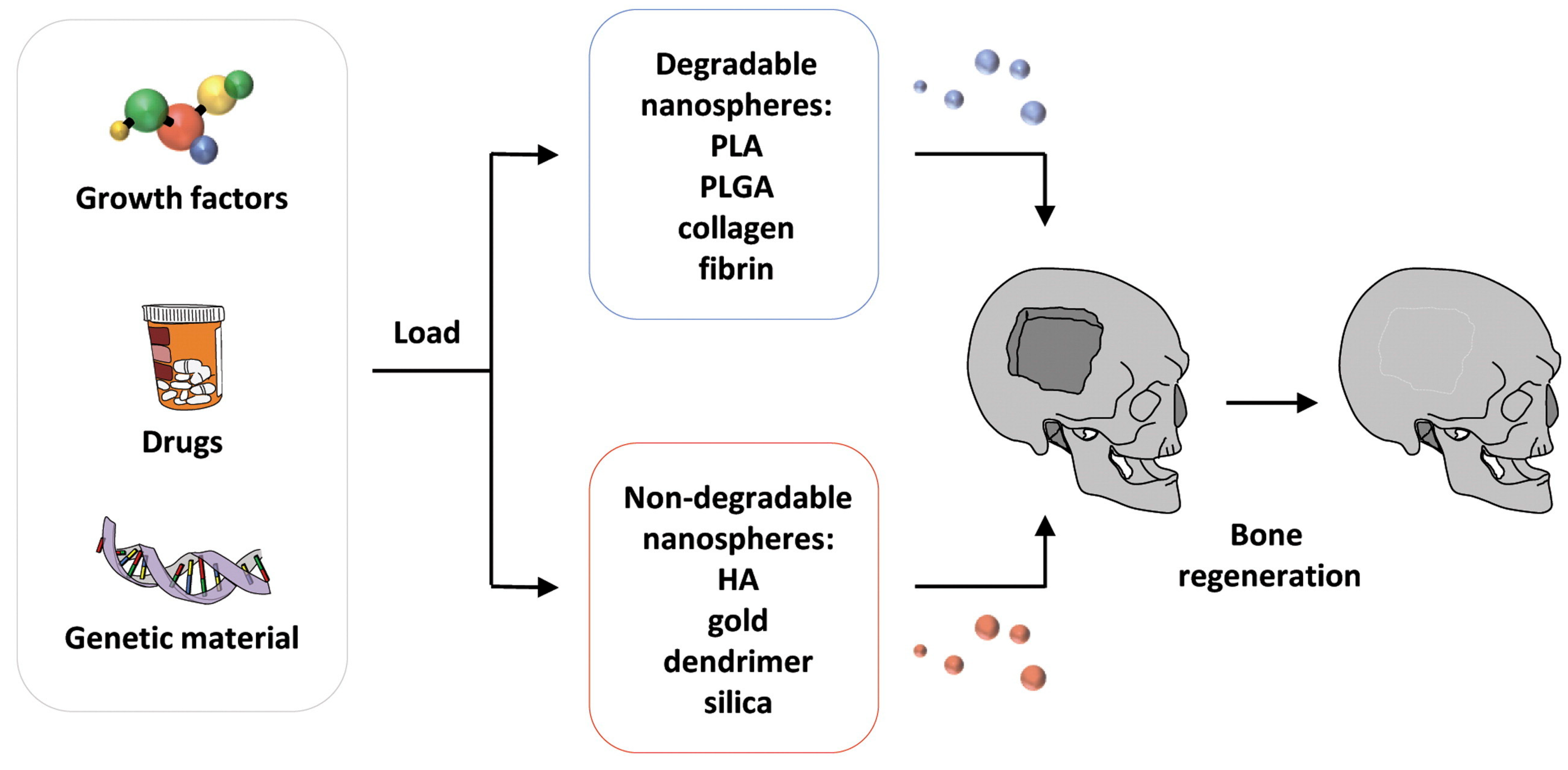
3.2. Nanotechnological Approaches
3.3. Advanced Manufacturing Techniques
3.3.1. Additive Manufacturing and 3D Printing Techniques
3.3.2. 3D Bioprinting
4. Clinical Applications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Migliorini, F.; La Padula, G.; Torsiello, E.; Spiezia, F.; Oliva, F.; Maffulli, N. Strategies for large bone defect reconstruction after trauma, infections or tumour excision: A comprehensive review of the literature. Eur. J. Med. Res. 2021, 26, 118. [Google Scholar] [CrossRef]
- Trumble, T.E.; Friedlaender, G.E. Allogeneic Bone in the Treatment of Tumors, Trauma, and Congenital Anomalies of the Hand. Orthop. Clin. N. Am. 1987, 18, 301–310. [Google Scholar] [CrossRef]
- Usui, M.; Onizuka, S.; Sato, T.; Kokabu, S.; Ariyoshi, W.; Nakashima, K. Mechanism of alveolar bone destruction in periodontitis—Periodontal bacteria and inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 201–208. [Google Scholar] [CrossRef]
- Yu, B.; Wang, C.Y. Osteoporosis and periodontal diseases—An update on their association and mechanistic links. Periodontology 2000 2022, 89, 99–113. [Google Scholar] [CrossRef]
- Gul, S.S. Prevalence and Severity of Circumferential Alveolar Bone Loss Using CBCT Images: A Retrospective Study of 20,620 Surfaces of 5155 Teeth. Diagnostics 2024, 14, 507. [Google Scholar] [CrossRef]
- Major, R.; Kowalczyk, P.; Surmiak, M.; Łojszczyk, I.; Podgórski, R.; Trzaskowska, P.; Ciach, T.; Russmueller, G.; Kasperkiewicz, K.; Major, Ł.; et al. Patient specific implants for jawbone reconstruction after tumor resection. Colloids Surf. B Biointerfaces 2020, 193, 111056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-L.; Liu, Y.; Shan, X.-F.; Xie, S.; Kang, Y.-F.; Cai, Z.-G. Clinical Characterization of Oral and Maxillofacial Tumors and Tumor-Like Lesions in Children and Adolescents. J. Craniofacial Surg. 2023, 34, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Berlin-Broner, Y.; Al Bawaliz, L.; Levin, L. Implications of Post-Traumatic Treatment of Immature Maxillary Incisors. Int. Dent. J. 2023, 73, 337–345. [Google Scholar] [CrossRef]
- Gutmacher, Z.; Peled, E.; Norman, D.; Lin, S. Alveolar Bone Fracture: Pathognomonic Sign for Clinical Diagnosis. Open Dent. J. 2017, 11, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Idowu, A.E.; Adedapo, A.O.; Akhiwu, B.I.; Agbara, R.; Olaniyi, T.O.; Alufohai, O.O. Causes of Dental Trauma: Results of Findings among Patients in a Secondary Oral Healthcare Center, Jos, Nigeria. J. West Afr. Coll. Surg. 2021, 11, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Fesseha, H.; Fesseha, Y. Bone Grafting, Its Principle and Application: A Review. Open J. 2020, 1, 43–50. [Google Scholar] [CrossRef]
- Kumar, P.; Vinitha, B.; Fathima, G. Bone grafts in dentistry. J. Pharm. Bioallied Sci. 2013, 5, S125–S127. [Google Scholar] [CrossRef]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. S2), S96–S101. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Pasha, Z.; Kale, P. Advances in bone grafting techniques for dental implants: A comprehensive review. IP Int. J. Periodontol. Implantol. 2023, 8, 195–199. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Ferraz, M.P. Bone Grafts in Dental Medicine: An Overview of Autografts, Allografts and Synthetic Materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Antoniac, I.; Cama, G.; Komlev, V.S.; Ravaglioli, A. Bioactive Materials for Bone Tissue Engineering. Biomed. Res. Int. 2016, 2016, 3741428. [Google Scholar] [CrossRef]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef]
- Sallent, I.; Capella-Monsonís, H.; Procter, P.; Bozo, I.Y.; Deev, R.V.; Zubov, D.; Vasyliev, R.; Perale, G.; Pertici, G.; Baker, J. The few who made it: Commercially and clinically successful innovative bone grafts. Front. Bioeng. Biotechnol. 2020, 8, 952. [Google Scholar] [CrossRef] [PubMed]
- Govoni, M.; Vivarelli, L.; Mazzotta, A.; Stagni, C.; Maso, A.; Dallari, D. Commercial Bone Grafts Claimed as an Alternative to Autografts: Current Trends for Clinical Applications in Orthopaedics. Materials 2021, 14, 3290. [Google Scholar] [CrossRef]
- Archunan, M.W.; Petronis, S. Bone Grafts in Trauma and Orthopaedics. Cureus 2021, 13, e17705. [Google Scholar] [CrossRef]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone tissue engineering graft substitutes: What is the future? Injury 2021, 52, S72–S77. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Hazballa, D.; Inchingolo, A.D.; Malcangi, G.; Marinelli, G.; Mancini, A.; Maggiore, M.E.; Bordea, I.R.; Scarano, A.; Farronato, M.; et al. Innovative Concepts and Recent Breakthrough for Engineered Graft and Constructs for Bone Regeneration: A Literature Systematic Review. Materials 2022, 15, 1120. [Google Scholar] [CrossRef]
- Titsinides, S.; Agrogiannis, G.; Karatzas, T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. Jpn. Dent. Sci. Rev. 2019, 55, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Moussa, N.T.; Dym, H. Maxillofacial bone grafting materials. Dent. Clin. N. Am. 2020, 64, 473–490. [Google Scholar] [CrossRef]
- Miron, R.J. Optimized bone grafting. Periodontology 2000 2024, 94, 143–160. [Google Scholar] [CrossRef]
- Kämmerer, P.W.; Al-Nawas, B. Bone reconstruction of extensive maxillomandibular defects in adults. Periodontology 2000 2023, 93, 340–357. [Google Scholar] [CrossRef]
- Patel, P.; Newman, M. Biomaterial for Osseous Reconstruction. In Innovative Perspectives in Oral and Maxillofacial Surgery; Stevens, M.R., Ghasemi, S., Tabrizi, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 11–17. [Google Scholar]
- Altiparmak, N.; Soydan, S.S.; Uckan, S. The effect of conventional surgery and piezoelectric surgery bone harvesting techniques on the donor site morbidity of the mandibular ramus and symphysis. Int. J. Oral Maxillofac. Surg. 2015, 44, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.; Li, D.J.; Auston, D.A.; Mir, H.S.; Yoon, R.S.; Koval, K.J. Autograft, Allograft, and Bone Graft Substitutes: Clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J. Orthop. Trauma 2019, 33, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Eras, V.; Pruß, A.; Perka, C.; Brune, J.; Vu-Han, T.L. Allografts: Expanding the surgeon’s armamentarium. Cell Tissue Bank. 2023, 24, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Sassard, W.R.; Eidman, D.K.; Gray, P.M.; Block, J.E.; Russo, R.; Russell, J.L.; Taboada, E.M. Augmenting local bone with Grafton demineralized bone matrix for posterolateral lumbar spine fusion: Avoiding second site autologous bone harvest. Orthopedics 2000, 23, 1059–1065. [Google Scholar] [CrossRef]
- Kim, D.M.; Nevins, M.L.; Camelo, M.; Camelo, J.M.B.; Schupbach, P.; Hanratty, J.J.; Utzel, N.G.; Nevins, M. The efficacy of demineralized bone matrix and cancellous bone chips for maxillary sinus augmentation. Int. J. Periodontics Restor. Dent. 2009, 29, 414. [Google Scholar]
- Reddy, B.R.; Sudhakar, J.; Rajesh, N.; Sandeep, V.; Reddy, Y.M.; Sagar, W.G. Comparative clinical and radiographic evaluation of mineralized cancellous bone allograft (puros®) and autogenous bone in the treatment of human periodontal intraosseous defects: 6-months follow-up study. J. Int. Soc. Prev. Community Dent. 2016, 6, S248–S253. [Google Scholar]
- Bhamb, N.; Kanim, L.E.; Drapeau, S.; Mohan, S.; Vasquez, E.; Shimko, D.; McKAY, W.; Bae, H.W. Comparative efficacy of commonly available human bone graft substitutes as tested for posterolateral fusion in an athymic rat model. Int. J. Spine Surg. 2019, 13, 437–458. [Google Scholar] [CrossRef]
- Giedraitis, A.; Arnoczky, S.P.; Bedi, A. Allografts in soft tissue reconstructive procedures: Important considerations. Sports Health 2014, 6, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Moon, K.; Du, W.; Cho, W.T.; Huh, J.B.; Bae, E.B. Effect of Porcine- and Bovine-Derived Xenografts with Hydroxypropyl Methylcellulose for Bone Formation in Rabbit Calvaria Defects. Materials 2023, 16, 1850. [Google Scholar] [CrossRef]
- Lu, T.; Yang, B.; Wang, R.; Qin, C. Xenotransplantation: Current Status in Preclinical Research. Front. Immunol. 2020, 10, 1850. [Google Scholar] [CrossRef]
- Kim, Y.J.; Saiki, C.E.T.; Silva, K.; Massuda, C.K.M.; de Souza Faloni, A.P.; Braz-Silva, P.H.; Pallos, D.; Sendyk, W.R. Bone Formation in Grafts with Bio-Oss and Autogenous Bone at Different Proportions in Rabbit Calvaria. Int. J. Dent. 2020, 2020, 2494128. [Google Scholar] [CrossRef]
- Scarano, A.; De Oliveira, P.S.; Traini, T.; Lorusso, F. Sinus membrane elevation with heterologous cortical lamina: A randomized study of a new surgical technique for maxillary sinus floor augmentation without bone graft. Materials 2018, 11, 1457. [Google Scholar] [CrossRef]
- Okumus, A.; Guven, E.; Ermis, I.; Olgac, V.; Arinci, A.; Metin, E. Comparative Analysis of Using Bone Graft, Hydroxyapatite Coralline (Biocoral®) and Porous Polyethylene (Medpor®) Implants for Cranioplasty in a Rat Model of Cranial Bone Defect. Turk. Neurosurg. 2020, 30, 263–270. [Google Scholar] [CrossRef]
- Trajkovski, B.; Jaunich, M.; Müller, W.-D.; Beuer, F.; Zafiropoulos, G.-G.; Houshmand, A. Hydrophilicity, viscoelastic, and physicochemical properties variations in dental bone grafting substitutes. Materials 2018, 11, 215. [Google Scholar] [CrossRef]
- Mazzoni, E.; D’Agostino, A.; Iaquinta, M.R.; Bononi, I.; Trevisiol, L.; Rotondo, J.C.; Patergnani, S.; Giorgi, C.; Gunson, M.J.; Arnett, G.W.; et al. Hydroxylapatite-collagen hybrid scaffold induces human adipose-derived mesenchymal stem cells to osteogenic differentiation in vitro and bone regrowth in patients. Stem Cells Transl. Med. 2020, 9, 377–388. [Google Scholar] [CrossRef]
- Honig, J.; Merten, H. Correspondence and Brief Communications-Reply-Risk of Transmission of Agents Associated with Creutzfeldt-Jakob Disease and Bovine Spongiform Encephalopathy. Plast. Reconstr. Surg.-Baltim. 2000, 105, 2274. [Google Scholar]
- Haggerty, C.J.; Vogel, C.T.; Fisher, G.R. Simple Bone Augmentation for Alveolar Ridge Defects. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 203–226. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Hernández-Cortés, P.; Mesa, F.; Carranza, N.; Juodzbalys, G.; Aguilar, M.; O’Valle, F. Slow resorption of anorganic bovine bone by osteoclasts in maxillary sinus augmentation. Clin. Implant Dent. Relat. Res. 2013, 15, 858–866. [Google Scholar] [CrossRef]
- Zimmermann, G.; Moghaddam, A. Allograft bone matrix versus synthetic bone graft substitutes. Injury 2011, 42, S16–S21. [Google Scholar] [CrossRef]
- Sohn, H.-S.; Oh, J.-K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Redondo, F.L.; Giaroli, M.C.; Ciolino, A.E.; Ninago, M.D. Preparation of Porous Poly (Lactic Acid)/Tricalcium Phosphate Composite Scaffolds for Tissue Engineering. Biointerface Res. Appl. Chem. 2022, 12, 5610–5624. [Google Scholar] [CrossRef]
- Kamboj, M.; Arora, R.; Gupta, H. Comparative evaluation of the efficacy of synthetic nanocrystalline hydroxyapatite bone graft (Ostim(®)) and synthetic microcrystalline hydroxyapatite bone graft (Osteogen(®)) in the treatment of human periodontal intrabony defects: A clinical and denta scan study. J. Indian Soc. Periodontol. 2016, 20, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Ogose, A.; Kondo, N.; Umezu, H.; Hotta, T.; Kawashima, H.; Tokunaga, K.; Ito, T.; Kudo, N.; Hoshino, M.; Gu, W.; et al. Histological assessment in grafts of highly purified beta-tricalcium phosphate (OSferion) in human bones. Biomaterials 2006, 27, 1542–1549. [Google Scholar] [CrossRef]
- Chacko, N.L.; Abraham, S.; Rao, H.N.; Sridhar, N.; Moon, N.; Barde, D.H. A Clinical and Radiographic Evaluation of Periodontal Regenerative Potential of PerioGlas®: A Synthetic, Resorbable Material in Treating Periodontal Infrabony Defects. J. Int. Oral Health 2014, 6, 20–26. [Google Scholar]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology-is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant Dent. 2017, 3, 23. [Google Scholar] [CrossRef]
- Georgeanu, V.A.; Gingu, O.; Antoniac, I.V.; Manolea, H.O. Current Options and Future Perspectives on Bone Graft and Biomaterials Substitutes for Bone Repair, from Clinical Needs to Advanced Biomaterials Research. Appl. Sci. 2023, 13, 8471. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Nowzari, H. The long-term risks and complications of bovine-derived xenografts: A case series. J. Indian Soc. Periodontol. 2019, 23, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.S.; Cabral, J.; Silva, C.; Vashishth, D. Bone Matrix Non-Collagenous Proteins in Tissue Engineering: Creating New Bone by Mimicking the Extracellular Matrix. Polymers 2021, 13, 1095. [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Budak, I.; Mirkovic, S.; Sokac, M.; Santosi, Z.; Puskar, T.; Vukelic, D. An approach to modelling of personalized bone grafts based on advanced technologies. Int. J. Simul. Model. 2016, 15, 637–648. [Google Scholar] [CrossRef]
- Huang, M.F.; Alfi, D.; Alfi, J.; Huang, A.T. The Use of Patient-Specific Implants in Oral and Maxillofacial Surgery. Oral Maxillofac. Surg. Clin. 2019, 31, 593–600. [Google Scholar] [CrossRef]
- Shujaat, S.; Riaz, M.; Jacobs, R. Synergy between artificial intelligence and precision medicine for computer-assisted oral and maxillofacial surgical planning. Clin. Oral Investig. 2023, 27, 897–906. [Google Scholar] [CrossRef]
- Brachet, A.; Bełżek, A.; Furtak, D.; Geworgjan, Z.; Tulej, D.; Kulczycka, K.; Karpiński, R.; Maciejewski, M.; Baj, J. Application of 3D Printing in Bone Grafts. Cells 2023, 12, 859. [Google Scholar] [CrossRef]
- Mirkhalaf, M.; Men, Y.; Wang, R.; No, Y.; Zreiqat, H. Personalized 3D printed bone scaffolds: A review. Acta Biomater. 2023, 156, 110–124. [Google Scholar] [CrossRef]
- Ivanovski, S.; Breik, O.; Carluccio, D.; Alayan, J.; Staples, R.; Vaquette, C. 3D printing for bone regeneration: Challenges and opportunities for achieving predictability. Periodontology 2000 2023, 93, 358–384. [Google Scholar] [CrossRef]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in bone tissue engineering. Nanomedicine 2015, 11, 1253–1263. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469. [Google Scholar] [CrossRef]
- Kulebyakin, K.Y.; Nimiritsky, P.P.; Makarevich, P.I. Growth Factors in Regeneration and Regenerative Medicine: “The Cure and the Cause”. Front. Endocrinol. 2020, 11, 384. [Google Scholar] [CrossRef]
- Ball, J.R.; Shelby, T.; Hernandez, F.; Mayfield, C.K.; Lieberman, J.R. Delivery of Growth Factors to Enhance Bone Repair. Bioengineering 2023, 10, 1252. [Google Scholar] [CrossRef]
- Donos, N.; Akcali, A.; Padhye, N.; Sculean, A.; Calciolari, E. Bone regeneration in implant dentistry: Which are the factors affecting the clinical outcome? Periodontology 2000 2023, 93, 26–55. [Google Scholar] [CrossRef]
- Shah, P.; Keppler, L.; Rutkowski, J. Bone morphogenic protein: An elixir for bone grafting—A review. J. Oral Implant 2012, 38, 767–778. [Google Scholar] [CrossRef]
- Chenard, K.E.; Teven, C.M.; He, T.C.; Reid, R.R. Bone morphogenetic proteins in craniofacial surgery: Current techniques, clinical experiences, and the future of personalized stem cell therapy. J. Biomed. Biotechnol. 2012, 2012, 601549. [Google Scholar] [CrossRef]
- Katagiri, T.; Watabe, T. Bone Morphogenetic Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Kawai, M.; Rosen, C.J. The insulin-like growth factor system in bone: Basic and clinical implications. Endocrinol. Metab. Clin. N. Am. 2012, 41, 323–333. [Google Scholar] [CrossRef]
- Adil, A.; Rouabhia, M.; Zhang, Z. Potential Use of Bone Tissue Engineering to Treat Human Bone Defects. In Bone Grafts: Procedures, Complications and Alternatives; Nova Science Publisher: New York, NY, USA, 2013. [Google Scholar]
- Lee, D.K.; Ki, M.-R.; Kim, E.H.; Park, C.-J.; Ryu, J.J.; Jang, H.S.; Pack, S.P.; Jo, Y.K.; Jun, S.H. Biosilicated collagen/β-tricalcium phosphate composites as a BMP-2-delivering bone-graft substitute for accelerated craniofacial bone regeneration. Biomater. Res. 2021, 25, 13. [Google Scholar] [CrossRef]
- Wadhwa, P.; Lee, J.H.; Zhao, B.C.; Cai, H.; Rim, J.-S.; Jang, H.-S.; Lee, E.-S. Microcomputed Tomography and Histological Study of Bone Regeneration Using Tooth Biomaterial with BMP-2 in Rabbit Calvarial Defects. Scanning 2021, 2021, 6690221. [Google Scholar] [CrossRef]
- Liu, B.; Yin, N.B.; Xiao, R.; Li, B.H.; Li, H.D.; Chen, S.X.; Li, S.L.; Wang, Y.Q. Evaluating the efficacy of recombinant human bone morphogenic protein-2 in the treatment of alveolar clefts with autologous bone grafting using computer-aided engineering techniques. Br. J. Oral Maxillofac. Surg. 2021, 59, 757–762. [Google Scholar] [CrossRef]
- Asparuhova, M.B.; Riedwyl, D.; Aizawa, R.; Raabe, C.; Couso-Queiruga, E.; Chappuis, V. Local Concentrations of TGF-β1 and IGF-1 Appear Determinant in Regulating Bone Regeneration in Human Postextraction Tooth Sockets. Int. J. Mol. Sci. 2023, 24, 8239. [Google Scholar] [CrossRef] [PubMed]
- Amaral Valladão, C.A.; Freitas Monteiro, M.; Joly, J.C. Guided bone regeneration in staged vertical and horizontal bone augmentation using platelet-rich fibrin associated with bone grafts: A retrospective clinical study. Int. J. Implant Dent. 2020, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-c.; Li, X.; Liu, H.; Wu, F.; Yang, L.; Su, Y.; Li, J.; Duan, S.-y. Clinical applications of concentrated growth factors combined with bone substitutes for alveolar ridge preservation in maxillary molar area: A randomized controlled trial. Int. J. Implant Dent. 2021, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Paolantonio, M.; Di Tullio, M.; Giraudi, M.; Romano, L.; Secondi, L.; Paolantonio, G.; Graziani, F.; Pilloni, A.; De Ninis, P.; Femminella, B. Periodontal regeneration by leukocyte and platelet-rich fibrin with autogenous bone graft versus enamel matrix derivative with autogenous bone graft in the treatment of periodontal intrabony defects: A randomized non-inferiority trial. J. Periodontol. 2020, 91, 1595–1608. [Google Scholar] [CrossRef]
- Liu, K.; Meng, C.-X.; Lv, Z.-Y.; Zhang, Y.-J.; Li, J.; Li, K.-Y.; Liu, F.-Z.; Zhang, B.; Cui, F.-Z. Enhancement of BMP-2 and VEGF carried by mineralized collagen for mandibular bone regeneration. Regen. Biomater. 2020, 7, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, H.; Duan, Q.; Bao, H.; Li, A.; Li, W.; Chen, J.; He, Y. A comparative study of the effects of platelet-rich fibrin, concentrated growth factor and platelet-poor plasma on the healing of tooth extraction sockets in rabbits. BMC Oral Health 2022, 22, 87. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Adult Stem Cells for Bone Regeneration and Repair. Front. Cell Dev. Biol. 2019, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Bertolai, R.; Catelani, C.; Aversa, A.; Rossi, A.; Giannini, D.; Bani, D. Bone graft and mesenchimal stem cells: Clinical observations and histological analysis. Clin. Cases Min. Bone Metab. 2015, 12, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Wofford, A.; Bow, A.; Newby, S.; Brooks, S.; Rodriguez, R.; Masi, T.; Stephenson, S.; Gotcher, J.; Anderson, D.E.; Campbell, J. Human fat-derived mesenchymal stem cells xenogenically implanted in a rat model show enhanced new bone formation in maxillary alveolar tooth defects. Stem Cells Int. 2020, 2020, 8142938. [Google Scholar] [CrossRef]
- Shahnaseri, S.; Sheikhi, M.; Hashemibeni, B.; Mousavi, S.A.; Soltani, P. Comparison of Autogenous Bone Graft and Tissue-Engineered Bone Graft in Alveolar Cleft Defects in Canine Animal Models Using Digital Radiography. Indian J. Dent. Res. 2020, 31, 118–123. [Google Scholar] [PubMed]
- Gutiérrez-Quintero, J.G.; Durán Riveros, J.Y.; Martínez Valbuena, C.A.; Pedraza Alonso, S.; Munévar, J.C.; Viafara-García, S.M. Critical-sized mandibular defect reconstruction using human dental pulp stem cells in a xenograft model-clinical, radiological, and histological evaluation. Oral Maxillofac. Surg. 2020, 24, 485–493. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef]
- Salem, S.S.; Hammad, E.N.; Mohamed, A.A.; El-Dougdoug, W. A comprehensive review of nanomaterials: Types, synthesis, characterization, and applications. Biointerface Res. Appl. Chem. 2022, 13, 41. [Google Scholar]
- Bauso, L.V.; La Fauci, V.; Longo, C.; Calabrese, G. Bone Tissue Engineering and Nanotechnology: A Promising Combination for Bone Regeneration. Biology 2024, 13, 237. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mousavi Shaegh, S.A.; Alibolandi, M.; Ebrahimzadeh, M.H.; Tamayol, A.; Jaafari, M.R.; Ramezani, M. Micro and nanotechnologies for bone regeneration: Recent advances and emerging designs. J. Control. Release 2018, 274, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.O.d. Overview of nanomaterials and cellular interactions. Biointerface Res. Appl. Chem. 2023, 13, 367. [Google Scholar]
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Z.; Yang, L.; Du, W.; Yu, T.; Tang, H.; Li, C.; Qiu, H. The role of natural polymers in bone tissue engineering. J. Control. Release 2021, 338, 571–582. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Critical Review of Biodegradable and Bioactive Polymer Composites for Bone Tissue Engineering and Drug Delivery Applications. Polymers 2021, 13, 2623. [Google Scholar] [CrossRef] [PubMed]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef]
- Pourhajrezaei, S.; Abbas, Z.; Khalili, M.A.; Madineh, H.; Jooya, H.; Babaeizad, A.; Gross, J.D.; Samadi, A. Bioactive polymers: A comprehensive review on bone grafting biomaterials. Int. J. Biol. Macromol. 2024, 278, 134615. [Google Scholar] [CrossRef] [PubMed]
- Stylios, G.; Wan, T.; Giannoudis, P. Present status and future potential of enhancing bone healing using nanotechnology. Injury 2007, 38, S63–S74. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 6, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Ghavimi, M.A.; Bani Shahabadi, A.; Jarolmasjed, S.; Memar, M.Y.; Maleki Dizaj, S.; Sharifi, S. Nanofibrous asymmetric collagen/curcumin membrane containing aspirin-loaded PLGA nanoparticles for guided bone regeneration. Sci. Rep. 2020, 10, 18200. [Google Scholar] [CrossRef]
- Zirak, N.; Maadani, A.M.; Salahinejad, E.; Abbasnezhad, N.; Shirinbayan, M. Fabrication, drug delivery kinetics and cell viability assay of PLGA-coated vancomycin-loaded silicate porous microspheres. Ceram. Int. 2022, 48, 48–54. [Google Scholar] [CrossRef]
- Fan, L.; Ren, Y.; Emmert, S.; Vučković, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef]
- Rastogi, K.; Vashishtha, R.; Shaloo, D.S. Scientific advances and pharmacological applications of marine derived-collagen and chitosan. Biointerface Res. Appl. Chem. 2022, 12, 3540–3558. [Google Scholar]
- Banche-Niclot, F.; Licini, C.; Montalbano, G.; Fiorilli, S.; Mattioli-Belmonte, M.; Vitale-Brovarone, C. 3D Printed Scaffold Based on Type I Collagen/PLGA_TGF-β1 Nanoparticles Mimicking the Growth Factor Footprint of Human Bone Tissue. Polymers 2022, 14, 857. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Jikan, S.S.B.; Adzila, S.; Murni, Z.; Badarulzaman, N.A.; Rosley, R.; Hameed, M.U. Synthesis and characterizations of hydroxyapatite using precursor extracted from chicken egg shell waste. Biointerface Res. Appl. Chem. 2022, 12, 5663–5671. [Google Scholar]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite Based Materials for Bone Tissue Engineering: A Brief and Comprehensive Introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Barbosa, F.; Garrudo, F.F.F.; Alberte, P.S.; Resina, L.; Carvalho, M.S.; Jain, A.; Marques, A.C.; Estrany, F.; Rawson, F.J.; Aléman, C.; et al. Hydroxyapatite-filled osteoinductive and piezoelectric nanofibers for bone tissue engineering. Sci. Technol. Adv. Mater. 2023, 24, 2242242. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.A.; Pickering, K.L.; Mucalo, M.R. A Review on the Use of Hydroxyapatite-Carbonaceous Structure Composites in Bone Replacement Materials for Strengthening Purposes. Materials 2018, 11, 1813. [Google Scholar] [CrossRef] [PubMed]
- Afridah, W.; Wikurendra, E.A.; Amalia, R.; Syafiuddin, A. Synthesis and Characterization of Hydroxyapatite Derived from Milkfish Bone by Simple Heat Treatments. Biointerface Res. Appl. Chem. 2022, 12, 2440–2449. [Google Scholar]
- Calabrese, G.; Petralia, S.; Fabbi, C.; Forte, S.; Franco, D.; Guglielmino, S.; Esposito, E.; Cuzzocrea, S.; Traina, F.; Conoci, S. Au, Pd and maghemite nanofunctionalized hydroxyapatite scaffolds for bone regeneration. Regen. Biomater. 2020, 7, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Narciso, A.M.; da Rosa, C.G.; Nunes, M.R.; Sganzerla, W.G.; Hansen, C.M.; de Melo, A.P.Z.; Paes, J.V.; Bertoldi, F.C.; Barreto, P.L.M.; Masiero, A.V. Antimicrobial green silver nanoparticles in bone grafts functionalization for biomedical applications. Biocatal. Agric. Biotechnol. 2021, 35, 102074. [Google Scholar] [CrossRef]
- Alsaeed, M.A.; Al-Ghaban, N.M.H. Chitosan Nanoparticle/Simvastatin for Experimental Maxillary Bony Defect Healing: A Histological and Histomorphometrical Study. Biomimetics 2023, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Afifi, O.H.; Ghoneim, S.M.; Youssef, D.A. The effect of titanium dioxide nanoparticles with or without platelet rich plasma on the healing of mandibular bony defects in rabbits. Tanta Dent. J. 2022, 19, 68–76. [Google Scholar] [CrossRef]
- Senthil, R.; Çakır, S. Nano apatite growth on demineralized bone matrix capped with curcumin and silver nanoparticles: Dental implant mechanical stability and optimal cell growth analysis. J. Oral Biosci. 2024, 66, 232–240. [Google Scholar] [CrossRef]
- Ren, S.; Zhou, Y.; Zheng, K.; Xu, X.; Yang, J.; Wang, X.; Miao, L.; Wei, H.; Xu, Y. Cerium oxide nanoparticles loaded nanofibrous membranes promote bone regeneration for periodontal tissue engineering. Bioact. Mater. 2022, 7, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Llano, C.H.; Solano, M.A.; Grande-Tovar, C.D. Nanocomposites of Chitosan/Graphene Oxide/Titanium Dioxide Nanoparticles/Blackberry Waste Extract as Potential Bone Substitutes. Polymers 2021, 13, 3877. [Google Scholar] [CrossRef]
- Pandey, R.K.; Prajapati, V.K. Molecular and immunological toxic effects of nanoparticles. Int. J. Biol. Macromol. 2018, 107, 1278–1293. [Google Scholar] [CrossRef]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanoparticle Res. 2023, 25, 43. [Google Scholar] [CrossRef]
- Bhushan, S.; Singh, S.; Maiti, T.K.; Sharma, C.; Dutt, D.; Sharma, S.; Li, C.; Tag Eldin, E.M. Scaffold Fabrication Techniques of Biomaterials for Bone Tissue Engineering: A Critical Review. Bioengineering 2022, 9, 728. [Google Scholar] [CrossRef] [PubMed]
- Steijvers, E.; Ghei, A.; Xia, Z. Manufacturing artificial bone allografts: A perspective. Biomater. Transl. 2022, 3, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.; Becerro, A.; Pardal-Peláez, B.; Quispe-López, N.; Blanco, J.-F.; Gómez-Polo, C. Main 3D Manufacturing Techniques for Customized Bone Substitutes. A Systematic Review. Materials 2021, 14, 2524. [Google Scholar] [CrossRef]
- Senusi, F.; Mahmood, S.; Ngadiman, N.H.A.; Zameri, M. Environmental Impact for 3D Bone Tissue Engineering Scaffolds Life Cycle: An Assessment. Biointerface Res. Appl. Chem. 2022, 12, 6504–6515. [Google Scholar]
- Sears, N.; Dhavalikar, P.; Whitely, M.; Cosgriff-Hernandez, E. Fabrication of biomimetic bone grafts with multi-material 3D printing. Biofabrication 2017, 9, 025020. [Google Scholar] [CrossRef] [PubMed]
- Anbu, R.T.; Suresh, V.; Gounder, R.; Kannan, A. Comparison of the efficacy of three different bone regeneration materials: An animal study. Eur. J. Dent. 2019, 13, 022–028. [Google Scholar] [CrossRef] [PubMed]
- Korn, P.; Ahlfeld, T.; Lahmeyer, F.; Kilian, D.; Sembdner, P.; Stelzer, R.; Pradel, W.; Franke, A.; Rauner, M.; Range, U.; et al. 3D Printing of Bone Grafts for Cleft Alveolar Osteoplasty—In vivo Evaluation in a Preclinical Model. Front. Bioeng. Biotechnol. 2020, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Bagheri Saed, A.; Behravesh, A.H.; Hasannia, S.; Akhoundi, B.; Hedayati, S.K.; Gashtasbi, F. An in vitro study on the key features of Poly L-lactic acid/biphasic calcium phosphate scaffolds fabricated via DLP 3D printing for bone grafting. Eur. Polym. J. 2020, 141, 110057. [Google Scholar] [CrossRef]
- Salah, M.; Tayebi, L.; Moharamzadeh, K.; Naini, F.B. Three-dimensional bio-printing and bone tissue engineering: Technical innovations and potential applications in maxillofacial reconstructive surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 18. [Google Scholar] [CrossRef]
- Genova, T.; Roato, I.; Carossa, M.; Motta, C.; Cavagnetto, D.; Mussano, F. Advances on Bone Substitutes through 3D Bioprinting. Int. J. Mol. Sci. 2020, 21, 7012. [Google Scholar] [CrossRef]
- Amler, A.-K.; Dinkelborg, P.H.; Schlauch, D.; Spinnen, J.; Stich, S.; Lauster, R.; Sittinger, M.; Nahles, S.; Heiland, M.; Kloke, L.; et al. Comparison of the Translational Potential of Human Mesenchymal Progenitor Cells from Different Bone Entities for Autologous 3D Bioprinted Bone Grafts. Int. J. Mol. Sci. 2021, 22, 796. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, T.; Tenorio, A.J.; Campbell, K.T.; Silva, E.A.; Leach, J.K. Alginate-based bioinks for 3D bioprinting and fabrication of anatomically accurate bone grafts. Tissue Eng. Part A 2021, 27, 1168–1181. [Google Scholar] [CrossRef]
- Dubey, N.; Ferreira, J.A.; Malda, J.; Bhaduri, S.B.; Bottino, M.C. Extracellular Matrix/Amorphous Magnesium Phosphate Bioink for 3D Bioprinting of Craniomaxillofacial Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 23752–23763. [Google Scholar] [CrossRef] [PubMed]
- Tharakan, S.; Khondkar, S.; Ilyas, A. Bioprinting of Stem Cells in Multimaterial Scaffolds and Their Applications in Bone Tissue Engineering. Sensors 2021, 21, 7477. [Google Scholar] [CrossRef]
- Yang, Z.; Yi, P.; Liu, Z.; Zhang, W.; Mei, L.; Feng, C.; Tu, C.; Li, Z. Stem cell-laden hydrogel-based 3D bioprinting for bone and cartilage tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 865770. [Google Scholar] [CrossRef]
- Gkika, D.A.; Maliaris, G.; Vordos, N.; Mitropoulos, A.C.; Kyzas, G.Z. Cost Profile of 3D Printing Using Biomaterials on a Lab Scale. Biointerface Res. Appl. Chem. 2023, 13, 93. [Google Scholar]
- Chiapasco, M.; Casentini, P.; Tommasato, G.; Dellavia, C.; Del Fabbro, M. Customized CAD/CAM titanium meshes for the guided bone regeneration of severe alveolar ridge defects: Preliminary results of a retrospective clinical study in humans. Clin. Oral Implant Res. 2021, 32, 498–510. [Google Scholar] [CrossRef]
- Sánchez-Labrador, L.; Martín-Ares, M.; Ortega-Aranegui, R.; López-Quiles, J.; Martínez-González, J.M. Autogenous Dentin Graft in Bone Defects after Lower Third Molar Extraction: A Split-Mouth Clinical Trial. Materials 2020, 13, 3090. [Google Scholar] [CrossRef]
- Kawai, T.; Kamakura, S.; Matsui, K.; Fukuda, M.; Takano, H.; Iino, M.; Ishikawa, S.; Kawana, H.; Soma, T.; Imamura, E.; et al. Clinical study of octacalcium phosphate and collagen composite in oral and maxillofacial surgery. J. Tissue Eng. 2020, 11, 2041731419896449. [Google Scholar] [CrossRef]
- Thoma, D.S.; Gasser, T.J.; Jung, R.E.; Hämmerle, C.H. Randomized controlled clinical trial comparing implant sites augmented with a volume-stable collagen matrix or an autogenous connective tissue graft: 3-year data after insertion of reconstructions. J. Clin. Periodontol. 2020, 47, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Kijartorn, P.; Wongpairojpanich, J.; Thammarakcharoen, F.; Suwanprateeb, J.; Buranawat, B. Clinical evaluation of 3D printed nano-porous hydroxyapatite bone graft for alveolar ridge preservation: A randomized controlled trial. J. Dent. Sci. 2022, 17, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.-L.; Lim, J.-Y.; Park, S.-N.; Choi, B.-H.; Kang, H.; Choi, W.-C. A Clinical Trial to Evaluate the Efficacy and Safety of 3D Printed Bioceramic Implants for the Reconstruction of Zygomatic Bone Defects. Materials 2020, 13, 4515. [Google Scholar] [CrossRef] [PubMed]
- Dragosloveanu, Ş.; Dragosloveanu, C.D.Μ.; Stanca, H.T.; Cotor, D.C.; Andrei, A.C.; Dragosloveanu, C.I.; Stoica, C.I. Tricalcium phosphate and hydroxyapatite treatment for benign cavitary bone lesions: A prospective clinical trial. Exp. Ther. Med. 2020, 20, 215. [Google Scholar] [CrossRef]
- Menezes, J.D.; Pereira, R.d.S.; Santos, A.M.d.S.; de Siqueira, N.B.; Boos-Lima, F.B.D.J.; Hochuli-Vieira, E. Three-dimensional volumetric changes of 5 different bone grafts in human maxillary sinuses reconstruction: A randomized clinical study. Oral Maxillofac. Surg. 2021, 25, 541–547. [Google Scholar] [CrossRef]
- Oral Surgery|Bone Grafting. Available online: https://clinicaltrials.gov/search?cond=Oral%20Surgery&intr=Bone%20grafting (accessed on 14 September 2024).
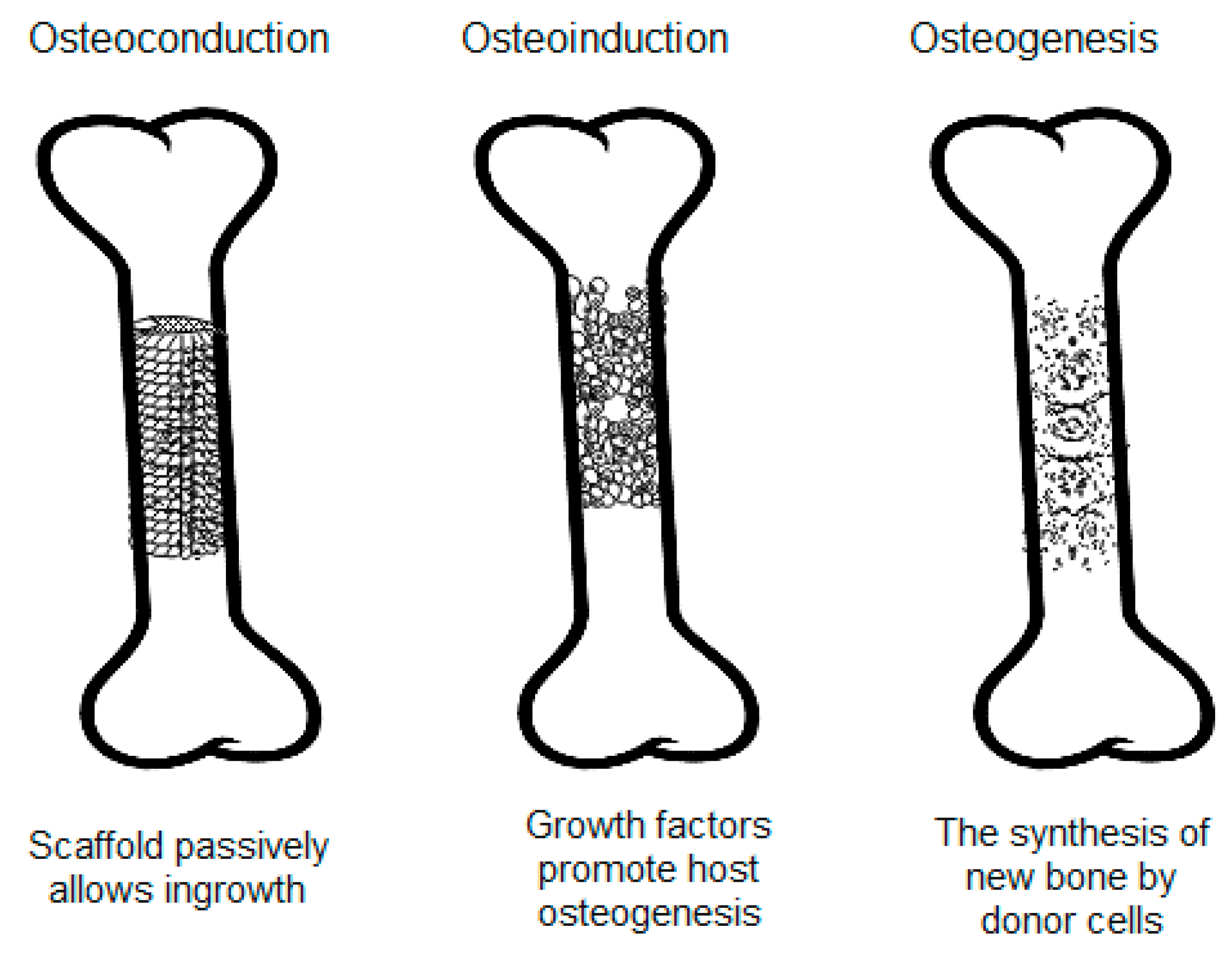
| Type of Graft | Source | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Autograft | Bone from the patient’s own body (e.g., from intraoral sites, iliac crest, cranium, mandible, radius, or tibia) | Osteogenic Osteoinductive Osteoconductive Biocompatible Very low risk of immune rejection No disease transmission Fast bone regeneration | Risk of infection Possibility of insufficient bone for transplant Need for extra surgery, which might result in pain, long recovery time, scars Donor site morbidity | [18,26,55] |
| Allografts | Freeze-dried bone, demineralized bone matrix (DBM), or mineralized bone extracted from living donors or cadavers | Osteoinductive Osteoconductive Single surgery needed Ease of harvesting | Risk of disease transmission Risk of immune rejection Blood incompatibility Diminished structural integrity due to irradiation | [13,18,26,56] |
| Xenografts | Deproteinized bone material extracted from non-human sources (bovine or porcine) | Osteoconductive Availability Similarity with human bone Low cost | Possibility of disease transmission Immunogenicity | [18,26,57] |
| Synthetic bone substitutes | HA, TCP, bioactive glasses, polymers, and some metals | Osteoconductive Availability Other advantages depend on the materials | Disadvantages depend on the materials | [16,26] |
| Graft | Targeted Defect | Findings | Refs. |
|---|---|---|---|
| Autogenous and xenogenous bone grafts with platelet-rich fibrin (PRF) | Vertical and horizontal bone augmentation prior to dental implant placement | Achieved average bone gains of 5.9 mm in thickness and 5.6 mm in height Improved graft handling and stability PRF enhanced soft tissue healing | [81] |
| Concentrated growth factors (CGFs) with deproteinized bovine bone mineral (DBBM) | Alveolar ridge preservation (ARP) following upper molar extraction | Bone area in the CGFs/DBBM group was better maintained compared to the control CGFs promoted better osteogenesis and bone integration CGFs as a membrane alternative to collagen—biocompatible; promoted soft and hard tissue growth without additional trauma | [82] |
| Leukocyte and PRF (L-PRF) with autogenous bone graft and the other Enamel Matrix Derivative (EMD) with autogenous bone graft | Intrabony defects (IBDs) | Both treatments were effective clinically and radiographically in promoting periodontal regeneration Stimulated cellular activities for bone regeneration and periodontal healing | [83] |
| Mineralized collagen loaded with BMP-2 and VEGF | Bone regeneration in mandibular defects | The combination of BMP-2 and VEGF resulted in the highest level of bone regeneration BMP-2 promoted osteogenesis by stimulating MSCs to become osteoblasts VEGF promoted angiogenesis BMP-2 and VEGF were more effective than using BMP-2 alone | [84] |
| Autologous platelet concentrates: PRF, CGF, and platelet-poor plasma (PPP) | Healing of tooth extraction sockets | All three platelet concentrates (PRF, CGF, PPP) improved healing and reduced bone resorption compared to control PPP showed the most significant early-stage bone healing effects CGF demonstrated superior results in later stages of healing RF was less effective compared to PPP and CGF Larger areas of bone formation were observed, the most significant being CGF | [85] |
| NPs/Graft | Application | Findings | Refs. |
|---|---|---|---|
| Bovine bone grafts with AgNPs | Dental bone grafts | Strong antimicrobial effects Potential of reducing infections in maxillofacial surgeries | [116] |
| Chitosan NPs (ChN)/Simvastatin (Sim) | Maxillary bony defect healing | ChN had the highest number of osteoblasts and osteoclasts Combining ChN with Sim did not enhance bone healing ChN’s effectiveness may be due to its ability to control drug release and its biocompatibility | [117] |
| Titanium dioxide NPs (TiO2NPs)/TiO2NPs-PRP | Healing large bone defects | TiO2NPs improved bone healing by promoting osteoblast functions PRP provides essential growth factors that promote vascularization and soft tissue healing TiO2NPs-PRP showed the most significant improvement in bone healing PRP enhanced early blood clot formation and sustained angiogenesis, while TiO2NPs provided a conducive environment for bone tissue development | [118] |
| Dental nano putty (D-nP) combining DBM, calcium sulfate hemihydrate (CSH), curcumin NPs (CU-NPs), AgNPs | Bone regeneration and prevent implant-associated infections | D-nP demonstrated effective antibacterial activity against both Gram-positive and Gram-negative bacteria D-nP had high cell viability (~95%) and promoted cellular adhesion and proliferation, which is important for bone regeneration The combination of DBM, CSH, CU-NPs, and AgNPs resulted in a material with improved mechanical strength | [119] |
| Cerium oxide (CeO2) NPs loaded nanofibrous membranes | Periodontal tissue repair | CeO2 NPs were biocompatible and promoted the proliferation of human periodontal ligament stem cells (hPDLSCs) Increased alkaline phosphatase activity, mineralized nodule formation, and the expression of osteogenic genes and proteins In rat cranial defect models, the composite membranes with CeO2 NPs accelerated new bone formation | [120] |
| Nanocomposites of Chitosan/Graphene Oxide/TiO2 NPs/Blackberry Waste Extract | Partial bone substitutes | Demonstrated good biocompatibility Soft tissue healing, hair regrowth, and no necrotic or inflammatory responses after 90 days of implantation in skull defects Promoted bone cell adhesion and proliferation | [121] |
| Bone Grafting Material | Description | Examples |
|---|---|---|
| Autogenous bone | Bone from the same individual | Block graft |
| Allogenic bone | Bone from the same species but from a different individual | Free frozen bone, freeze-dried bone allograft, demineralized freeze-dried bone allograft, and deproteinized bone allograft |
| Xenogenic bone | Material of biological origin but from a different species | Materials derived from animal bone, corals, calcifying algae, and wood |
| Alloplastic bone | Material of synthetic origin | Calcium phosphates, glass ceramics, polymers, and metals |
| Clinical Study Graft | Targeted Defect | Clinical Study Details | Results | Refs. |
|---|---|---|---|---|
| CAD/CAM titanium meshes filled with graft material (from patient and bovine source) | Guided bone regeneration of severe alveolar ridge defects | 41 patients enrolled between 2018 and 2019 The mean duration of mesh maintenance was 7 months | Eight of these sites integrated the graft uneventfully, and three showed partial bone loss 100% survival rate of implants after a follow-up of 10.6 months | [140] |
| Autogenous dentin as a graft | Periodontal defects caused by the extraction of impacted lower third molars | 15 patients recruited and selected over a period of 12 months Evaluations performed at 3 and 6 months post-operatively | The dentin graft showed significant improvements in probing depth (PD), bone density, and maintenance of the alveolar bone crest compared to the control Reduced periodontal pocket depth and improved bone healing | [141] |
| Octacalcium phosphate (OCP) and its collagen composite (OCP/Col) as bone substitutes | Oral and maxillofacial surgeries: sinus floor elevation, socket preservation, cyst removal, and alveolar cleft repair | 60 patients Abutments were exchanged, and prosthetic treatment started 6 months after implant placement | OCP/Col was successful in sinus floor elevation (1-stage), cyst, and alveolar cleft procedures, meeting the criteria for success OCP/Col was deemed safe, with adverse events like pain and swelling being typical of normal treatment | [142] |
| Synthetic graft volume-stable collagen matrix (VCMX) versus autogenous subepithelial connective tissue graft (SCTG) | Grafting around dental implants | 20 patients Re-examinations at 6 months, 1 year, and 3 years | Both VCMX and SCTG led to slight increases in buccal mucosal thickness over three years Initially, patient morbidity was higher with SCTG, but over time, satisfaction levels for both groups were high Both VCMX and SCTG provided stable, healthy, and aesthetically pleasing outcomes, with VCMX offering the advantage of reduced patient morbidity | [143] |
| Novel 3D-printed nano-porous HA (3DP HA) versus nano-crystalline bone graft (NanoBone®) | Preserving the alveolar ridge after tooth extraction | 30 patients Evaluation at 4 months after implant placement | After four months, both groups showed similar outcomes in terms of bone resorption, soft tissue changes, and new bone formation Both materials effectively minimized ridge resorption and provided stable conditions for implant placement | [144] |
| AM CaOSiO2-P2O5-B2O3 glass-ceramic (BGS-7) implants | Reconstructing zygomatic bone defects | 8 patients Follow-up study performed after 4 years | CT scans showed 100% bone fusion at 6 months The mean displacement of implants was minimal, indicating good stability Patients reported high satisfaction with both aesthetic and functional outcomes No adverse effects were reported | [145] |
| Allograft versus TCP/HA bone substitute | Benign cavitary bone lesions | 15 patients included in the study between 2016 and 2019 Follow up every 3 weeks until 6 months, and then at 2-month intervals until one year | Both treatments resulted in similar healing times, with all lesions disappearing after 12 months Both types of grafts showed good integration and similar clinical outcomes The TCP/HA graft is a cost-effective alternative but is more fragile | [146] |
| 5 grafts: (1) Autogenous graft (2) β-TCP graft (3) β-TCP + Autogenous Bone Graft (4) Bioactive Glass (5) Bioactive Glass + Autogenous Bone Graft | Reconstructing maxillary sinuses | 40 patients Evaluation performed after 6 months of bone healing | Bioactive glass + Autogenous bone graft outperformed other combinations with 45.8% new bone formation and 37.9% bone volume change Bone graft resorption rates were similar across the different graft materials Combination grafts generally had better outcomes compared to single-material grafts, but were similar to autogenous graft | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolae, C.-L.; Pîrvulescu, D.-C.; Niculescu, A.-G.; Epistatu, D.; Mihaiescu, D.E.; Antohi, A.M.; Grumezescu, A.M.; Croitoru, G.-A. An Up-to-Date Review of Materials Science Advances in Bone Grafting for Oral and Maxillofacial Pathology. Materials 2024, 17, 4782. https://doi.org/10.3390/ma17194782
Nicolae C-L, Pîrvulescu D-C, Niculescu A-G, Epistatu D, Mihaiescu DE, Antohi AM, Grumezescu AM, Croitoru G-A. An Up-to-Date Review of Materials Science Advances in Bone Grafting for Oral and Maxillofacial Pathology. Materials. 2024; 17(19):4782. https://doi.org/10.3390/ma17194782
Chicago/Turabian StyleNicolae, Carmen-Larisa, Diana-Cristina Pîrvulescu, Adelina-Gabriela Niculescu, Dragoș Epistatu, Dan Eduard Mihaiescu, Alexandru Mihai Antohi, Alexandru Mihai Grumezescu, and George-Alexandru Croitoru. 2024. "An Up-to-Date Review of Materials Science Advances in Bone Grafting for Oral and Maxillofacial Pathology" Materials 17, no. 19: 4782. https://doi.org/10.3390/ma17194782










