Polymerization of Potassium Azide in Liquid Nitrogen Using Nanosecond-Pulsed Spark Plasma
Abstract
:1. Introduction
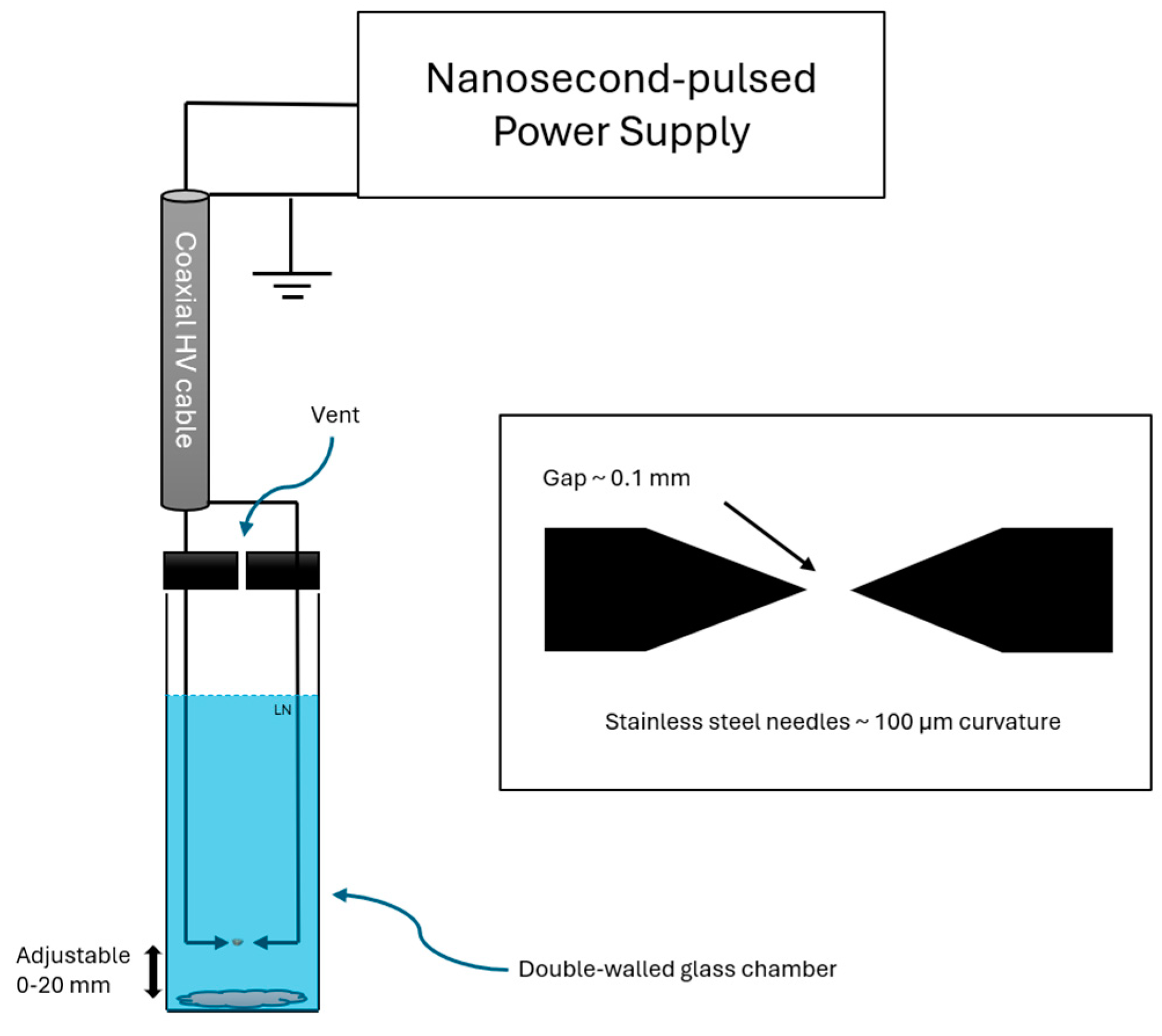
2. Materials and Methods
3. Results and Discussions
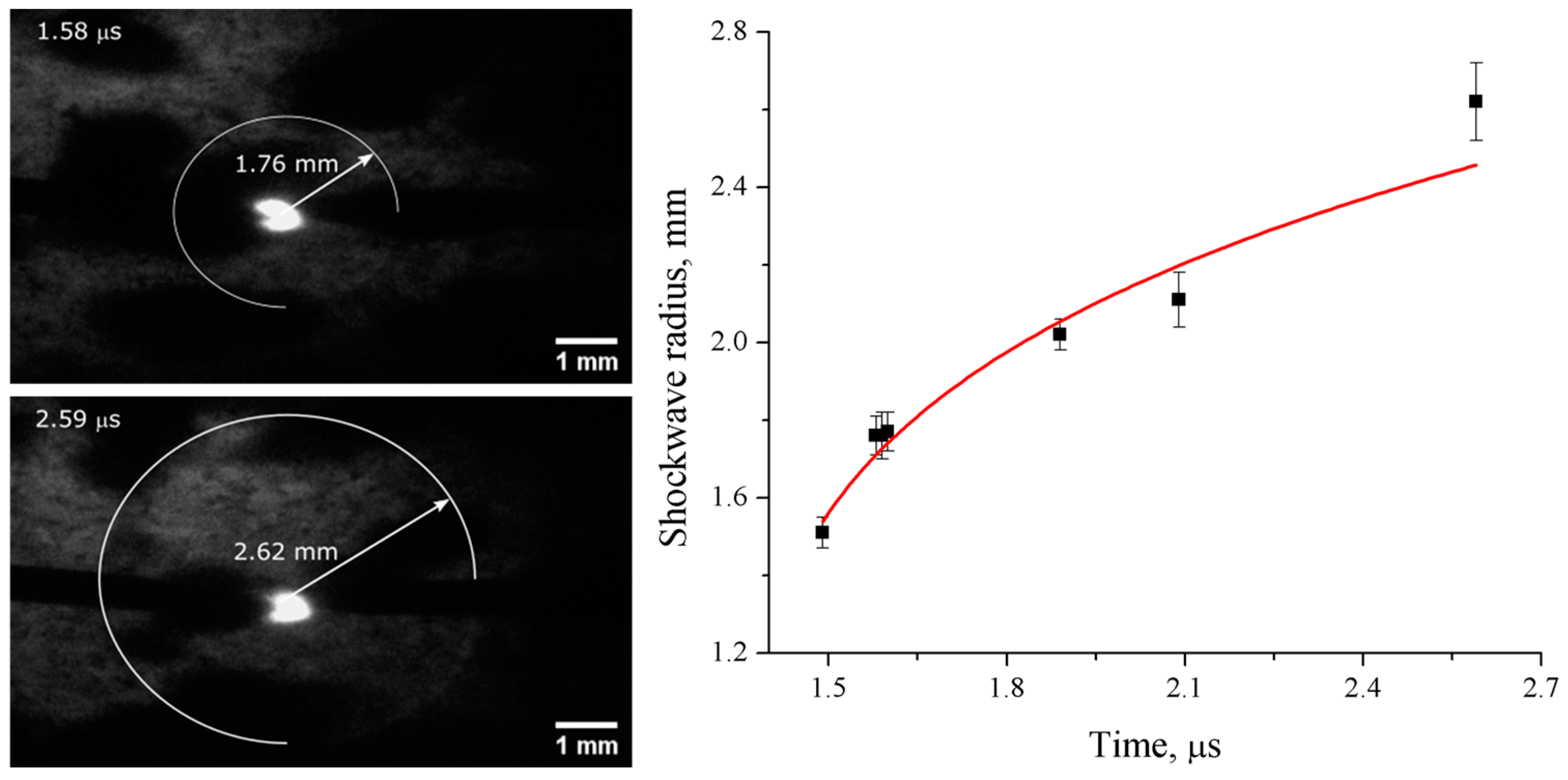
3.1. Characterization of Spark Discharge in Liquid Nitrogen
3.2. Treatment of Potassium Azide with Nanosecond-Pulsed Plasma in Liquid Nitrogen—Color Change
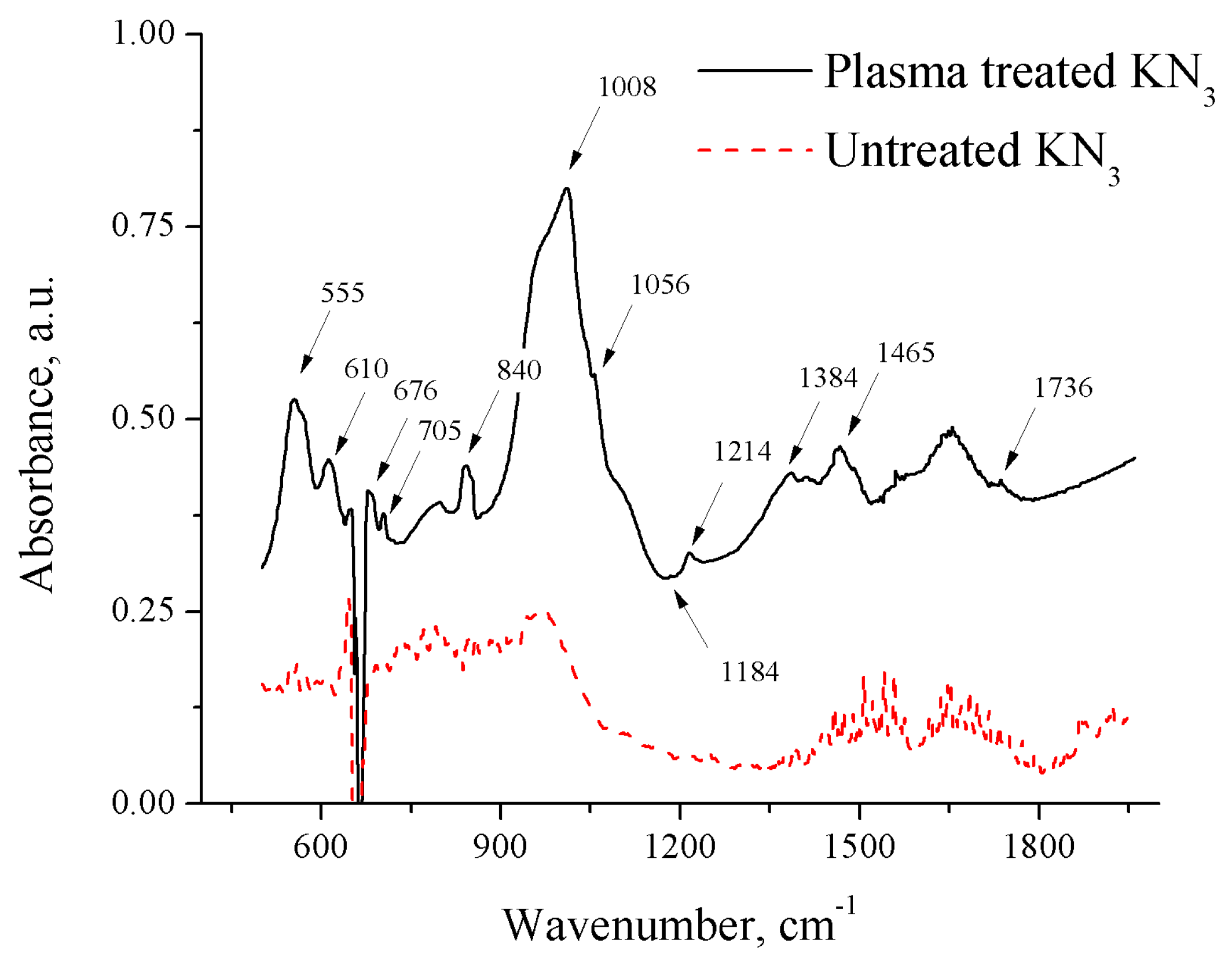
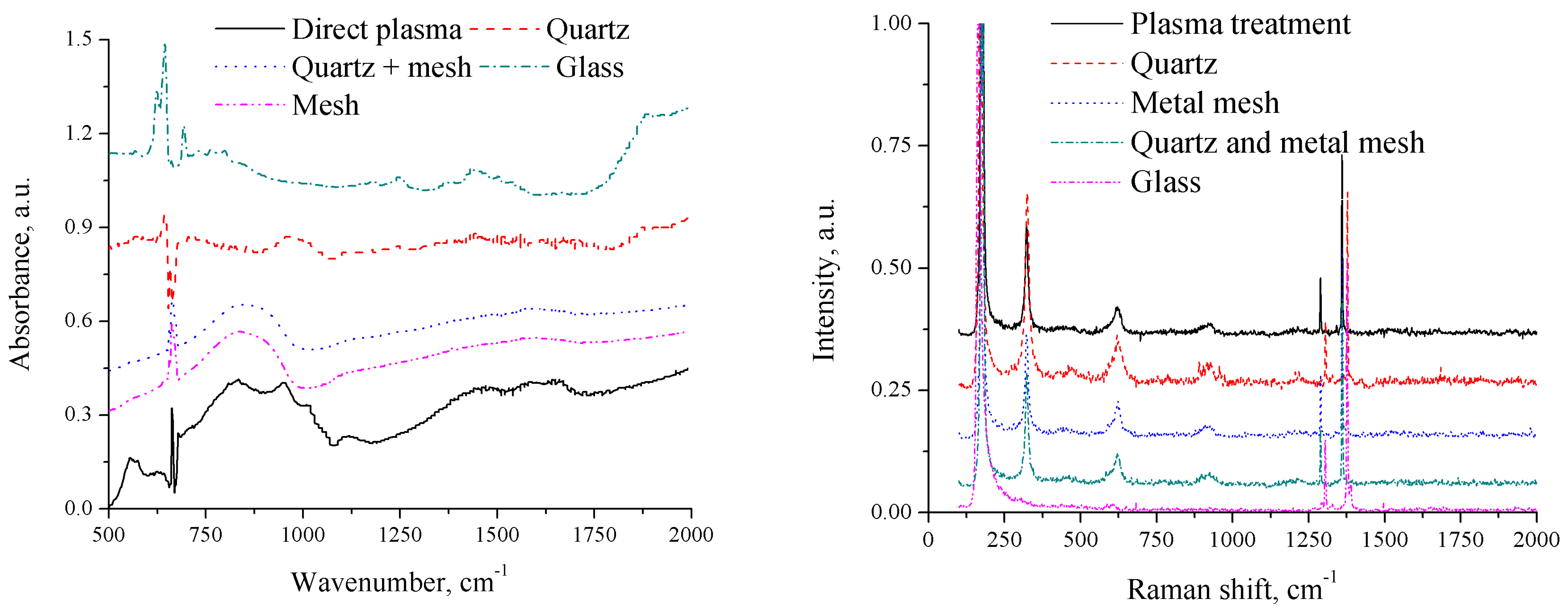
3.3. FTIR Spectrum
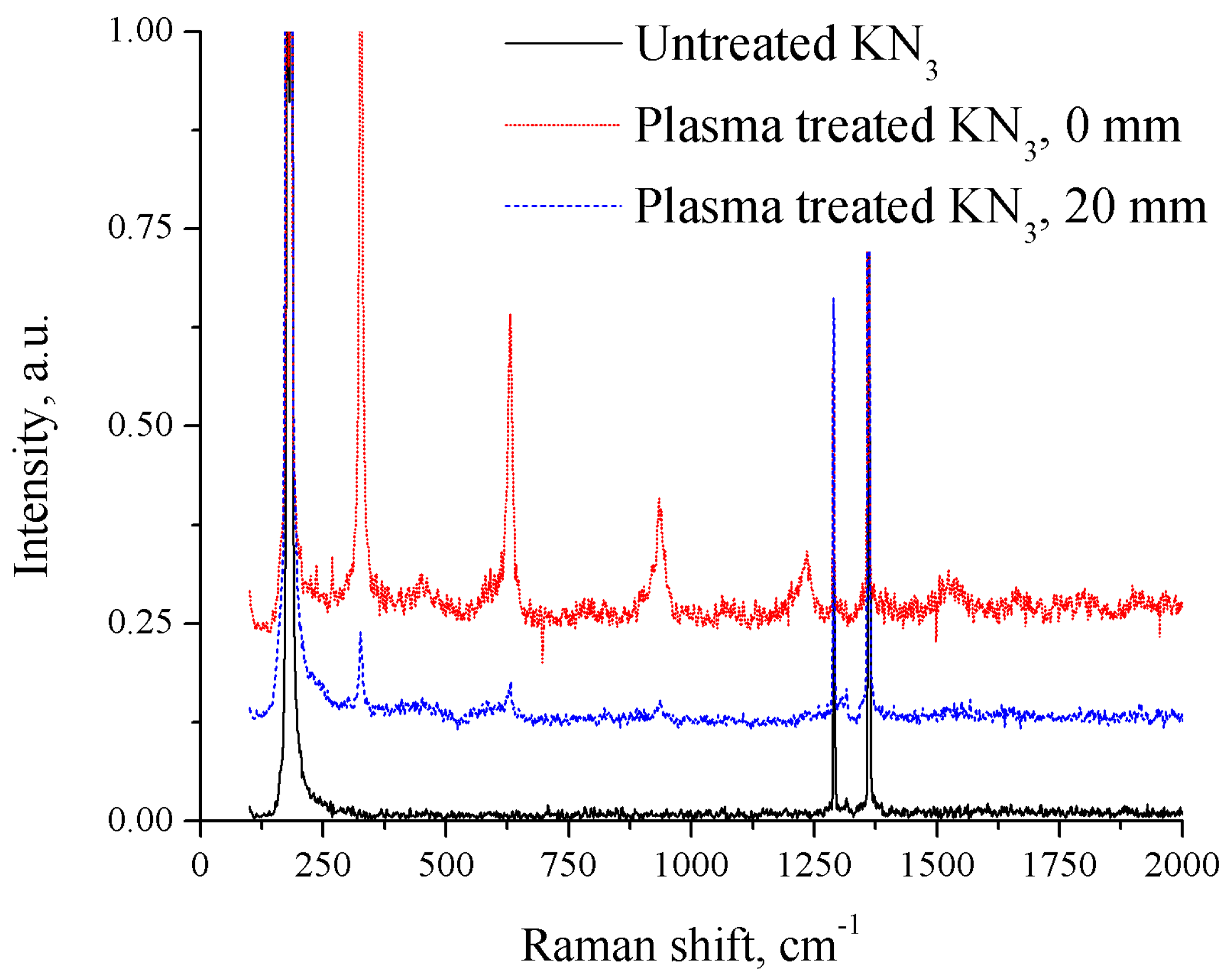
3.4. Raman Spectrum
3.5. Mechanism Study of Color Change, Crystal Structure Change, and Polymerization
- Covered with a quartz window, allowing certain wavelengths of UV, visible light, and IR, as well as electric fields to pass through but blocking the direct effects of plasma-generated reactive species;
- Enclosed with a metal mesh, which, being a conductor, nullifies the electric field within it while allowing delivery of all other factors;
- Shielded by both quartz and metal mesh, eliminating the effects of reactive species and electric fields;
- Covered with a glass window, eliminating the effects of UV and reactive species.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safari, J.; Gandomi-Ravandi, S. Structure, synthesis, and application of azines: A historical perspective. RSC Adv. 2014, 4, 46224–46249. [Google Scholar] [CrossRef]
- Peng, X.M.; Cai, G.X.; Zhou, C.H. Recent developments in azole compounds as antibacterial and antifungal agents. Curr. Top. Med. Chem. 2013, 13, 1963–2010. [Google Scholar] [CrossRef]
- Olofson, R.A.; Michelman, J.S. Furazan. J. Org. Chem. 1965, 30, 1854–1859. [Google Scholar] [CrossRef]
- Gagliardi, L.; Orlandi, G.; Evangelisti, S.; Roos, B.O. A theoretical study of the nitrogen clusters formed from the ion N3−, N5+, and N5−. J. Chem. Phys. 2001, 114, 10733–10737. [Google Scholar] [CrossRef]
- Capitelli, M.; Colonna, G.; D’Ammando, G.; Laporta, V.; Laricchiuta, A. The role of electron scattering with vibrationally excited nitrogen molecules on non-equilibrium plasma kinetics. Phys. Plasmas 2013, 20, 101609. [Google Scholar] [CrossRef]
- Yongjin, C.; Shuhong, B. High Energy Density Material (HEDM)—Progress in Research Azine Energetic Compounds. Johns. Matthey Technol. Rev. 2019, 63, 51–72. [Google Scholar] [CrossRef]
- McMahan, A.K.; LeSar, R. Pressure dissociation of solid nitrogen under 1 Mbar. Phys. Rev. Lett. 1985, 54, 1929. [Google Scholar] [CrossRef]
- Mailhiot, C.; Yang, L.H.; McMahan, A.K. Polymeric nitrogen. Phys. Rev. B 1992, 46, 14419. [Google Scholar] [CrossRef]
- Eremets, M.I.; Hemley, R.J.; Mao, H.-K.; Gregoryanz, E. Semiconducting non-molecular nitrogen up to 240 GPa and its low-pressure stability. Nature 2001, 411, 170–174. [Google Scholar] [CrossRef]
- Graham, W.G.; Stalder, K.R. Plasmas in liquids and some of their applications in nanoscience. J. Phys. D Appl. Phys. 2011, 44, 174037. [Google Scholar] [CrossRef]
- Dobrynin, D.; Seepersad, Y.; Pekker, M.; Shneider, M.; Friedman, G.; Fridman, A. Non-equilibrium nanosecond-pulsed plasma generation in the liquid phase (water, PDMS) without bubbles: Fast imaging, spectroscopy, and leader-type model. J. Phys. D Appl. Phys. 2013, 46, 105201. [Google Scholar] [CrossRef]
- Eremets, M.I.; Gavriliuk, A.G.; Trojan, I.A.; Dzivenko, D.A.; Boehler, R. Single-bonded cubic form of nitrogen. Nat. Mater. 2004, 3, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Eremets, M.I.; Popov, M.Y.; Trojan, I.A.; Denisov, V.N.; Boehler, R.; Hemley, R.J. Polymerization of nitrogen in sodium azide. J. Chem. Phys. 2004, 120, 10618–10623. [Google Scholar] [CrossRef] [PubMed]
- Pistorius, C.W.F.T. Phase Diagrams to High Pressures of the Univalent Azides Belonging to the Space Group D 4h 18-I4/mcm. J. Chem. Phys. 1969, 51, 2604–2609. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Sang, D.; Wang, X.; Liu, C.; Hu, H.; Wang, W.; Zhang, B.; Fan, Q.; Han, Y.; et al. Ionic conduction in sodium azide under high pressure: Experimental and theoretical approaches. Appl. Phys. Lett. 2018, 112, 173903. [Google Scholar] [CrossRef]
- Williams, A.S.; Steele, B.A.; Oleynik, I.I. Novel rubidium poly-nitrogen materials at high pressure. J. Chem. Phys. 2017, 147, 234701. [Google Scholar] [CrossRef]
- Dobrynin, D.; Rakhmanov, R.; Fridman, A. Nanosecond-pulsed spark discharge plasma in liquid nitrogen: Synthesis of polynitrogen from NaN3. J. Phys. D Appl. Phys. 2019, 52, 455502. [Google Scholar] [CrossRef]
- Song, Z.; Fridman, A.; Dobrynin, D. Effects of liquid properties on the development of nanosecond-pulsed plasma inside of liquid: Comparison of water and liquid nitrogen. J. Phys. D Appl. Phys. 2024, 57, 175203. [Google Scholar] [CrossRef]
- Katsuki, S.; Tanaka, K.; Fudamoto, T.; Namihira, T.; Akiyama, H.; Bluhm, H. Shock waves due to pulsed streamer discharges in water Japan. J. Appl. Phys. 2006, 45, 239–242. [Google Scholar] [CrossRef]
- Yahya, S.M. Fundamentals of Compressible flow: SI Units with Aircraft and Rocket Propulsion; New Age International: New Delhi, India, 2003. [Google Scholar]
- Taylor, N.D.; Fridman, G.; Fridman, A.; Dobrynin, D. Non-equilibrium microsecond pulsed spark discharge in liquid as a source of pressure waves. Int. J. Heat Mass Transf. 2018, 126, 1104–1110. [Google Scholar] [CrossRef]
- Belmonte, T.; Kabbara, H.; Noël, C.; Pflieger, R. Analysis of Zn I emission lines observed during a spark discharge in liquid nitrogen for zinc nanosheet synthesis. Plasma Sources Sci. Technol. 2018, 27, 074004. [Google Scholar] [CrossRef]
- Dobrynin, D.; Song, Z.; Fridman, A. Synthesis of Highly Energetic PolyNitrogen by Nanosecond-Pulsed Plasma in Liquid Nitrogen. Materials 2021, 14, 4292. [Google Scholar] [CrossRef]
- Dobrynin, D.; Rakhmanov, R.; Fridman, A. Nanosecond-pulsed discharge in liquid nitrogen: Optical characterization and production of an energetic non-molecular form of nitrogen-rich material. J. Phys. D Appl. Phys. 2019, 52, 39LT01. [Google Scholar] [CrossRef]
- Maycock, J.N. Electrical Effects due to the F Center in the Potassium Halides. J. Appl. Phys. 1964, 35, 1512–1515. [Google Scholar] [CrossRef]
- Tilley, R.J.D. Defects in Solids; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Nassau, K. The Physics and Chemistry of Color: The Fifteen Causes of Color; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Hayes, W.; Stoneham, A.M. Defects and Defect Processes in Nonmetallic Solids; Courier Corporation: North Chelmsford, MA, USA, 2012. [Google Scholar]
- Hersh, H.N. Proposed excitonic mechanism of color-center formation in alkali halides. Phys. Rev. 1966, 148, 928. [Google Scholar] [CrossRef]
- Blatchley, E.R., III. Numerical modelling of UV intensity: Application to collimated-beam reactors and continuous-flow systems. Water Res. 1997, 31, 2205–2218. [Google Scholar] [CrossRef]
- Bryant, J.I. Infrared Vibrations of Crystalline Potassium Azide. J. Chem. Phys. 1963, 38, 2845–2854. [Google Scholar] [CrossRef]
- Tobita, M.; Bartlett, R.J. Structure and stability of N6 isomers and their spectroscopic characteristics. J. Phys. Chem. A 2001, 105, 4107–4113. [Google Scholar] [CrossRef]
- Workentin, M.S.; Wagner, B.D.; Negri, F.; Zgierski, M.Z.; Lusztyk, J.; Siebrand, W.; Wayner, D.D.M. N6. bul.-. Spectroscopic and Theoretical Studies of an Unusual Pseudohalogen Radical Anion. J. Phys. Chem. 1995, 99, 94–101. [Google Scholar] [CrossRef]
- Bryant, J.I.; Brooks, R.L., III. Raman Spectrum of Potassium Azide Single Crystals. J. Chem. Phys. 1965, 43, 880–882. [Google Scholar] [CrossRef]
- Ji, C.; Zheng, R.; Hou, D.; Zhu, H.; Wu, J.; Chyu, M.-C.; Ma, Y. Pressure-induced phase transition in potassium azide up to 55 GPa. J. Appl. Phys. 2012, 111, 112613. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, Z.; Lin, H.Q.; Li, Y.L. Pressure-induced planar N6 rings in potassium azide. Sci. Rep. 2014, 4, 4358. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Xu, N.; Li, D.; Wang, D.; Chen, L. Pressure-induced polymerization of nitrogen in potassium azides. Europhys. Lett. 2013, 104, 16005. [Google Scholar] [CrossRef]
- Zhang, M.; Yan, H.; Wei, Q.; Liu, H. A new high-pressure polymeric nitrogen phase in potassium azide. RSC Adv. 2015, 5, 11825–11830. [Google Scholar] [CrossRef]
- Wang, Y.; Bykov, M.; Chepkasov, I.; Samtsevich, A.; Bykova, E.; Zhang, X.; Jiang, S.-Q.; Greenberg, E.; Chariton, S.; Prakapenka, V.B.; et al. Stabilization of hexazine rings in potassium polynitride at high pressure. Nat. Chem. 2022, 14, 794–800. [Google Scholar] [CrossRef]

| Observed IR Frequency, cm−1 | Symmetry | Prediction Method | |||||
|---|---|---|---|---|---|---|---|
| PW91 | B3LYP | CCSD(T) | |||||
| Frequency, cm−1 | Intensity | Frequency, cm−1 | Intensity | Frequency, cm−1 | Intensity | ||
| 555 | B3 | 588.5 | 11.4 | ||||
| 705 | B2 | 710.4 | 9.7 | 735.3 | 6.8 | 724 | 7.2 |
| 840 | B3 | 872.3 | 33.6 | 858.8 | 9.2 | 850.1 | 8.4 |
| 1008 | B1 | 1002.8 | 12.3 | ||||
| 1184 | B1 | 1144.1 | 29.2 | 1150 | 2.1 | ||
| B3 | 1184.3 | 24.1 | |||||
| 1384 | B2 | 1367.7 | 2.8 | 1330.5 | 2.5 | ||
| Setup | Filter Material Used | Factors Eliminated | Factors in Function | Color Change Observed | Crystal Structure Change | Polymerization |
|---|---|---|---|---|---|---|
| 0 | N/A | Active species, electric field, UV, visible light, IR | Yes | Yes | Yes | |
| 1 | Quartz | Active species | UV, electric field, visible light, IR | Yes | Yes | No |
| 2 | Metal mesh | Electric field | Active species, UV, visible light, IR | Yes | Yes | No |
| 3 | Quartz and metal mesh | Active species, electric field | UV, visible light, IR | Yes | Yes | No |
| 4 | Glass | Active species, UV | Electric field, visible light, IR | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Fridman, A.; Dobrynin, D. Polymerization of Potassium Azide in Liquid Nitrogen Using Nanosecond-Pulsed Spark Plasma. Materials 2024, 17, 4787. https://doi.org/10.3390/ma17194787
Song Z, Fridman A, Dobrynin D. Polymerization of Potassium Azide in Liquid Nitrogen Using Nanosecond-Pulsed Spark Plasma. Materials. 2024; 17(19):4787. https://doi.org/10.3390/ma17194787
Chicago/Turabian StyleSong, Zhiheng, Alexander Fridman, and Danil Dobrynin. 2024. "Polymerization of Potassium Azide in Liquid Nitrogen Using Nanosecond-Pulsed Spark Plasma" Materials 17, no. 19: 4787. https://doi.org/10.3390/ma17194787





