Mathematical Modeling of Initial Exothermic Behavior and Thixotropic Properties in Nanoclay-Enhanced Cementitious Materials
Abstract
:1. Introduction
2. Mathematical Model Establishment
2.1. The First Exothermic Peak
2.2. The Rheological Behavior
3. Results and Discussion
3.1. The First Exothermic Peak—Calibration and Validation 1
3.2. The First Exothermic Peak—Calibration and Validation 2
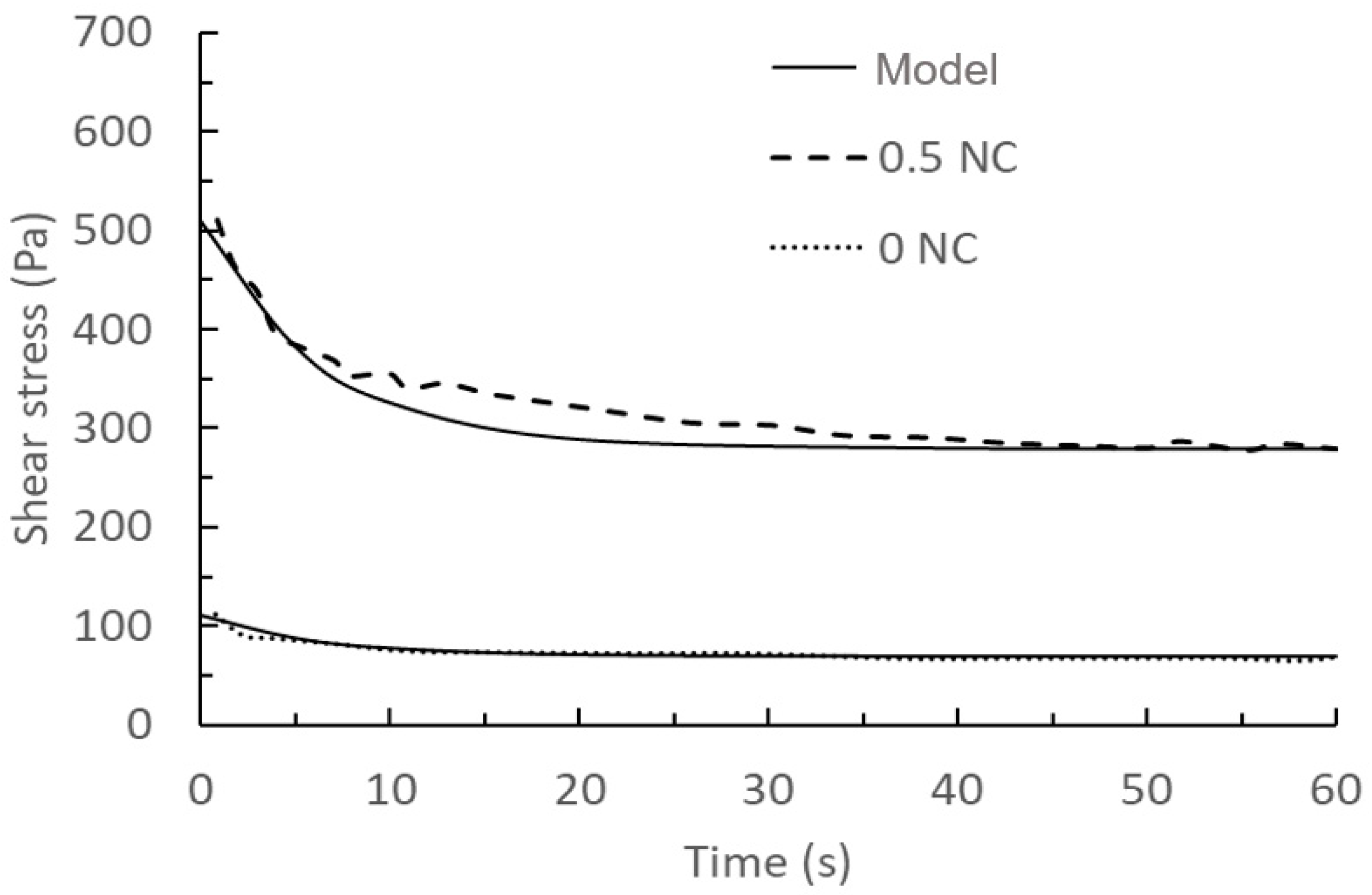
3.3. The Rheological Behavior—Calibration and Validation 3
3.4. The Rheological Behavior—Calibration and Validation 4
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le, T.T.; Austin, S.A.; Lim, S.; Buswell, R.A.; Law, R.; Gibb, A.G.; Thorpe, T. Hardened properties of high-performance printing concrete. Cem. Concr. Res. 2012, 42, 558–566. [Google Scholar] [CrossRef]
- Kazemian, A.; Yuan, X.; Cochran, E.; Khoshnevis, B. Cementitious materials for construction-scale 3D printing: Laboratory testing of fresh printing mixture. Constr. Build. Mater. 2017, 145, 639–647. [Google Scholar] [CrossRef]
- Reales, O.A.M.; Duda, P.; Silva, E.C.; Paiva, M.D.; Toledo Filho, R.D. Nanosilica particles as structural buildup agents for 3D printing with Portland cement pastes. Constr. Build. Mater. 2019, 219, 91–100. [Google Scholar] [CrossRef]
- Lim, S.; Buswell, R.A.; Le, T.T.; Austin, S.A.; Gibb, A.G.; Thorpe, T. Developments in construction-scale additive manufacturing processes. Autom. Constr. 2012, 21, 262–268. [Google Scholar] [CrossRef]
- Buswell, R.A.; De Silva, W.L.; Jones, S.Z.; Dirrenberger, J. 3D printing using concrete extrusion: A roadmap for research. Cem. Concr. Res. 2018, 112, 37–49. [Google Scholar] [CrossRef]
- Yang, H.; Che, Y.; Shi, M. Influences of calcium carbonate nanoparticles on the workability and strength of 3D printing cementitious materials containing limestone powder. J. Build. Eng. 2021, 44, 102976. [Google Scholar] [CrossRef]
- Guo, X.; Yang, J.; Xiong, G. Influence of supplementary cementitious materials on rheological properties of 3D printed fly ash based geopolymer. Cem. Concr. Compos. 2020, 114, 103820. [Google Scholar] [CrossRef]
- Link, J.; Sowoidnich, T.; Pfitzner, C.; Gil-Diaz, T.; Heberling, F.; Lützenkirchen, J.; Schäfer, T.; Ludwig, H.; Haist, M. The influences of cement hydration and temperature on the thixotropy of cement paste. Materials 2020, 13, 1853. [Google Scholar] [CrossRef] [PubMed]
- Wallevik, J.E. Rheological properties of cement paste: Thixotropic behavior and structural breakdown. Cem. Concr. Res. 2009, 39, 14–29. [Google Scholar] [CrossRef]
- Thiedeitz, M.; Crasselt, C.; Xiao, P.; Ukrainczyk, N.; Schmidt, W.; Kränkel, T. Multidisciplinary approach for the prediction of cement paste rheological properties: Physical analysis, experimental rheology and microstructural modelling. Ce/Papers 2023, 6, 715–724. [Google Scholar] [CrossRef]
- Panda, B.; Ruan, S.; Unluer, C.; Tan, M.J. Investigation of the properties of alkali-activated slag mixes involving the use of nanoclay and nucleation seeds for 3D printing. Compos. Part B Eng. 2020, 186, 107826. [Google Scholar] [CrossRef]
- Yıldız, N.; Erol, M.; Baran, B.; Sarıkaya, Y.; Çalımlı, A. Modification of rheology and permeability of Turkish ceramic clays using sodium silicate. Appl. Clay Sci. 1998, 13, 65–77. [Google Scholar] [CrossRef]
- Ferron, R.P.; Gregori, A.; Sun, Z.; Shah, S.P. Rheological method to evaluate structural buildup in self-consolidating concrete cement pastes. ACI Mater. J. 2007, 104, 242. [Google Scholar]
- Quanji, Z.; Lomboy, G.R.; Wang, K. Influence of nano-sized highly purified magnesium alumino silicate clay on thixotropic behavior of fresh cement pastes. Constr. Build. Mater. 2014, 69, 295–300. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in Understanding Hydration of Portland Cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Zhang, J.; Ke, G.; Liu, Y. Early hydration heat of calcium sulfoaluminate cement with influences of supplementary cementitious materials and water to binder ratio. Materials 2021, 14, 642. [Google Scholar] [CrossRef]
- Kaufmann, J.; Winnefeld, F.; Lothenbach, B. Stability of ettringite in CSA cement at elevated temperatures. Adv. Cem. Res. 2016, 28, 251–261. [Google Scholar] [CrossRef]
- Jarny, S.; Roussel, N.; Le Roy, R.; Coussot, P. Modelling thixotropic behavior of fresh cement pastes from MRI measurements. Cem. Concr. Res. 2008, 38, 616–623. [Google Scholar] [CrossRef]
- Kozior, T.; Kundera, C. Viscoelastic properties of cell structures manufactured using a photo-curable additive technology—PJM. Polymers 2021, 13, 1895. [Google Scholar] [CrossRef]
- Ayegba, B.O.; Egbe, K.J.I.; Matin Nazar, A.; Huang, M.; Hariri-Ardebili, M.A. Resource Efficiency and Thermal Comfort of 3D Printable Concrete Building Envelopes Optimized by Performance Enhancing Insulation: A Numerical Study. Energies 2022, 15, 1069. [Google Scholar] [CrossRef]
- Nodehi, M.; Aguayo, F.; Nodehi, S.E.; Gholampour, A.; Ozbakkaloglu, T.; Gencel, O. Durability properties of 3D printed concrete (3DPC). Autom. Constr. 2022, 142, 104479. [Google Scholar] [CrossRef]
- Han, N.; Xiao, J.; Zhang, L.; Peng, Y. A microscale-based numerical model for investigating hygro-thermo-mechanical behaviour of 3D printed concrete at elevated temperatures. Constr. Build. Mater. 2022, 344, 128231. [Google Scholar] [CrossRef]
- Şahin, H.G.; Mardani, A. How does rheological behaviour affect the interlayer-bonding strength of 3DPC mixtures? J. Adhes. Sci. Technol. 2023, 1–25. [Google Scholar] [CrossRef]
- Dai, X.; Tao, Y.; Van Tittelboom, K.; De Schutter, G. Rheological and mechanical properties of 3D printable alkali-activated slag mixtures with addition of nano clay. Cem. Concr. Compos. 2023, 139, 104995. [Google Scholar] [CrossRef]
- Hattori, K.; Izumi, K. A rheological expression of coagulation rate theory. J. Dispers. Sci. Technol. 1982, 3, 129–145. [Google Scholar] [CrossRef]
- Ukrainczyk, N.; Thiedeitz, M.; Kränkel, T.; Koenders, E.; Gehlen, C. Modeling SAOS yield stress of cement suspensions: Microstructure-based computational approach. Materials 2020, 13, 2769. [Google Scholar] [CrossRef]
- Flatt, R.J.; Bowen, P. Yield stress of multimodal powder suspensions: An extension of the YODEL (Yield Stress mODEL). J. Am. Ceram. Soc. 2007, 90, 1038–1044. [Google Scholar] [CrossRef]
- Flatt, R.J.; Bowen, P. Yodel: A yield stress model for suspensions. J. Am. Ceram. Soc. 2006, 89, 1244–1256. [Google Scholar] [CrossRef]
- Kapur, P.C.; Scales, P.J.; Boger, D.V.; Healy, T.W. Yield Stress of Suspensions Loaded with Size Distributed Particles. AIChE J. 1997, 43, 1171–1179. [Google Scholar] [CrossRef]
- Ridi, F.; Fratini, E.; Luciani, P.; Winnefeld, F.; Baglioni, P. Hydration kinetics of tricalcium silicate by calorimetric methods. J. Colloid Interface Sci. 2011, 364, 118–124. [Google Scholar] [CrossRef]
- Bai, S.; Guan, X.; Li, G. Early-age hydration heat evolution and kinetics of Portland cement containing nano-silica at different temperatures. Constr. Build. Mater. 2022, 334, 127363. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Cahn, J.W. The kinetics of grain boundary nucleated reactions. Acta Metall. 1956, 4, 449–459. [Google Scholar] [CrossRef]
- Kashchiev, D. Nucleation; Butterworth-Heinemann: Oxford, UK, 2000; pp. 115–132. [Google Scholar]
- Cheng, D.C.-H.; Evans, F. Phenomenological characterization of the rheological behaviour of inelastic reversible thixotropic and antithixotropic fluids. Br. J. Appl. Phys. 1965, 16, 1599–1617. [Google Scholar] [CrossRef]
- Sang, Q.Z.; Chen, X.; Zhang, J.-L.; Yuan, Y. Modelling the First Exothermic Peak during Hydration of Cement incorporating Blast Furnace Slag. In Proceedings of the Seventeenth International Conference on Civil, Structural and Environmental Engineering Computing, Pécs, Hungary, 28–31 August 2023; Volume CCC 6. Paper 4.1. [Google Scholar]
- Cuesta, A.; Santacruz, I.; Sanfélix, S.G.; Fauth, F.; Aranda, M.A.G.; De la Torre, A.G. Hydration of C4AF in the presence of other phases: A synchrotron X-ray powder diffraction study. Constr. Build. Mater. 2015, 101, 818–827. [Google Scholar] [CrossRef]
- Jakob, C.; Jansen, D.; Ukrainczyk, N.; Koenders, E.; Pott, U.; Stephan, D.; Neubauer, J. Relating ettringite formation and rheological changes during the initial cement hydration: A comparative study applying XRD analysis, rheological measurements and modeling. Materials 2019, 12, 2957. [Google Scholar] [CrossRef] [PubMed]
- Jakob, C.; Jansen, D.; Dengler, J.; Neubauer, J. Controlling ettringite precipitation and rheological behavior in ordinary Portland cement paste by hydration control agent, temperature and mixing. Cem. Concr. Res. 2023, 166, 107095. [Google Scholar] [CrossRef]
- Jiang, W.; Li, W.; Chen, X. Effects on the initial hydration exotherm: An extend numerical model of the first exothermic peak for 3D printed cement-based materials. Front. Struct. Civ. Eng. 2023, 8, 291. [Google Scholar]
- Papo, A. The thixotropic behavior of white Portland cement pastes. Cem. Concr. Res. 1988, 18, 595–603. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Wu, A.; Klein, B.; Zhang, X. A constitutive model for thixotropic cemented tailings backfill pastes. J. Non-Newton. Fluid Mech. 2021, 295, 104548. [Google Scholar] [CrossRef]
- Vachaparambil, K.J.; Mårtensson, G.; Essén, L. Rheological characterization of non-Brownian suspensions based on structure kinetics. Solder. Surf. Mt. Technol. 2018, 30, 57–64. [Google Scholar] [CrossRef]
- Teng, L.; Zhu, J.; Khayat, K.H.; Liu, J. Effect of welan gum and nanoclay on thixotropy of UHPC. Cem. Concr. Res. 2020, 138, 106238. [Google Scholar] [CrossRef]
- Cheung, J.; Jeknavorian, A.; Roberts, L.; Silva, D. Impact of admixtures on the hydration kinetics of Portland cement. Cem. Concr. Res. 2011, 41, 1289–1309. [Google Scholar] [CrossRef]
- Kawashima, S.; Chaouche, M.; Corr, D.J.; Shah, S.P. Rate of thixotropic rebuilding of cement pastes modified with highly purified attapulgite clays. Cem. Concr. Res. 2013, 53, 112–118. [Google Scholar] [CrossRef]
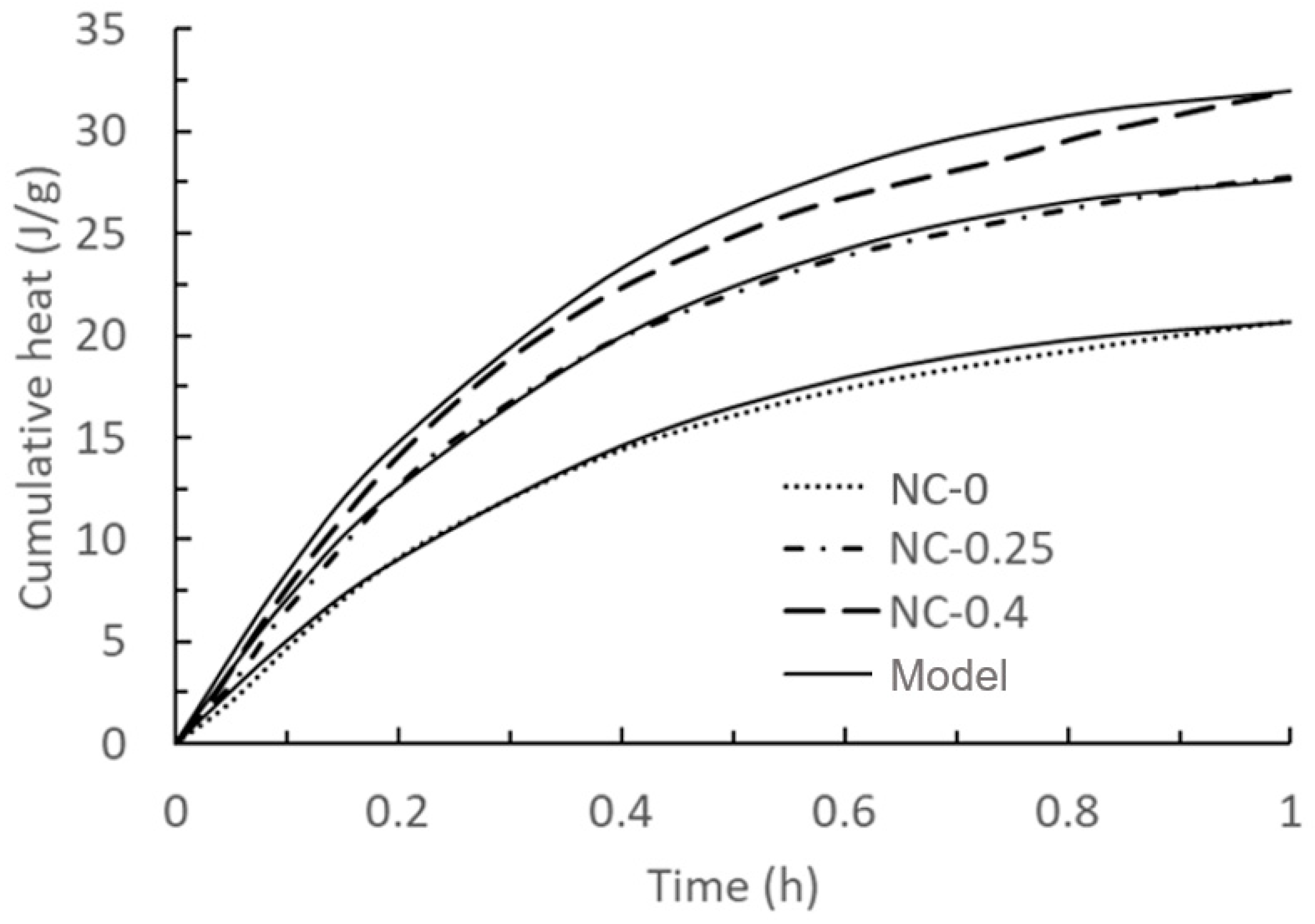
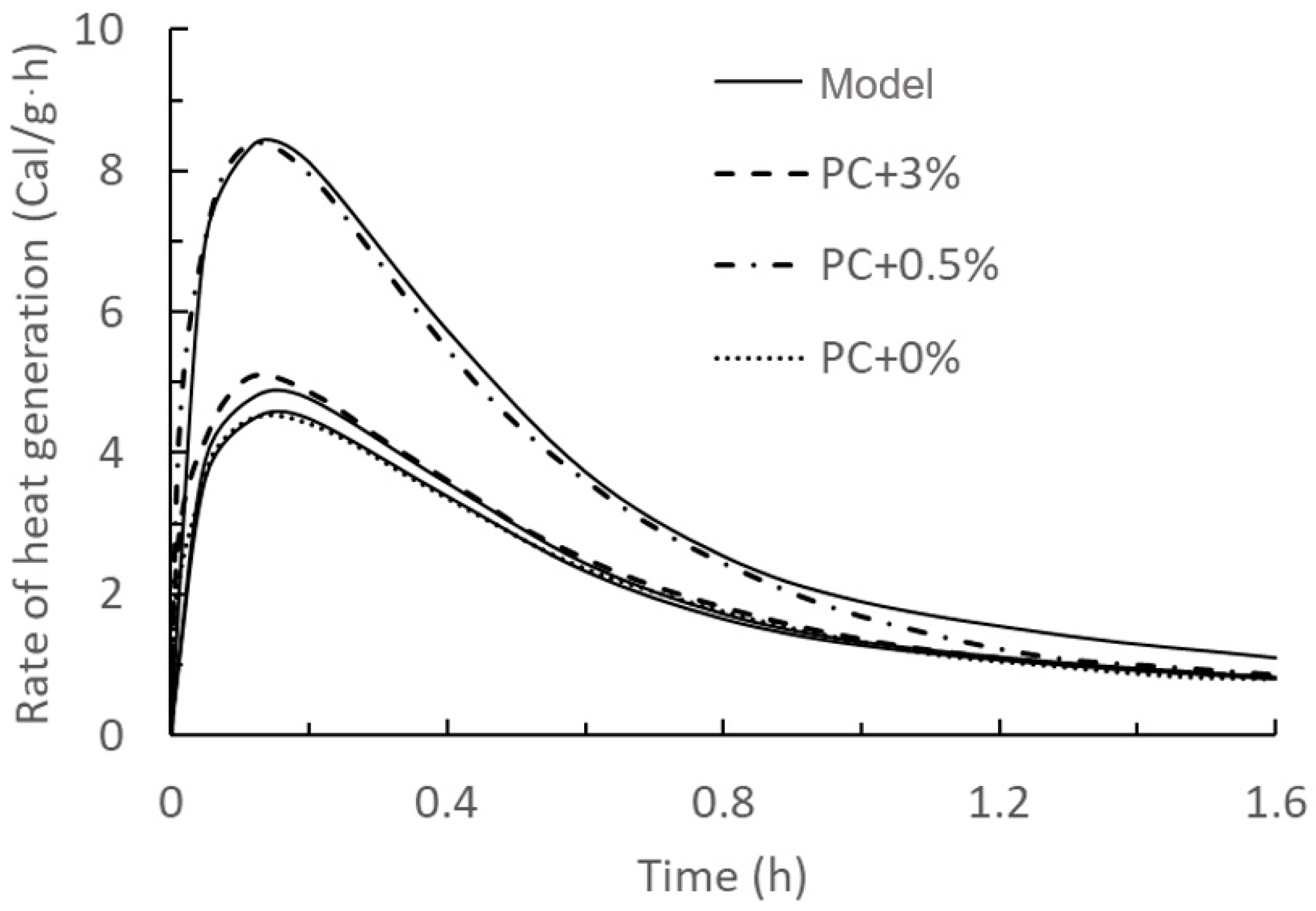



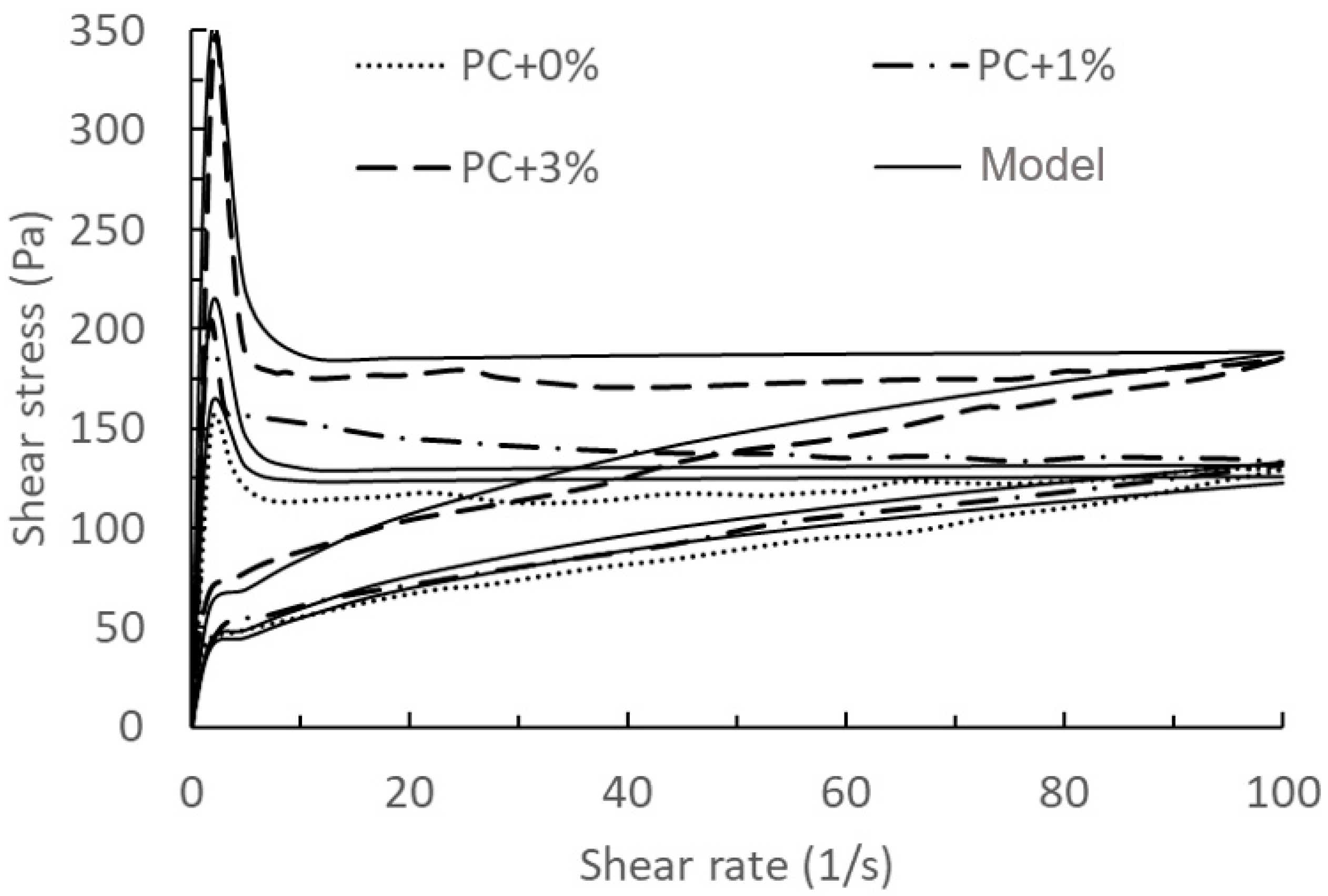
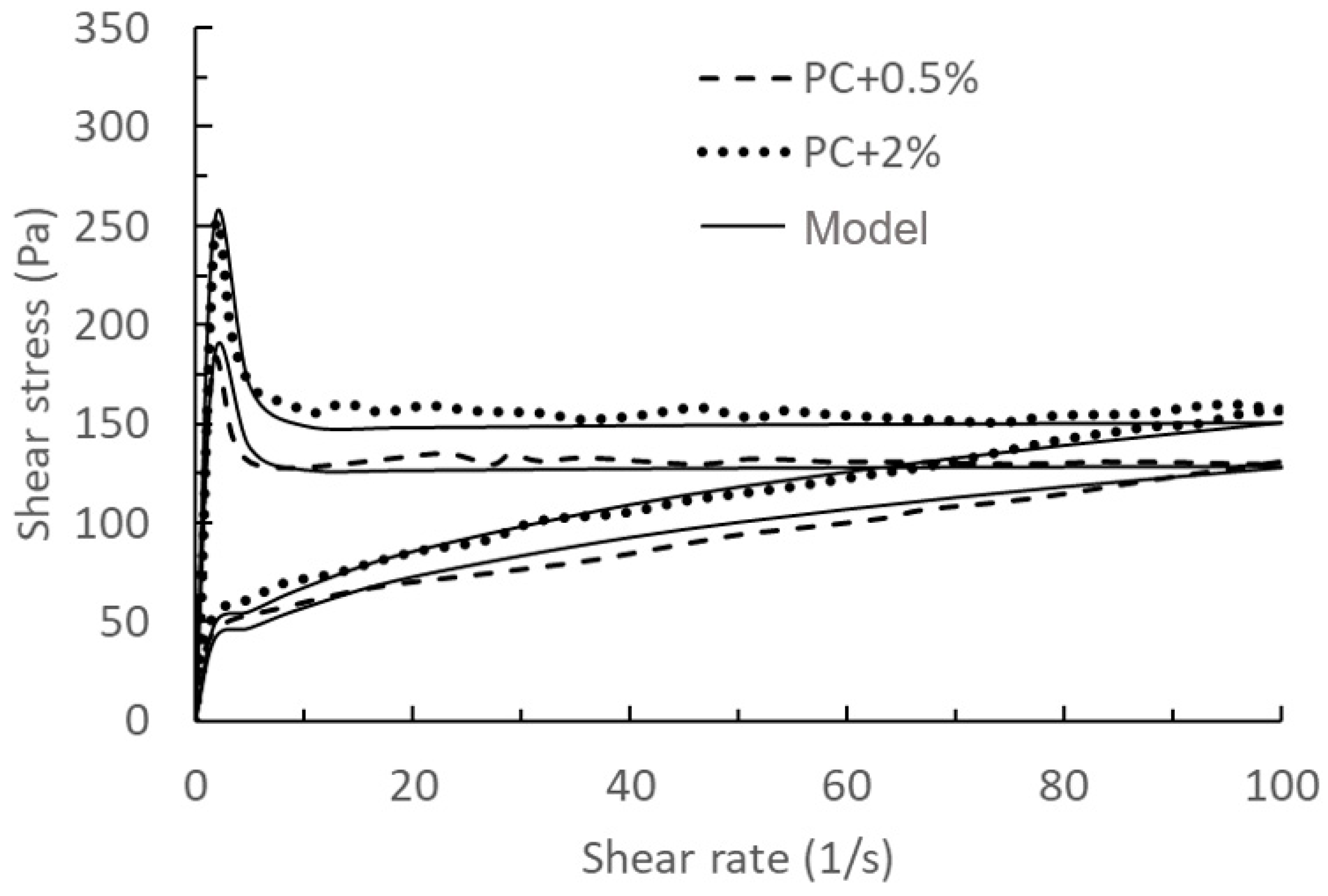

| Figure | Name | RMSE | Figure | Name | RMSE |
|---|---|---|---|---|---|
| Figure 1 | NC-0 | 0.13 J/g | Figure 5 | PC + 0.5% | 2.92 Pa |
| NC-0.25 | 0.05 J/g | PC + 2% | 9.07 Pa | ||
| NC-0.4 | 0.35 J/g | Figure 6 | PC + 0% | 7.52 Pa | |
| Figure 2 | PC + 0% | 0.11 Cal/g·h | PC + 1% | 1.88 Pa | |
| PC + 0.5% | 0.18 Cal/g·h | PC + 3% | 12.90 Pa | ||
| PC + 3% | 0.26 Cal/g·h | Figure 7 | PC + 0.5% | 8.37 Pa | |
| Figure 3 | PC + 1% | 0.21 Cal/g·h | PC + 2% | 9.92 Pa | |
| PC + 2% | 0.34 Cal/g·h | Figure 8 | 0 NC | 4.12 Pa | |
| Figure 4 | PC + 0% | 3.68 Pa | 0.5 NC | 25.69 Pa | |
| PC + 1% | 6.78 Pa | Figure 9 | 0 NC | 3.32 Pa | |
| PC + 3% | 11.27 Pa | 0.5 NC | 21.45 Pa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, P.; Chen, X.; Cao, D.; Yuan, Y.; Dai, Y.; Ukrainczyk, N.; Koenders, E. Mathematical Modeling of Initial Exothermic Behavior and Thixotropic Properties in Nanoclay-Enhanced Cementitious Materials. Materials 2024, 17, 1502. https://doi.org/10.3390/ma17071502
Xiao P, Chen X, Cao D, Yuan Y, Dai Y, Ukrainczyk N, Koenders E. Mathematical Modeling of Initial Exothermic Behavior and Thixotropic Properties in Nanoclay-Enhanced Cementitious Materials. Materials. 2024; 17(7):1502. https://doi.org/10.3390/ma17071502
Chicago/Turabian StyleXiao, Peng, Xi Chen, Donglin Cao, Yong Yuan, Ying Dai, Neven Ukrainczyk, and Eddie Koenders. 2024. "Mathematical Modeling of Initial Exothermic Behavior and Thixotropic Properties in Nanoclay-Enhanced Cementitious Materials" Materials 17, no. 7: 1502. https://doi.org/10.3390/ma17071502








