Hardness and Corrosion Behavior of CrMnFeCoNi Alloy Fabricated by Ball Milling and Spark Plasma Sintering
Abstract
:1. Introduction
2. Experimental Details
2.1. Ball Milling and Sintering
2.2. Analysis of Density, Crystal Structure, and Composition Distribution
2.3. Measure of Hardness and Corrosion Resistance
3. Results and Discussion
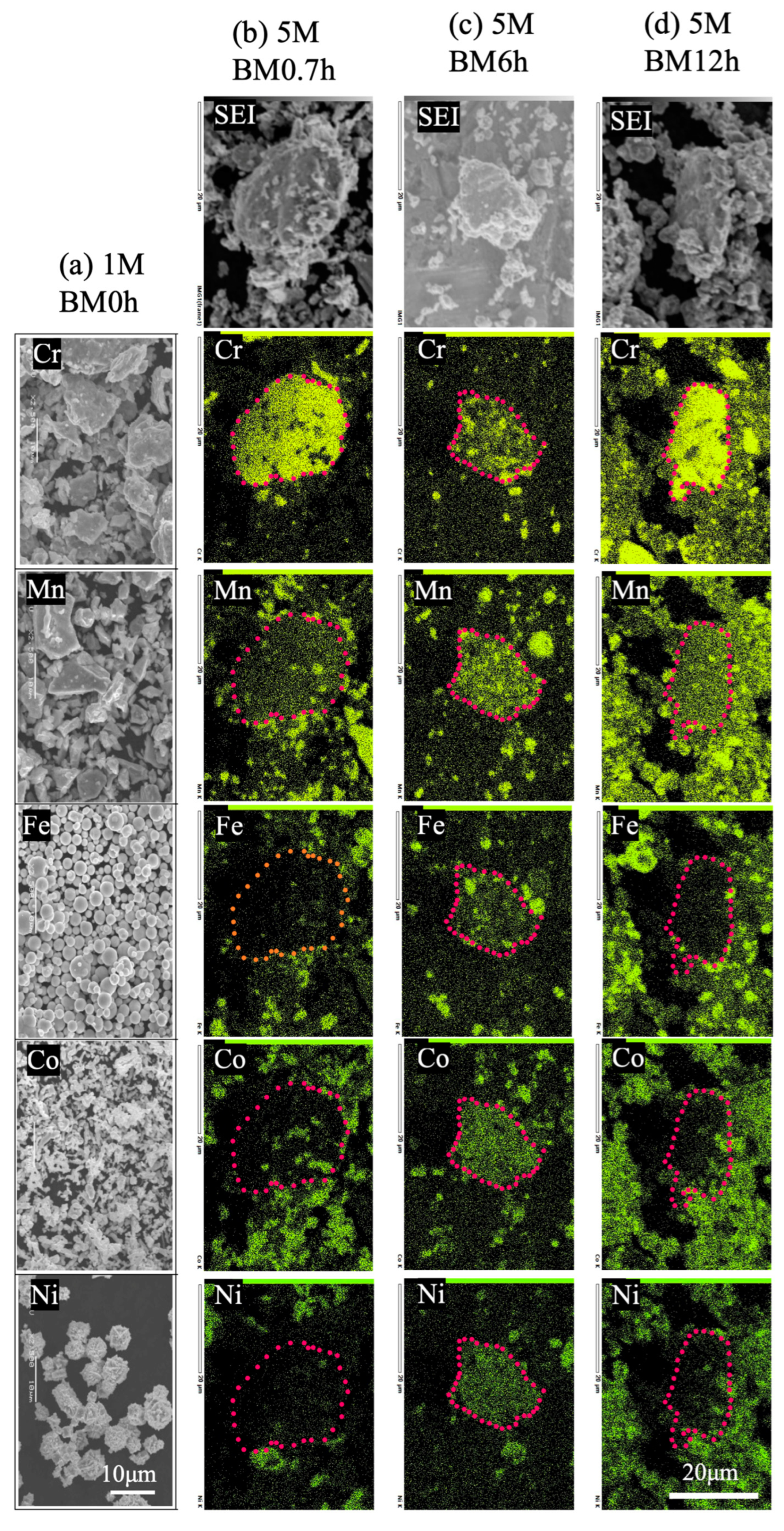
3.1. Morphology, Composition, and Crystal Structure of Raw and Mixed Powders
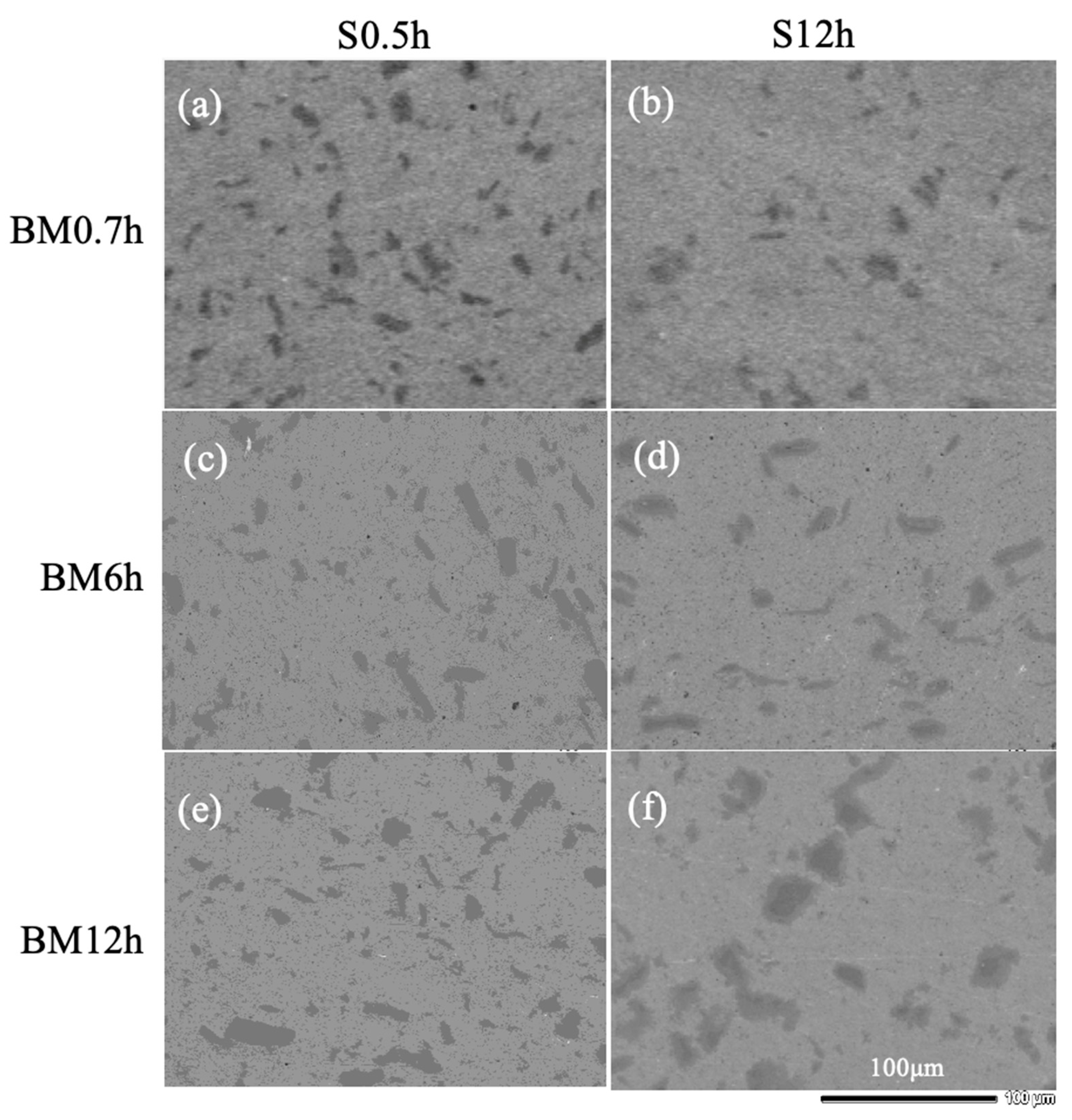
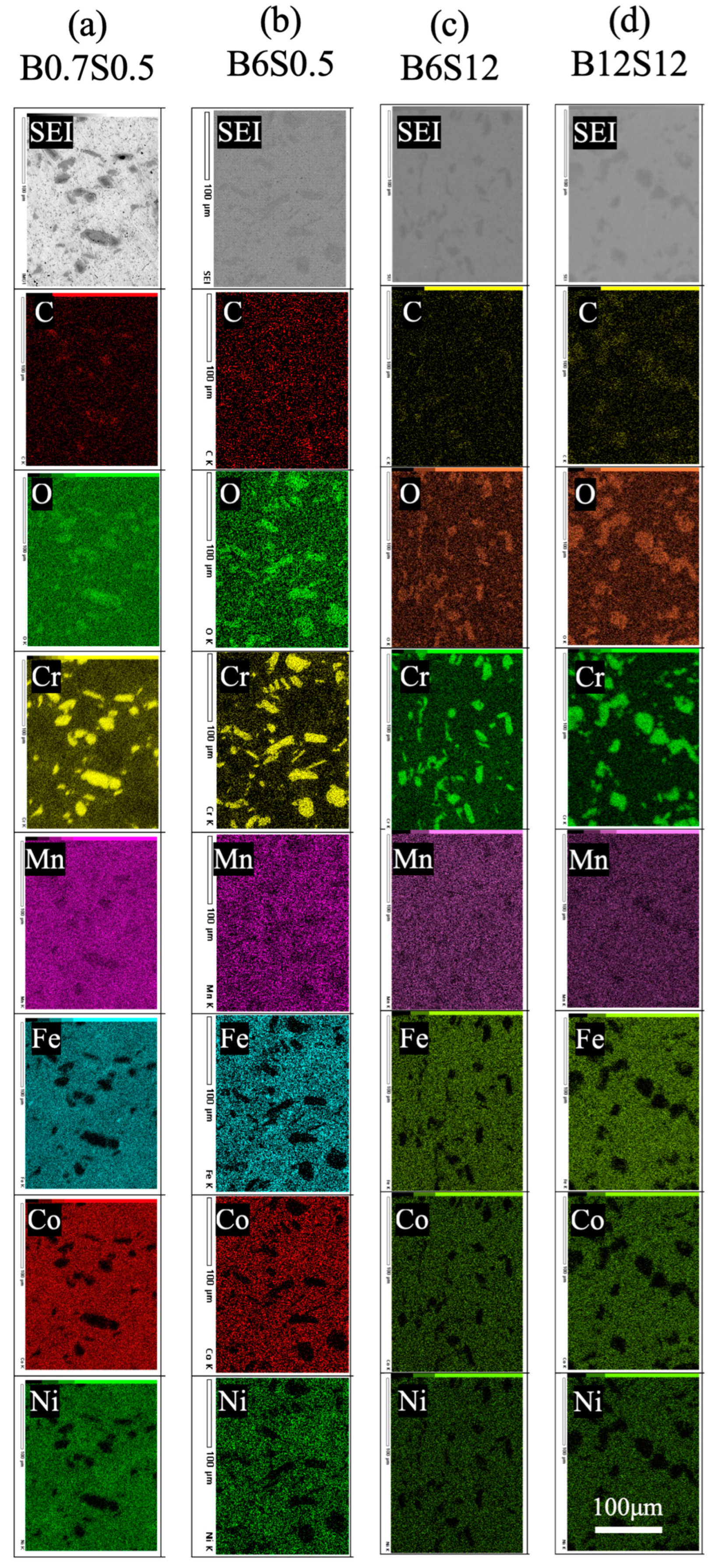
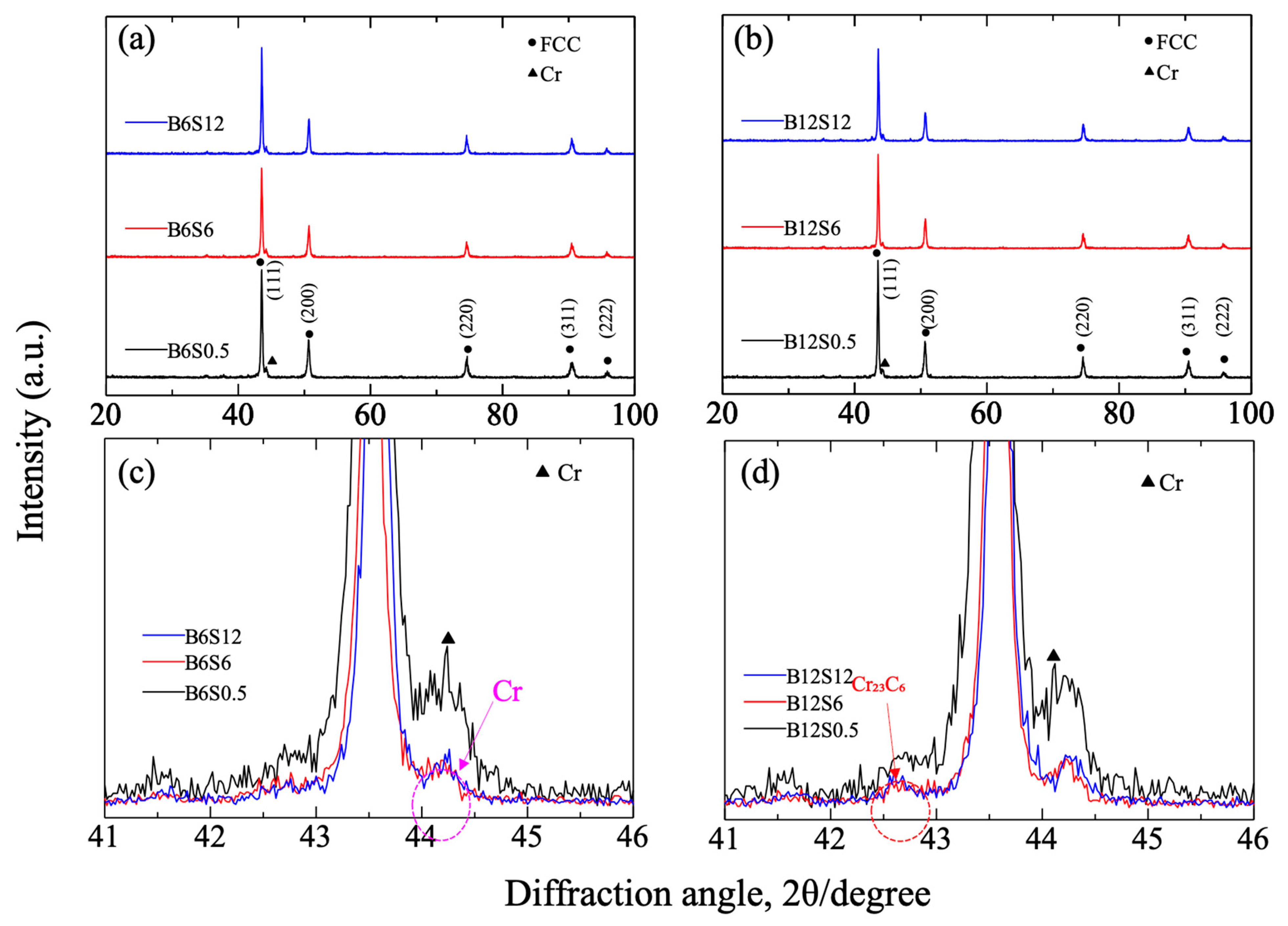
3.2. Composition and Crystal Structure of Sintered Materials
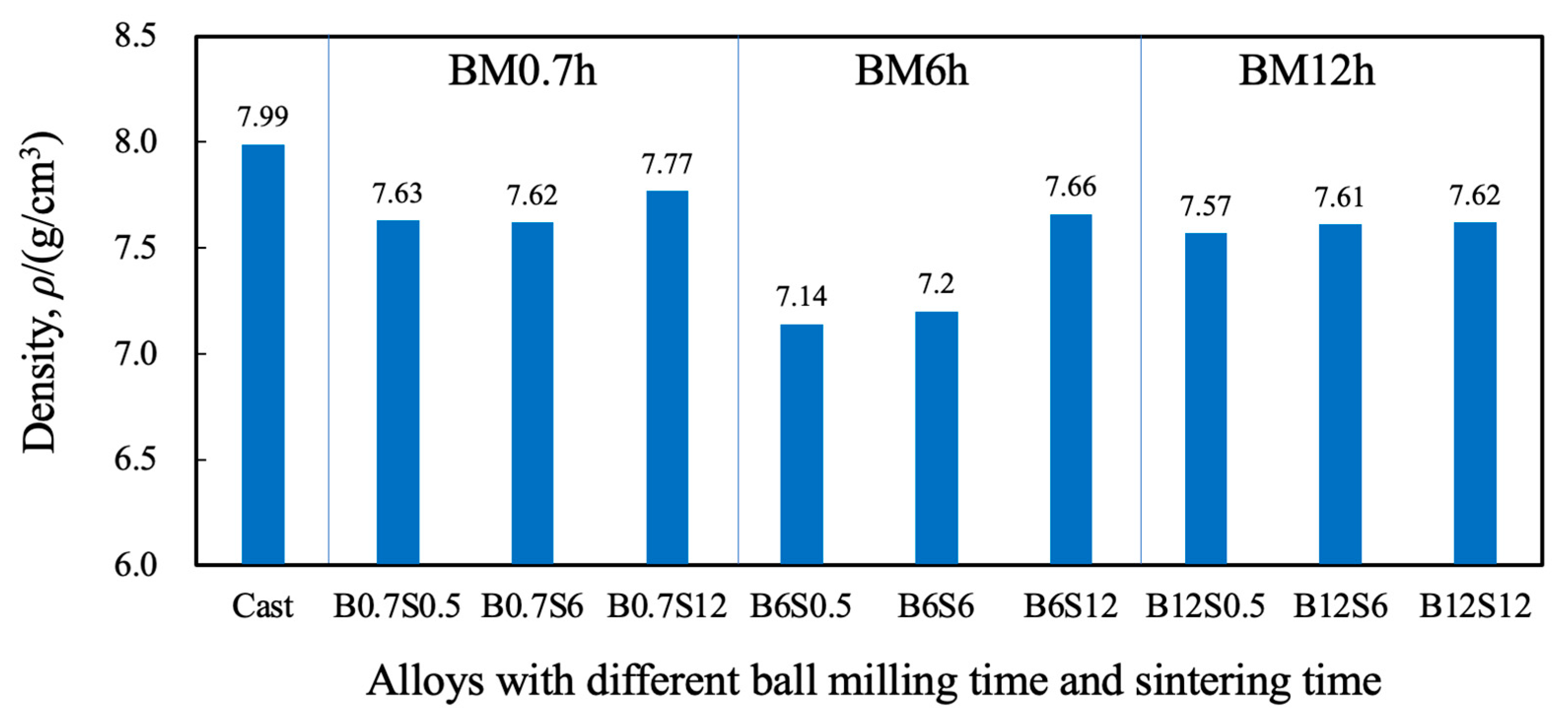
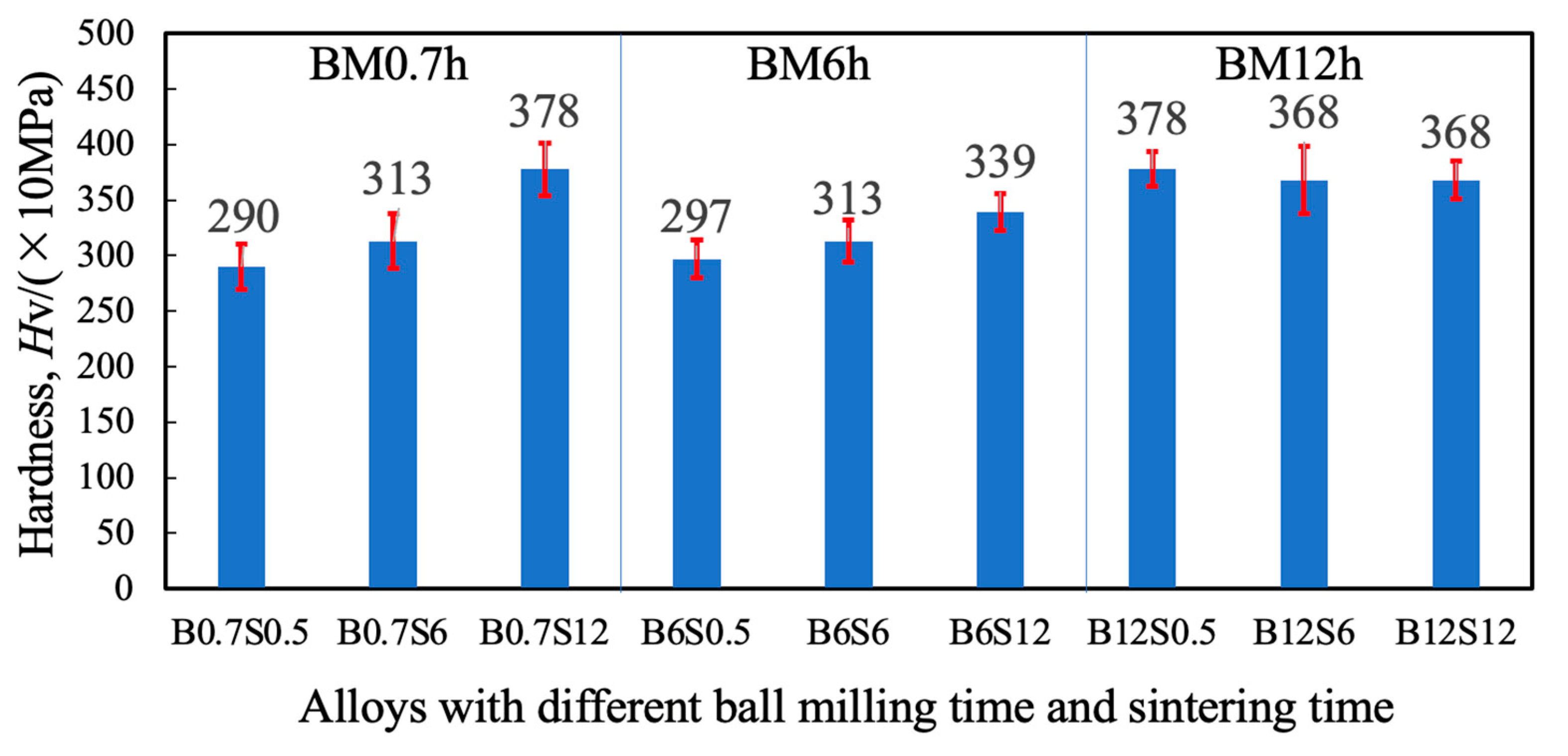
3.3. Density and Hardness of Sintered Materials
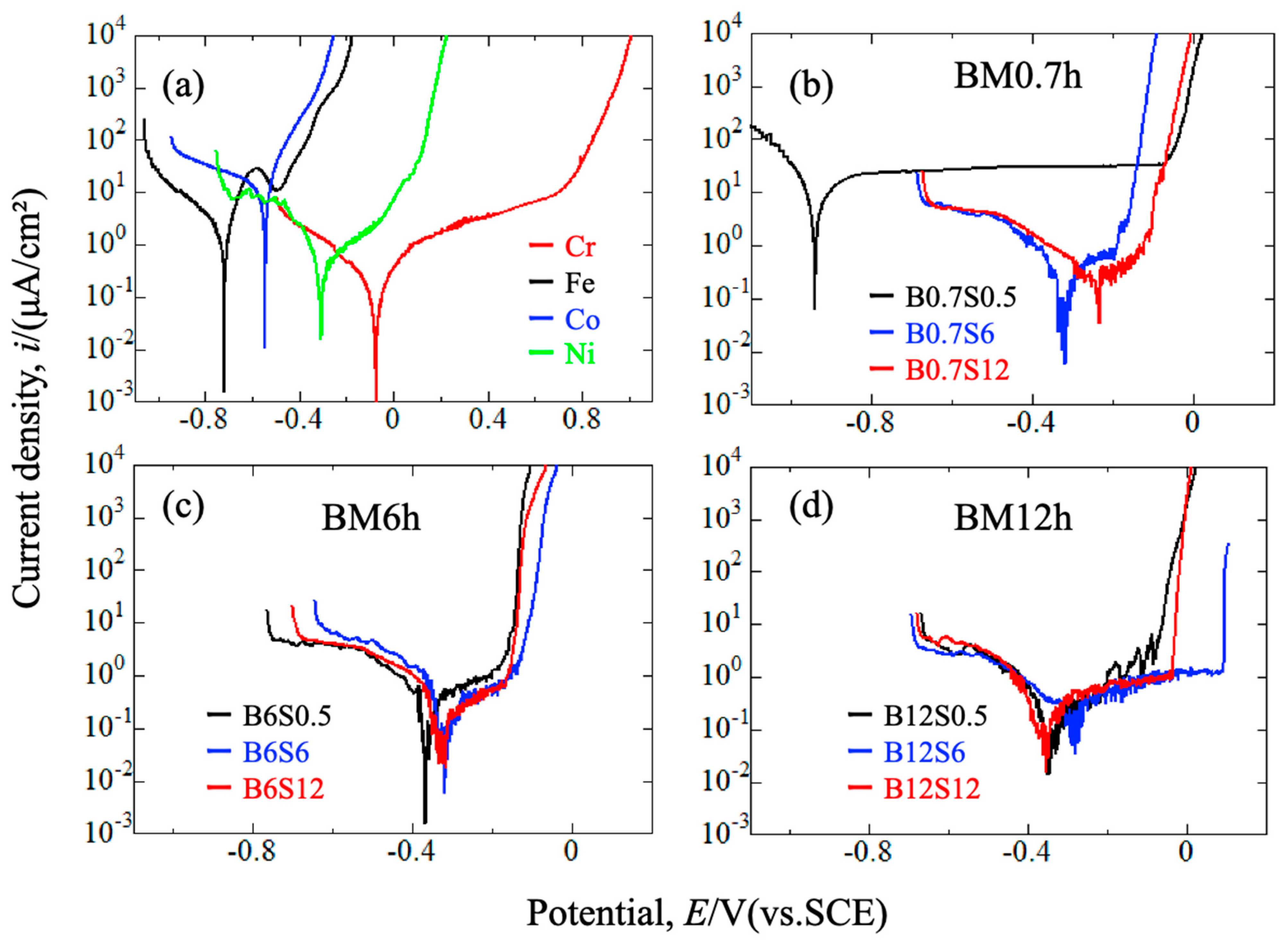
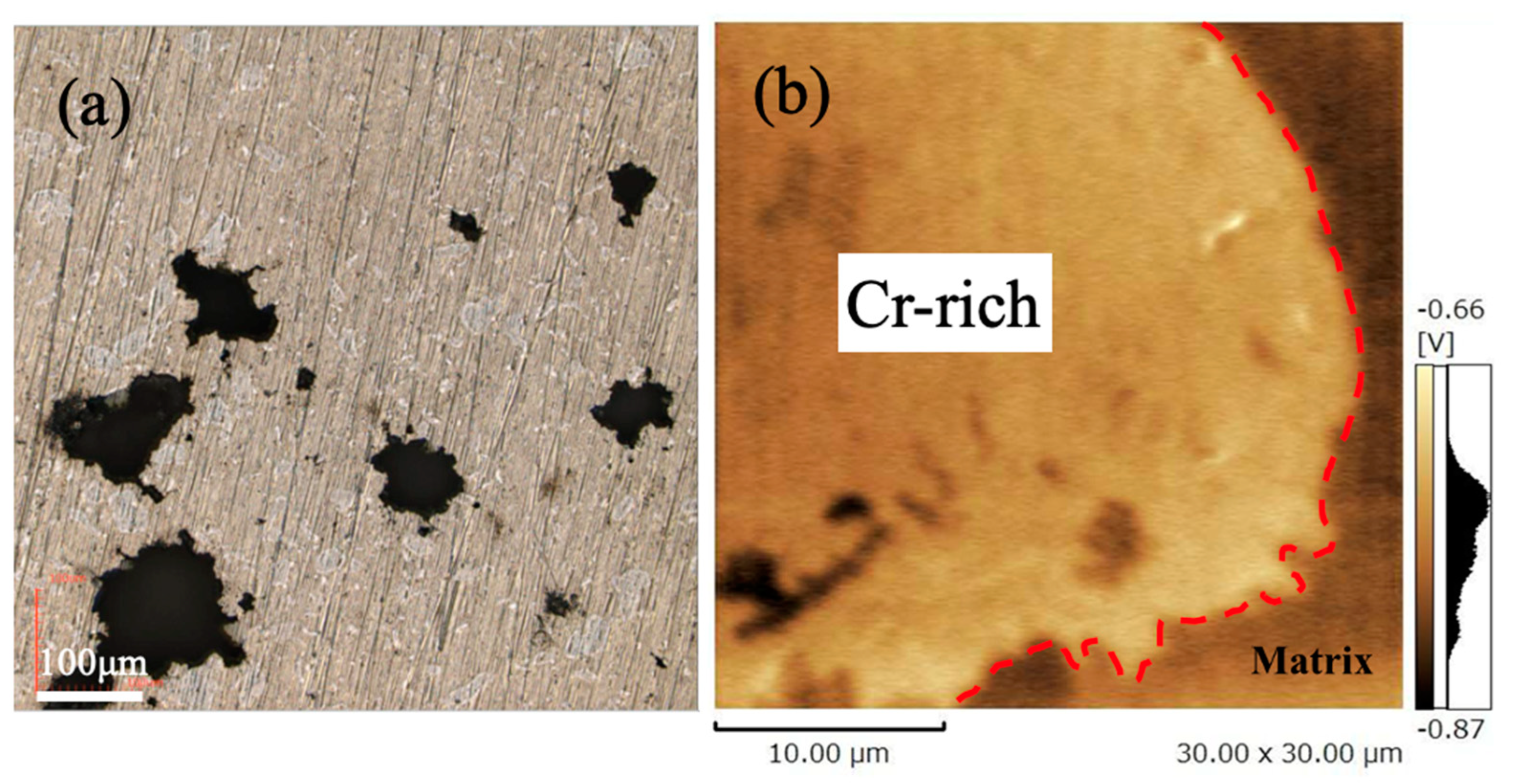
3.4. Corrosion Resistance of Sintered Materials
4. Conclusions
- Ball milling achieved a partial alloying of Cr, Mn, Fe, Co, and Ni at 300 rpm, with a higher alloying degree for a longer milling time.
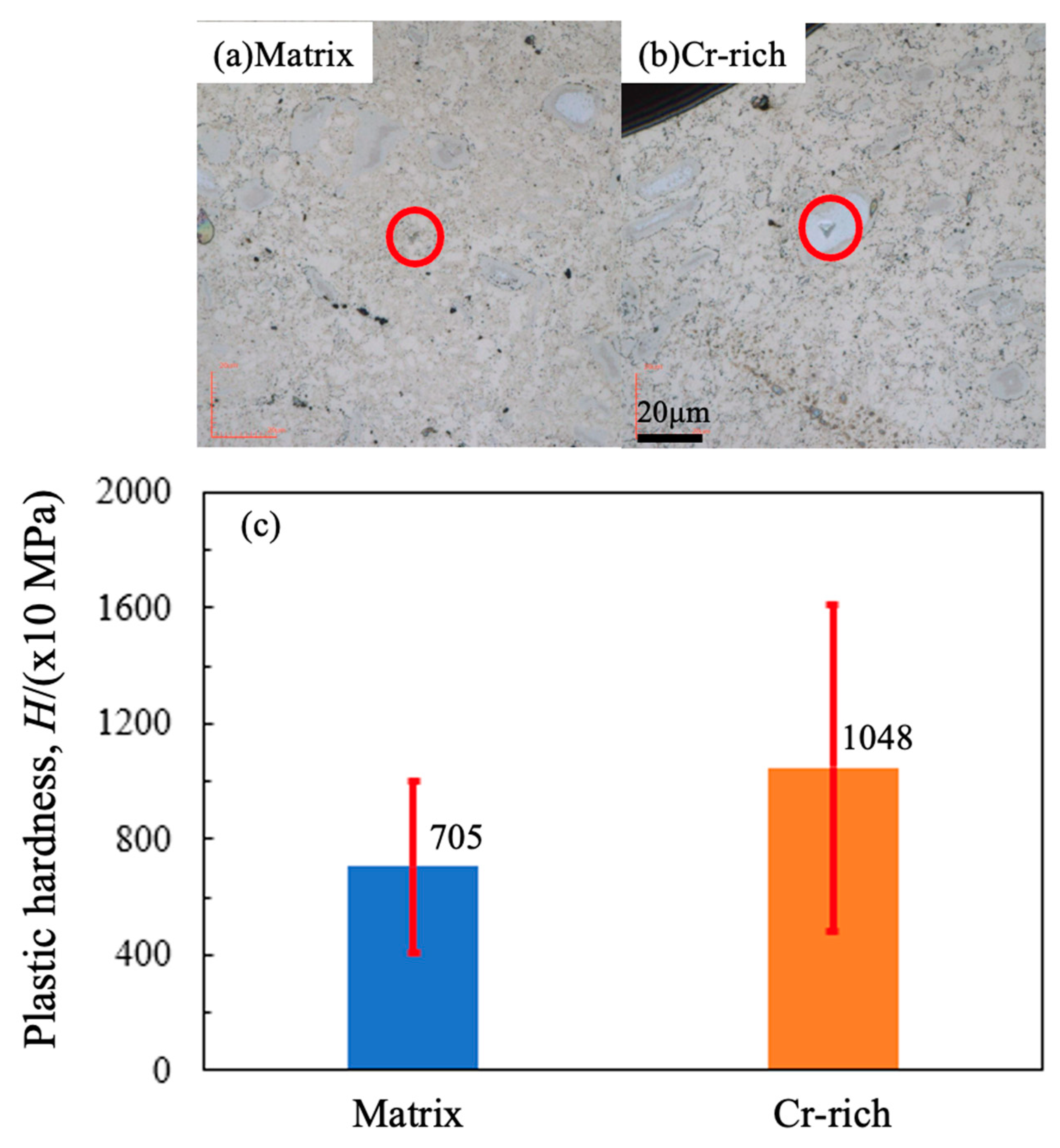
- Cr-rich zones, containing Mn, C, and O, formed in all sintered alloys, with inheritance from the ball-milled particles. Chromium carbide was confirmed in sintered alloys, whose amount was independent of milling time but decreased with the extended sintering.
- Extended milling or long sintering can achieve high hardness and corrosion resistance for sintered alloys. The Cr-enriched zones, showing high hardness and potential, affect hardness and corrosion resistance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Gan, J.Y.; Lin, S.J.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Metall. Mater. Trans. A 2004, 35, 2533–2536. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar] [CrossRef]
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-entropy alloys fabricated via powder metallurgy. A critical review. Powder Metall. 2019, 62, 84–114. [Google Scholar] [CrossRef]
- Li, L.; Chen, Z.; Yuge, K.; Kishida, K.; Inui, H.; Heilmaier, M.; George, E.P. Plastic deformation of single crystals of the equiatomic Cr-Fe-Co-Ni medium entropy alloy—A comparison with Cr-Mn-Fe-Co-Ni and Cr-Co-Ni alloys. Int. J. Plast. 2023, 169, 103732. [Google Scholar] [CrossRef]
- Inui, H. New Perspective and development in understanding crystal plasticity in advanced structural materials-with special reference to high-entropy alloys. Mater. Jpn. 2024, 63, 321–327. [Google Scholar] [CrossRef]
- Ye, Q.; Feng, K.; Li, Z.; Lu, F.; Li, R.; Huang, J.; Wu, Y. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Han, Z.; Ren, W.; Yang, J.; Tian, A.; Du, Y.; Liu, G.; Wei, R.; Zhang, G.; Chen, Y. The corrosion behavior of ultra-fine grained CoNiFeCrMn high-entropy alloys. J. Alloys Compd. 2020, 816, 152583. [Google Scholar] [CrossRef]
- Fujita, K.; Fujiwara, H.; Kikuchi, S. Bimodal microstructure design of CrMnFeCoNi high-entropy alloy using powder metallurgy. J. Soc. Mater. Sci. Jpn. 2021, 70, 648–655. [Google Scholar] [CrossRef]
- Mane, R.B.; Panigrahi, B.B. Effect of alloying order on non-isothermal sintering kinetics of mechanically alloyed high entropy alloy powders. Mater. Lett. 2018, 217, 131–134. [Google Scholar] [CrossRef]
- Ji, W.; Wang, W.; Wang, H.; Zhang, J.; Wang, Y.; Zhang, F.; Fu, Z. Alloying behavior and novel properties of CoCrFeNiMn high-entropy alloy fabricated by mechanical alloying and spark plasma sintering. Intermetallics 2015, 56, 24–27. [Google Scholar] [CrossRef]
- Gao, L.; Liao, W.; Zhang, H.; Surjadi, J.; Sun, D.; Lu, Y. Microstructure, mechanical and corrosion behaviors of CoCrFeNiAl0.3 high entropy alloy (HEA) films. Coatings 2017, 7, 156. [Google Scholar] [CrossRef]
- Li, W.; Liu, P.; Liaw, P.K. Microstructures and properties of high-entropy alloy films and coatings: A review. Mater. Res. Lett. 2018, 6, 199–229. [Google Scholar] [CrossRef]
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion of high entropy alloys. NPJ Mater. Degrad. 2017, 1, 1–18. [Google Scholar] [CrossRef]
- Nascimento, C.B.; Donatus, U.; Ríos, C.T.; Oliveira, M.C.L.D.; Antunes, R.A. A review on Corrosion of High Entropy Alloys: Exploring the Interplay Between Corrosion Properties, Alloy Composition, Passive Film Stability and Materials Selection. Mater. Res. 2022, 25, e20210442. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Ivanov, E.; Boldyrev, V.V. The science and technology of mechanical alloying. Mater. Sci. Eng. A 2001, 304–306, 151–158. [Google Scholar] [CrossRef]
- Joo, S.H.; Kato, H.; Jang, M.J.; Moon, J.; Kim, E.B.; Hong, S.J.; Kim, H.S. Structure and properties of ultrafine-grained CoCrFeMnNi high-entropy alloys produced by mechanical alloying and spark plasma sintering. J. Alloys Compd. 2017, 698, 591–604. [Google Scholar] [CrossRef]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. China Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef]
- Ujah, C.O.; Kallon, D.V.V.; Aigbodion, V.S. Corrosion characteristics of high-entropy alloys prepared by spark plasma sintering. Int. J. Adv. Manuf. Technol. 2024, 132, 63–82. [Google Scholar] [CrossRef]
- Aiso, T.; Nishimoto, M.; Muto, I.; Sugawara, Y. Roles of alloying elements in the corrosion resistance of equiatomic CoCrFeMnNi high-entropy alloy and application to corrosion-resistant alloy design. Mater. Trans. 2021, 62, 1677–1680. [Google Scholar] [CrossRef]
- Chmielak, L.; Roncery, L.M.; Niederhofer, P.; Weber, S.; Theisen, W. CrMnFeCoNi high entropy alloys with carbon and nitrogen: Mechanical properties, wear and corrosion resistance. SN Appl. Sci. 2021, 3, 835. [Google Scholar] [CrossRef]
- Pao, L.; Muto, I.; Sugawara, Y. Pitting at inclusions of the equiatomic CoCrFeMnNi alloy and improving corrosion resistance by potentiodynamic polarization in H2SO4. Corros. Sci. 2021, 191, 109748. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, B.; Yuan, Y.; Yin, S.; Guo, S. Effect of annealing time on microstructure and corrosion behavior of CoCrFeMnNi high-entropy alloy in alkaline soil simulation solution. Corros. Commun. 2021, 3, 45–61. [Google Scholar] [CrossRef]
- Hashimoto, N. Irradiation damage and corrosion behavior in high entropy alloys. J. Surf. Finish. Soc. Jpn. 2024, 75, 123–130. [Google Scholar] [CrossRef]
- Wetzel, A.; von der Au, M.; Dietrich, P.M.; Radnik, J.; Ozcan, O.; Witt, J. The comparison of the corrosion behavior of the CrCoNi medium entropy alloy and CrMnFeCoNi high entropy alloy. Appl. Surf. Sci. 2022, 601, 154171. [Google Scholar] [CrossRef]
- Zhao, R.-F.; Ren, B.; Cai, B.; Liu, Z.-X.; Zhang, G.-P.; Zhang, J.-J. Corrosion behavior of CoxCrCuFeMnNi high-entropy alloys prepared by hot pressing sintered in 3.5% NaCl solution. Results Phys. 2019, 15, 102667. [Google Scholar] [CrossRef]
- Hryha, E.; Nyborg, L. Oxide Transformation in Cr-Mn-Prealloyed Sintered Steels: Thermodynamic and Kinetic Aspects. Metall. Mater. Trans. A 2014, 45, 1736–1747. [Google Scholar] [CrossRef]
- Benjamin, J.S.; Volin, T.E. The mechanism of mechanical alloying. Metall. Trans. 1974, 5, 1929–1934. [Google Scholar] [CrossRef]
- Ishida, T. Sintered Materials Process; Morikita Publishing: Tokyo, Japan, 2011. [Google Scholar]
- Kikuchi, T.; Takarada, Y. Relationship between hardness and tensile strength of austenitic stainless steel. J. High Press. Inst. Jpn. 2020, 58, 216–221. [Google Scholar]
- Wang, R.; Li, Y.; Xiao, T.; Cong, L.; Ling, Y.; Lu, Z.; Fukushima, C.; Tsuchitori, I.; Bazzaoui, M. Using atomic force microscopy to measure thickness of passive film on stainless steel immersed in aqueous solution. Sci. Rep. 2019, 9, 13094. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ohgata, Y.; Li, Y.; Xiao, T.; Honda, M. Tribocorrosion behaviour of SUS430 stainless steel in aqueous solutions with different pH. Coatings 2023, 13, 1539. [Google Scholar] [CrossRef]

| BM0.7h | BM6h | BM12h | |
|---|---|---|---|
| Lattice parameter | 0.3525 | 0.3529 | 0.3532 |
| Crystalline size | 22 | 24 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Kamada, S. Hardness and Corrosion Behavior of CrMnFeCoNi Alloy Fabricated by Ball Milling and Spark Plasma Sintering. Materials 2024, 17, 4793. https://doi.org/10.3390/ma17194793
Wang R, Kamada S. Hardness and Corrosion Behavior of CrMnFeCoNi Alloy Fabricated by Ball Milling and Spark Plasma Sintering. Materials. 2024; 17(19):4793. https://doi.org/10.3390/ma17194793
Chicago/Turabian StyleWang, Rongguang, and Sohei Kamada. 2024. "Hardness and Corrosion Behavior of CrMnFeCoNi Alloy Fabricated by Ball Milling and Spark Plasma Sintering" Materials 17, no. 19: 4793. https://doi.org/10.3390/ma17194793








