On the Use of the Fricke-Pluronic F-127 Gel Dosimeter for Radiation Isocenter Testing of a Medical Linear Accelerator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dosimeter Preparation
2.2. Sample Preparation
2.3. Irradiation
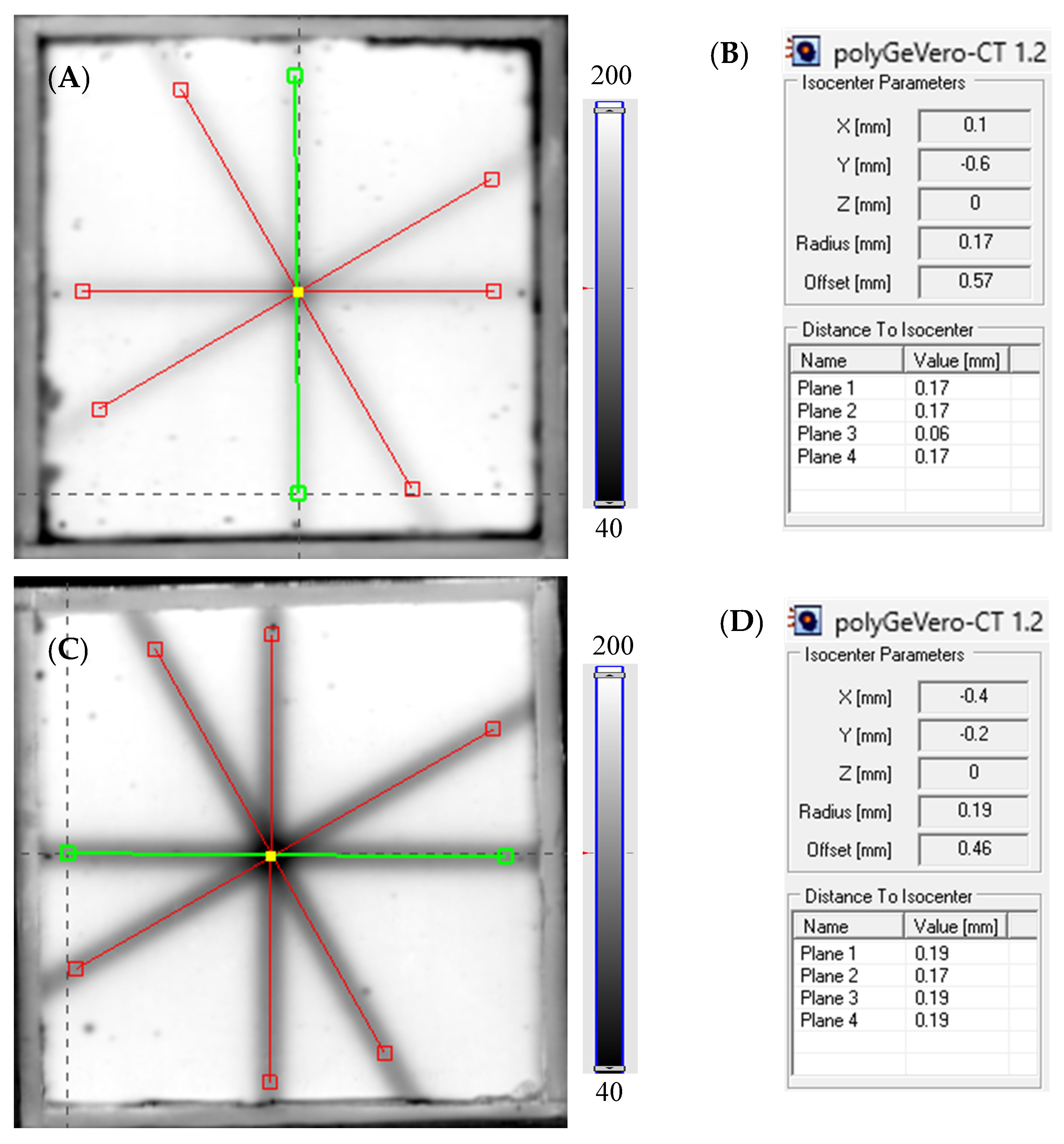
2.4. Readout and Determining the Radiation Isocenter
3. Results and Discussion
3.1. Stability and Optimal MU per Field
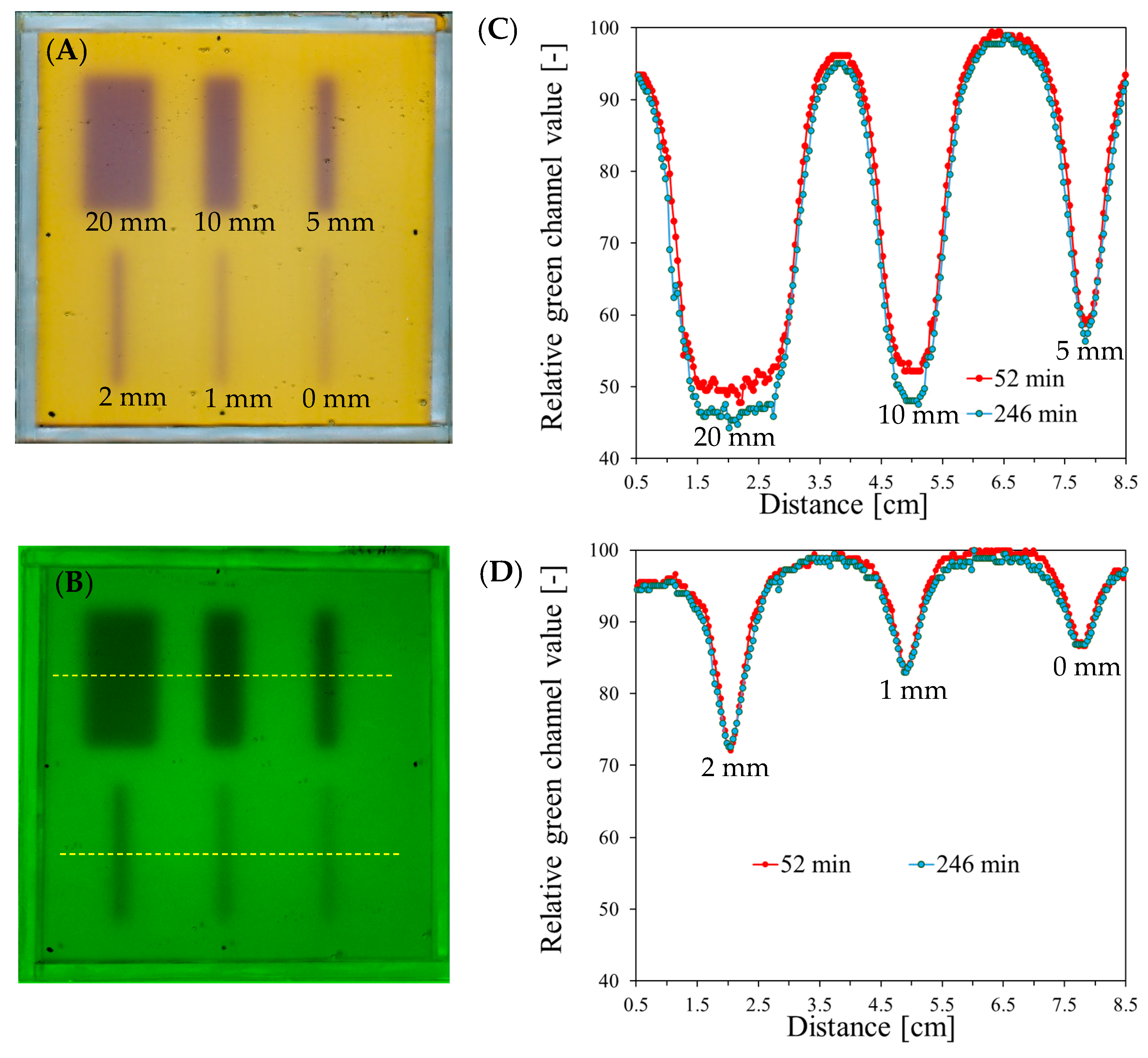
3.2. Evaluation of Beam Size
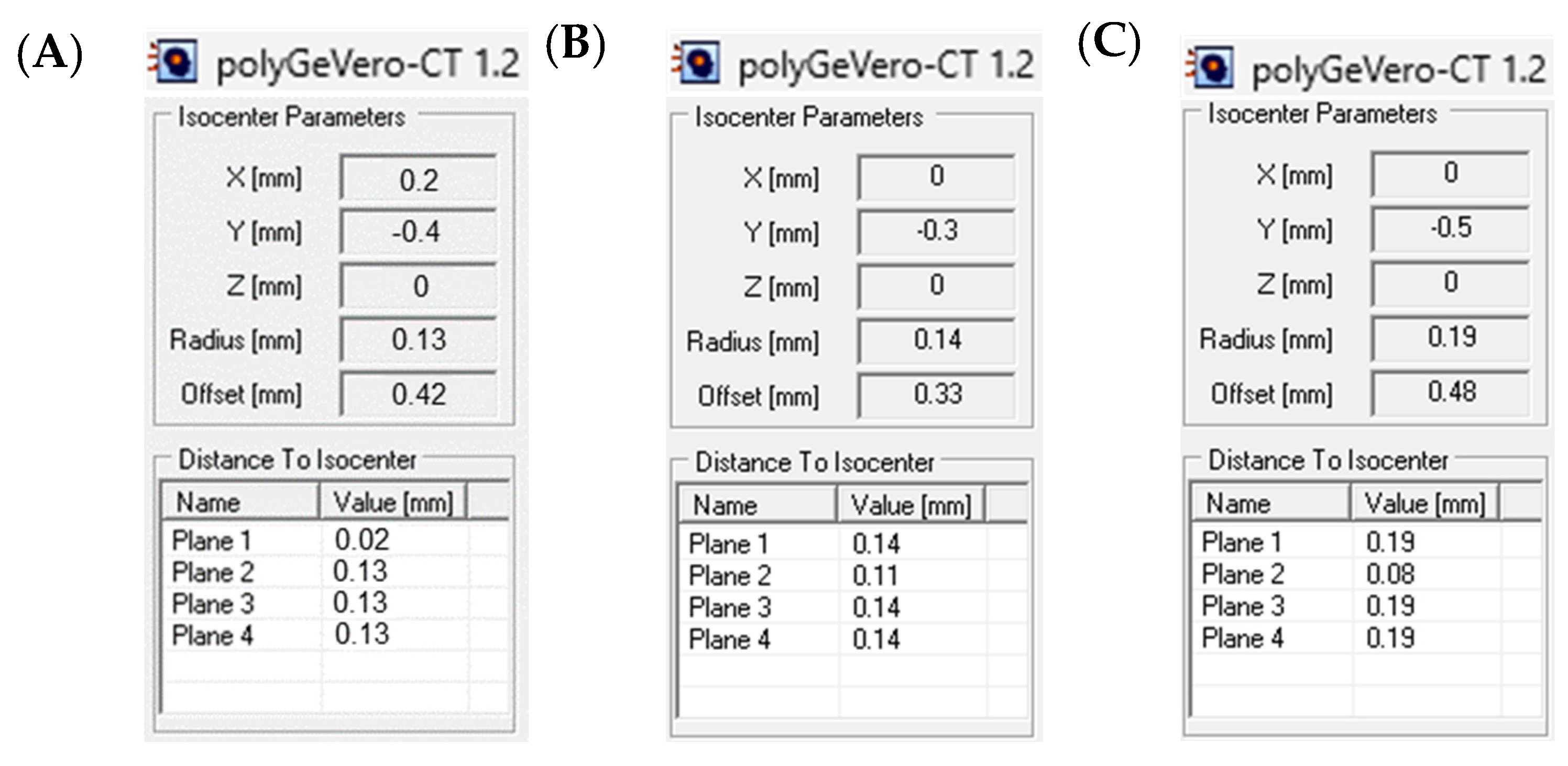
3.3. Example Application: The Coincidence Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venning, A.; Healy, B.; Nitschke, K.; Baldock, C. Investigation of the MAGAS normoxic polymer gel dosimeter with Pyrex glass walls for clinical radiotherapy dosimetry. Nucl. Instrum. Methods Phys. Res. A 2005, 555, 396–402. [Google Scholar] [CrossRef]
- Pappas, E.; Maris, T.; Angelopoulos, A.; Paparigopoulou, M.; Sakelliou, L.; Sandilos, P.; Voyiatzi, S.; Vlachos, L. A new polymer gel for magnetic resonance imaging (MRI) radiation dosimetry. Phys. Med. Biol. 1999, 44, 2677–2684. [Google Scholar] [CrossRef]
- Venning, A.; Brindha, S.; Hill, B.; Baldock, C. Preliminary study of a normoxic PAG gel dosimeter with tetrakis (hydroxymethyl) phosphonium chloride as an antioxidant. J. Phys. Conf. Ser. 2004, 3, 155–158. [Google Scholar] [CrossRef]
- Kozicki, M.; Jaszczak, M.; Maras, P.; Dudek, M.; Cłapa, M. On the development of a VIPARnd radiotherapy 3D polymer gel dosimeter. Phys. Med. Biol. 2017, 62, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Kwiatos, K.; Kadlubowski, S.; Dudek, M. TTC-Pluronic 3D radiochromic gel dosimetry of ionizing radiation. Phys. Med. Biol. 2017, 62, 5668–5690. [Google Scholar] [CrossRef] [PubMed]
- Kouvati, K.; Jaszczak, M.; Papagiannis, P.; Kadlubowski, S.; Wach, R.; Maras, P.; Dudek, M.; Kozicki, M. Leuco crystal violet-Pluronic F–127 3D radiochromic gel dosimeter. Phys. Med. Biol. 2019, 64, 175017. [Google Scholar] [CrossRef]
- Hayashi, S.; Ono, K.; Fujino, K.; Ikeda, S.; Tanaka, K. Novel radiochromic gel dosimeter based on a polyvinyl alcohol—Iodide complex. Radiat. Meas. 2020, 131, 106226. [Google Scholar] [CrossRef]
- De Deene, Y.; Wheatley, M.; Dong, B.; Roberts, N.; Jelen, U.; Waddington, D.; Liney, G. Towards real-time 4D radiation dosimetry on an MRI-Linac. Phys. Med. Biol. 2020, 65, 225031. [Google Scholar] [CrossRef] [PubMed]
- Dorsh, S.; Mann, P.; Lang, C.; Hearing, P.; Runz, A.; Karger, C.P. Feasibility of polymer gel-based measurements of radiation isocenter accuracy in magnetic fields. Phys. Med. Biol. 2018, 63, 11NT02. [Google Scholar] [CrossRef]
- Dorsh, S.; Mann, P.; Elter, A.; Runz, A.; Klüter, S.; Karger, C.P. Polymer gel-based measurements of the isocenter accuracy in an MR-LINAC. J. Phys. Conf. Ser. 2019, 1305, 012007. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, S.-M.; Qu, T. What do we mean when we talk about the linac isocenter? Int. J. Med. Phys. Clin. Eng. Radiat. Oncol. 2015, 4, 233–242. [Google Scholar] [CrossRef]
- Fan, Q.; Zhou, S.; Lei, Y.; Li, S.; Zhang, M.-A. Quality assurance approach for linear accelerator mechanical isocenters with portal images. Int. J. Med. Phys. Clin. Eng. Radiat. Oncol. 2018, 7, 100–114. [Google Scholar] [CrossRef]
- Peace, T.; Subramanian, B.; Ravindran, P. An experimental study on using a diagnostic computed radiography system as a quality assurance tool in radiotherapy. Australas. Phys. Eng. Sci. Med. 2008, 31, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Lutz, W.; Winston, K.R.; Maleki, N. A system for stereotactic radiosurgery with a linear accelerator. Int. J. Radiat. Oncol. Biol. Phys. 1988, 14, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, A.; Castro, I.; Martinez, J.A. A procedure to determine the radiation isocenter size in a linear accelerator. Med. Phys. 2004, 31, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, T.; Penne, R.; Verellen, D.; Hrbacek, J.; Lang, S.; Leysen, K.; Vandevondel, I.; Poels, K.; Reynders, T.; Gevaert, T.; et al. Computer-aided analysis of star shot films for high-accuracy radiation therapy treatment units. Phys. Med. Biol. 2012, 57, 2997–3011. [Google Scholar] [CrossRef] [PubMed]
- Treuer, H.; Hoevels, M.; Luyken, K.; Gierich, A.; Kocher, M.; Muller, R.-P.; Sturm, V. On isocentre adjustment and quality control in linear accelerator based radiosurgery with circular collimators and room lasers. Phys. Med. Biol. 2000, 45, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Dorsh, S.; Mann, P.; Elter, A.; Runz, A.; Spindeldreier, C.K.; Klüter, S.; Karger, C.P. Measurement of isocenter alignment accuracy and image distortion of an 0.35 T RM-linac system. Phys. Med. Biol. 2019, 64, 205011. [Google Scholar]
- Pant, K.; Umeh, C.; Oldham, M.; Floyd, S.; Giles, W.; Adamson, J. Comprehensive radiation and imaging isocenter verification using NIPAM kV-CBCT dosimetry. Med. Phys. 2020, 47, 927–936. [Google Scholar] [CrossRef]
- Maras, P.; Kozicki, M. Fast isocenter determination using 3D polymer gel dosimetry with kilovoltage cone-beam CT reading and the polyGeVero-CT software package for linac quality assurance in radiotherapy. Materials 2022, 15, 6807. [Google Scholar] [CrossRef]
- Kozicki, M.; Maras, P. On the measurement of radiation isocenter for medical accelerators using 3D polymer gel dosimetry. Introduction, application, and good practices. Measurement 2023, 221, 113585. [Google Scholar] [CrossRef]
- Kozicki, M.; Jaszczak, M.; Maras, P.; Kadłubowski, S. Measurement of the radiation dose and radiation isocenter of the TrueBeam accelerator using 3D polymer gel dosimeters from the VIPAR family with different chemical history. Measurement 2023, 221, 113452. [Google Scholar] [CrossRef]
- Fricke, H.; Morse, S. The chemical action of Roentgen rays on dilute ferrous sulphate solutions as a measure of radiation dose. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1927, 18, 430–432. [Google Scholar]
- Gore, J.C.; Kang, Y.S.; Schultz, R.J. Measurement of radiation dose distributions by nuclear magnetic resonance (NMR) imaging. Phys. Med. Biol. 1984, 29, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Bero, M.A.; Gilboy, W.B.; Glover, P.M. Radiochromic gel dosemeter for three-dimensional dosimetry. Radiat. Phys. Chem. 2001, 61, 433–435. [Google Scholar] [CrossRef]
- Baldock, C.; Harris, P.J.; Piercy, A.R.; Healy, B. Experimental determination of the diffusion coefficient in two-dimensions in ferrous sulphate gels using the finite element method. Australas. Phys. Eng. Sci. Med. 2001, 24, 19–30. [Google Scholar] [CrossRef]
- Doran, S.J. The history and principles of optical computed tomography for scanning 3-D radiation dosimeters: 2008 update. J. Phys. Conf. Ser. 2009, 164, 012020. [Google Scholar] [CrossRef]
- Pérez, P.; Torres, P.R.; Bruna, A.; Brunetto, M.; Aon, E.; Franco, D.; Mattea, F.; Figueroa, R.; Santibáñez, M.; Valente, M. Fricke gel xylenol orange dosimeter layers for stereotactic radiosurgery: A preliminary approach. Appl. Radiat. Isot. 2021, 178, 109936. [Google Scholar] [CrossRef]
- Penev, K.; Mequanint, K. Controlling sensitivity and stability of ferrous–xylenol orange–gelatin 3D gel dosimeters by doping with phenanthroline-type ligands and glyoxal. Phys. Med. Biol. 2013, 58, 1823–1838. [Google Scholar] [CrossRef]
- Jordan, K.; Sekimoto, M. Effects of adding glycerol and sucrose to ferrous xylenol orange hydrogel. J. Phys. Conf. Ser. 2010, 250, 012048. [Google Scholar] [CrossRef]
- d’Errico, F.; Lazzeri, L.; Dondi, D.; Mariani, M.; Marrale, M.; Souza, S.O.; Gambarini, S. Novel GTA-PVA Fricke gels for three-dimensional dose mapping in radiotherapy. Radiat. Meas. 2017, 106, 612–617. [Google Scholar] [CrossRef]
- Eyadeh, M.M.; Rabaeh, K.E.; Hailat, T.F.; Al-Shorman, M.Y.; Aldweri, F.M.; Kanan, H.M.; Awad, S.I. Investigation of a novel chemically cross-linked Fricke-Methythymol blue–synthetic polymer gel dosimeter with glutaraldehyde cross-linker. Radiat. Measur. 2018, 118, 77–85. [Google Scholar] [CrossRef]
- Piotrowski, M.; Maras, P.; Kadłubowski, S.; Kozicki, M. Study of the Optimal Composition and Storage Conditions of the Fricke–XO–Pluronic F–127 Radiochromic Dosimeter. Materials 2022, 15, 984. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Piotrowski, M.; Maras, P.; Jaszczak, M.; Kozicki, M. Anisotropic diffusion of Fe ions in Fricke-XO-Pluronic F-127 and Fricke-XO-gelatine 3D radiotherapy dosimeters. Phys. Med. Biol. 2021, 66, 155005. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, M.; Maras, P.; Wach, R.; Kadlubowski, S.; Kozicki, M. Impact of Salt on Thermal Stability and Dose Response of the Fricke-XO-Pluronic F-127 3D Radiotherapy Dosimeter. Materials 2022, 15, 5223. [Google Scholar] [CrossRef] [PubMed]
- Maras, P.; Jaszczak, M.; Kozicki, M. Basic features of VIC-T dosimeter with spiral CT readout, CT scanning conditions and data processing with a new polyGeVero-CT software package. Radiat. Phys. Chem. 2021, 189, 109730. [Google Scholar] [CrossRef]
- Kwiatos, K.; Maras, P.; Kadłubowski, S.; Stempień, Z.; Dudek, M.; Kozicki, M. Tetrazolium salts-Pluronic F-127 gels for 3D radiotherapy dosimetry. Phys. Med. Biol. 2018, 63, 095012. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.E.; Hanley, J.; Bayouth, J.; Yin, F.F.; Simon, W.; Dresser, S.; Serago, C.; Aguirre, F.; Ma, L.; Arjomandy, B.; et al. Task Group 142 report: Quality assurance of medical accelerators. Med. Phys. 2009, 36, 4197–4212. [Google Scholar] [CrossRef] [PubMed]
- UNIQUE Linear Accelerator Installation Product Acceptance (IPA-LE-UNIQUE-K), December 2021, Varian, Palo Alto, USA.
- Diniz, I.M.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F–127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef]
- JCGM 100:2008; Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurement. The Joint Committee for Guides in Metrology (JCGM): Research Triangle Park, NC, USA, 2008.

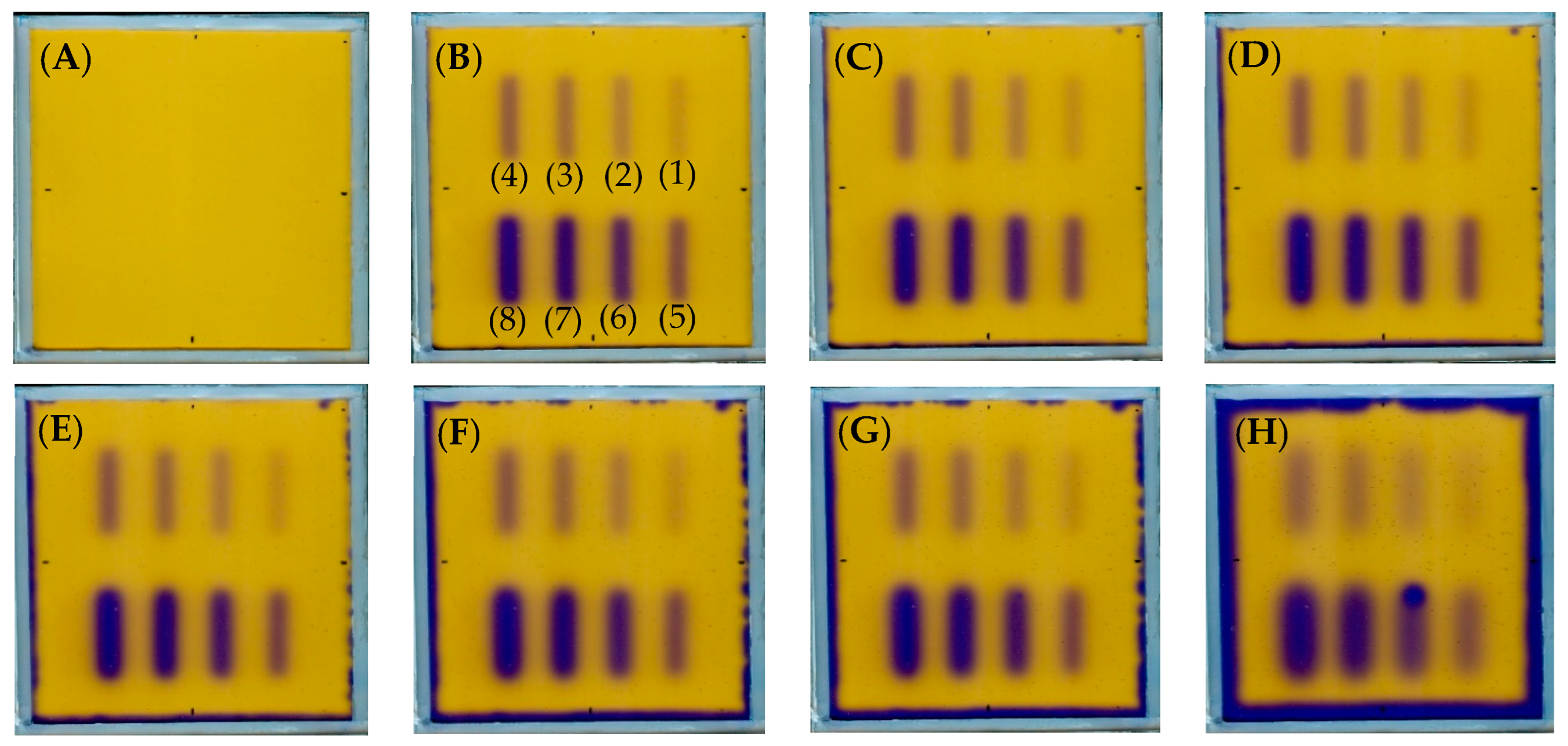
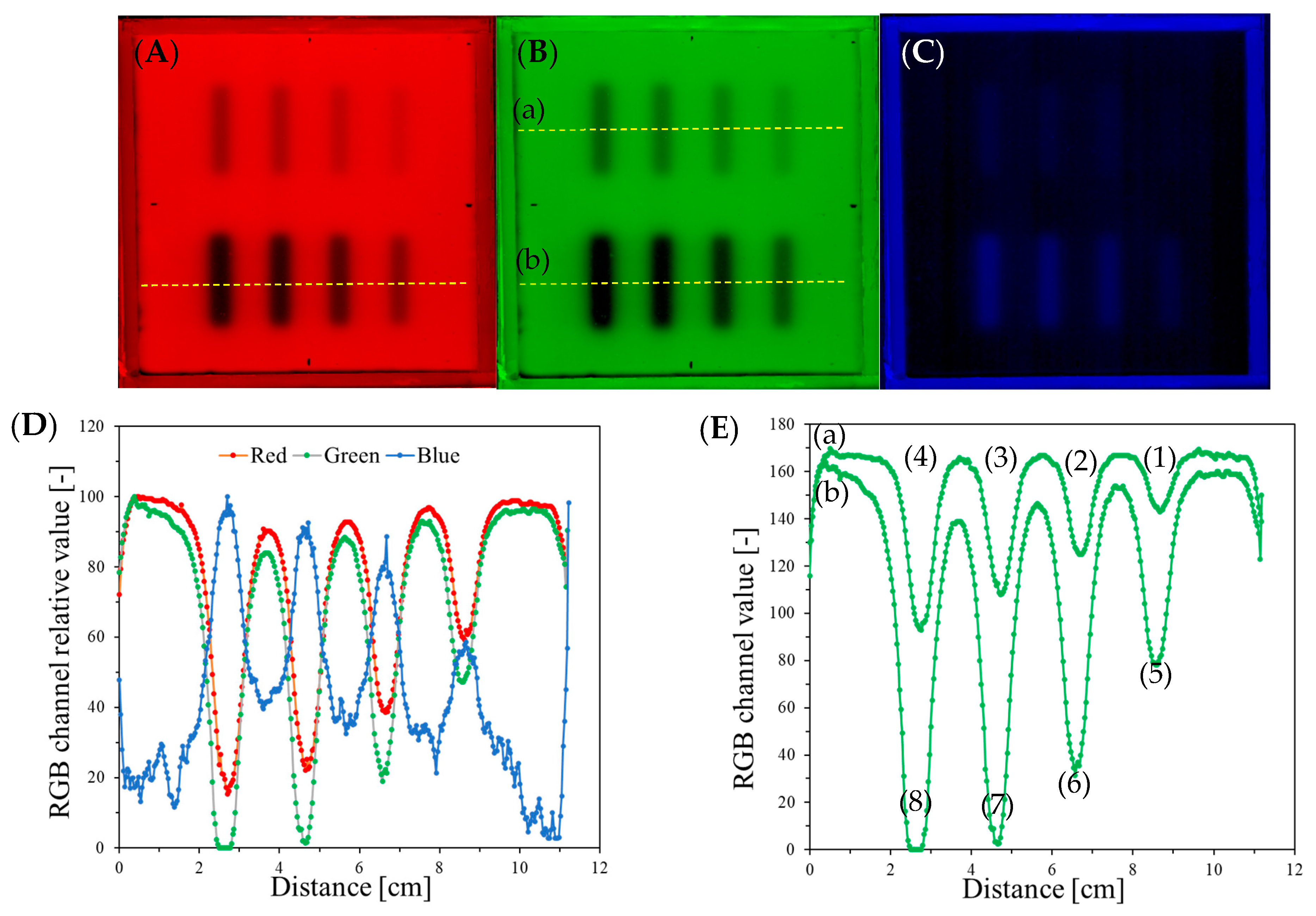
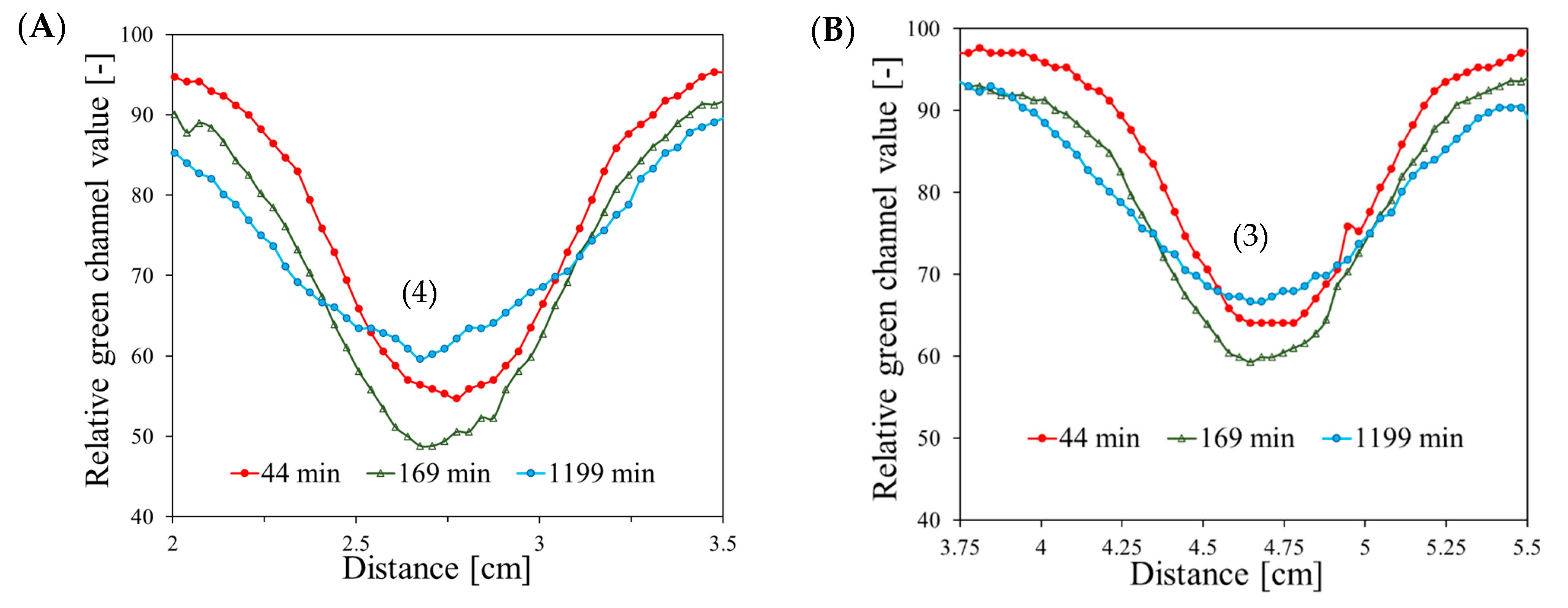

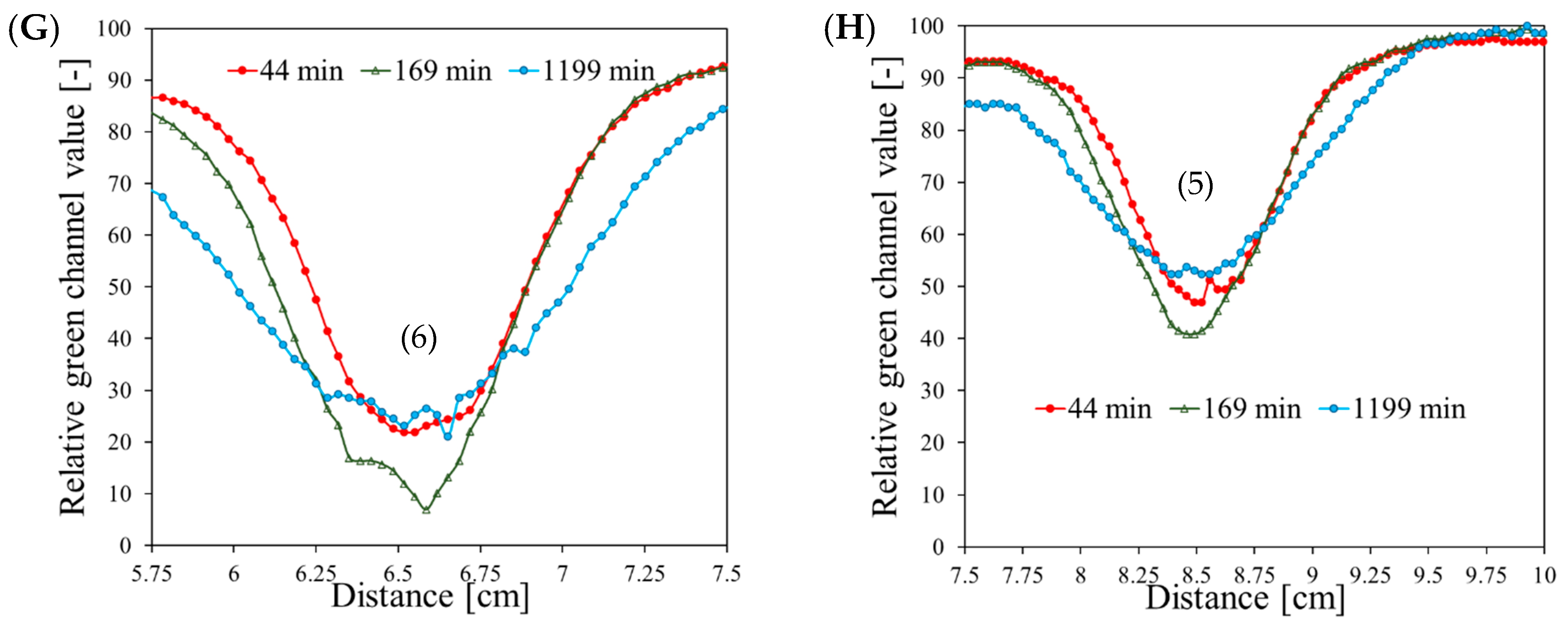
| Planes | Planned Angles [°] | Monitor Units | |
|---|---|---|---|
| 750 | 2500 | ||
| Measured Angles [°] | |||
| Plane 1 | 0 | 359.57 | 0.34 |
| Plane 2 | 90 | 89.93 | 90.47 |
| Plane 3 | 150 | 150.00 | 150.39 |
| Plane 4 | 240 | 239.62 | 240.19 |
| Difference between planned and measured angles [°] | |||
| Plane 1 | 0 | 0.43 | 0.34 |
| Plane 2 | 90 | −0.07 | 0.47 |
| Plane 3 | 150 | 0 | 0.39 |
| Plane 4 | 240 | −0.38 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowski, M.; Maras, P.; Kozicki, M. On the Use of the Fricke-Pluronic F-127 Gel Dosimeter for Radiation Isocenter Testing of a Medical Linear Accelerator. Materials 2024, 17, 1521. https://doi.org/10.3390/ma17071521
Piotrowski M, Maras P, Kozicki M. On the Use of the Fricke-Pluronic F-127 Gel Dosimeter for Radiation Isocenter Testing of a Medical Linear Accelerator. Materials. 2024; 17(7):1521. https://doi.org/10.3390/ma17071521
Chicago/Turabian StylePiotrowski, Michał, Piotr Maras, and Marek Kozicki. 2024. "On the Use of the Fricke-Pluronic F-127 Gel Dosimeter for Radiation Isocenter Testing of a Medical Linear Accelerator" Materials 17, no. 7: 1521. https://doi.org/10.3390/ma17071521







