Synergistic Effect of Dwarf Bamboo Flowering and Wild Boar Rooting on Forest Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Species
2.2. Experimental Layout and Permanent Plot Set Up
2.3. Vegetation Survey
2.4. Data Analysis
3. Results
3.1. Species Diversity
3.2. Species Composition
3.3. Regeneration of Tree Species
3.4. Reestablishment of S. borealis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Royo, A.A.; Carson, W.P. On the formation of dense understory layers in forests worldwide: Consequences and implications for forest dynamics, biodiversity, and succession. Can. J. For. Res. 2006, 36, 1345–1362. [Google Scholar] [CrossRef]
- George, L.O.; Bazzaz, F.A. The fern understory as an ecological filter: Growth and survival of canopy-tree seedlings. Ecology 1999, 80, 846–856. [Google Scholar] [CrossRef]
- Abe, M.; Miguchi, H.; Nakashizuka, T. An interactive effect of simultaneous death of dwarf bamboo, canopy gap, and predatory rodents on beech regeneration. Oecologia 2001, 127, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Izaki, J.; Miguchi, H.; Masaki, T.; Makita, A.; Nakashizuka, T. The effects of Sasa and canopy gap formation on tree regeneration in an old beech forest. J. Veg. Sci. 2002, 13, 565–574. [Google Scholar] [CrossRef]
- Abe, M.; Miguchi, H.; Honda, A.; Makita, A.; Nakashizuka, T. Short-term changes affecting regeneration of Fagus crenata after the simultaneous death of Sasa kurilensis. J. Veg. Sci. 2005, 16, 49–56. [Google Scholar] [CrossRef]
- Caccia, F.D.; Chaneton, E.J.; Kitzberger, T. Direct and indirect effects of understorey bamboo shape tree regeneration niches in a mixed temperate forest. Oecologia 2009, 161, 771–780. [Google Scholar] [CrossRef]
- Letcher, S.G.; Chazdon, R.L. Lianas and self-supporting plants during tropical forest succession. For. Ecol. Manag. 2009, 257, 2150–2156. [Google Scholar] [CrossRef]
- Montti, L.; Campanello, P.I.; Gatti, M.G.; Blundo, C.; Austin, A.T.; Sala, O.E.; Goldstein, G. Understory bamboo flowering provides a very narrow light window of opportunity for canopy-tree recruitment in a neotropical forest of Misiones, Argentina. For. Ecol. Manag. 2011, 262, 1360–1369. [Google Scholar] [CrossRef]
- Messier, C.; Parent, S.; Bergeron, Y. Effects of overstory and understory vegetation on the understory light environment in mixed boreal forests. J. Veg. Sci. 1998, 9, 511–520. [Google Scholar] [CrossRef]
- Watt, A.S. Pattern and process in the plant community. J. Ecol. 1947, 35, 1–22. [Google Scholar] [CrossRef] [Green Version]
- McGee, C.E.; Smith, R.C. Undisturbed rhododendron thickets are not spreading. J. For. 1967, 65, 334–335. [Google Scholar]
- Masaki, T.; Tanaka, H.; Tanouchi, H.; Sakai, T.; Nakashizuka, T. Structure, dynamics and disturbance regime of temperate broad-leaved forests in Japan. J. Veg. Sci. 1999, 10, 805–814. [Google Scholar] [CrossRef]
- Kudo, G.; Amagai, Y.; Hoshino, B.; Kaneko, M. Invasion of dwarf bamboo into alpine snow-meadows in northern Japan: Pattern of expansion and impact on species diversity. Ecol. Evol. 2011, 1, 85–96. [Google Scholar] [CrossRef]
- Cho, S.; Lee, K.; Choung, Y. Distribution, abundance, and effect on plant species diversity of Sasa borealis in Korean forests. J. Ecol. Environ. 2018, 42, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Horsley, S.B. Mechanisms of interference between hayscented fern and black cherry. Can. J. For. Res. 1993, 23, 2059–2069. [Google Scholar] [CrossRef]
- Umeki, K.; Kikuzawa, K. Long-term growth dynamics of natural forests in Hokkaido, northern Japan. J. Veg. Sci. 1999, 10, 815–824. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Dalling, J.W.; Carson, W.P. The impact of lianas on tree regeneration in tropical forest canopy gaps: Evidence for an alternative pathway of gap-phase regeneration. J. Ecol. 2000, 8, 655–666. [Google Scholar] [CrossRef]
- George, L.O.; Bazzaz, F.A. The herbaceous layer as a filter determining spatial pattern in forest tree regeneration. In The Herbaceous Layer in Forests of Eastern North America; Gilliam, F.S., Roberts, M.R., Eds.; Oxford University Press: New York, NY, USA, 2003; pp. 265–282. [Google Scholar]
- Mallik, A.U. Conifer regeneration problems in boreal and temperate forests with ericaceous understory: Role of disturbance, seedbed limitation, and keystone species change. Crit. Rev. Plant Sci. 2003, 22, 341–366. [Google Scholar] [CrossRef]
- Taylor, A.H.; Jinyan, H.; ShiQiang, Z. Canopy tree development and undergrowth bamboo dynamics in old-growth Abies–Betula forests in southwestern China: A 12-year study. For. Ecol. Manag. 2004, 200, 347–360. [Google Scholar] [CrossRef]
- Doležal, J.; Matsuki, S.; Hara, T. Effects of dwarf-bamboo understory on tree seedling emergence and survival in a mixed-oak forest in northern Japan: A multi-site experimental study. Community Ecol. 2009, 10, 225–235. [Google Scholar] [CrossRef]
- Hirobe, M.; Miyamoto, S.; Sakamoto, K.; Kondo, J.; Otoda, T.; Akaji, Y.; Yamanaka, N. The spatial distributions of understory trees in relation to dwarf bamboo cover in a cool-temperate deciduous broadleaf forest in Japan. J. For. Res. 2015, 20, 357–362. [Google Scholar] [CrossRef]
- Suzuki, S. Index to Japanese Bambusaceae; Gakken: Tokyo, Japan, 1978; pp. 1–384. [Google Scholar]
- Toyooka, H.; Sato, M.; Ishizuka, S. Distribution Map of the Sasa Group in Hokkaido, Explanatory Note; Hokkaido Branch Forestry and Forest Products Research Institute: Sapporo, Japan, 1983; pp. 1–36. (In Japanese) [Google Scholar]
- Saitoh, T.; Seiwa, K.; Nishiwaki, A.; Kanno, H.; Akasaka, S. Spatial distribution patterns of Sasa palmata in relation to light conditions across gap-understory continuum in a beech (Fagus crenata) forest. J. Jpn. For. Soc. 2000, 82, 342–348. [Google Scholar]
- Fukuzawa, K.; Shibata, H.; Takagi, K.; Satoh, F.; Koike, T.; Sasa, K. Roles of dominant understory Sasa bamboo in carbon and nitrogen dynamics following canopy tree removal in a cool-temperate forest in northern Japan. Plant Species Biol. 2015, 30, 104–115. [Google Scholar] [CrossRef] [Green Version]
- Masaki, T.; Tanaka, N.; Yagihashi, T.; Ogawa, M.; Tanaka, H.; Sugita, H.; Sato, T.; Nagaike, T. Dynamics of dwarf bamboo populations and tree regeneration over 40 years in a clear-cut beech forest: Effects of advance weeding and herbicide application. J. For. Res. 2021, 26, 1–11. [Google Scholar] [CrossRef]
- Lee, W.T.; Lim, Y. Plant Geography; Kangwon National University Press: Chuncheon, Korea, 2002; pp. 1–412. (In Korean) [Google Scholar]
- Černý, T.; Doležal, J.; Janeček, Š.; Šrůtek, M.; Valachovič, M.; Petřík, P.; Altman, J.; Bartoš, M.; Song, J.S. Environmental correlates of plant diversity in Korean temperate forests. Acta Oecologica 2013, 47, 37–45. [Google Scholar] [CrossRef]
- Černý, T.; Kopecký, M.; Petřík, P.; Song, J.S.; Šrůtek, M.; Valachovič, M.; Altman, J.; Doležal, J. Classification of Korean forests: Patterns along geographic and environmental gradients. Appl. Veg. Sci. 2015, 18, 5–22. [Google Scholar] [CrossRef]
- Cho, S.; Kim, Y.; Choung, Y. Distribution and synchronized massive flowering of Sasa borealis in the forests of Korean National Parks. J. Ecol. Environ. 2018, 42, 1–9. [Google Scholar] [CrossRef]
- Park, S.G.; Yi, M.H.; Yoon, J.W.; Sin, H.T. Environmental factors and growth properties of Sasa borealis (Hack.) Makino community and effect its distribution on the development of lower vegetation in Jirisan National Park. Korean J. Environ. Ecol. 2012, 26, 82–90. (In Korean) [Google Scholar]
- Halpern, C.B. Early successional patterns of forest species: Interactions of life history traits and disturbance. Ecology 1989, 70, 704–720. [Google Scholar] [CrossRef]
- Hughes, J.W.; Fahey, T.J. Colonization dynamics of herbs and shrubs in a disturbed northern hardwood forest. J. Ecol. 1991, 79, 605–616. [Google Scholar] [CrossRef]
- Beckage, B.; Clark, J.S.; Clinton, B.D.; Haines, B.L. A long-term study of tree seedling recruitment in southern Appalachian forests: The effects of canopy gaps and shrub understories. Can. J. For. Res. 2000, 30, 1617–1631. [Google Scholar] [CrossRef]
- Webb, S.L.; Scanga, S.E. Windstorm disturbance without patch dynamics: Twelve years of change in a Minnesota forest. Ecology 2001, 82, 893–897. [Google Scholar] [CrossRef]
- Pacala, S.W.; Canham, C.D.; Silander, J.A., Jr.; Kobe, R.K. Sapling growth as a function of resources in a north temperate forest. Can. J. For. Res. 1994, 24, 2172–2183. [Google Scholar] [CrossRef]
- Finzi, A.C.; Canham, C.D. Sapling growth in response to light and nitrogen availability in a southern New England forest. For. Ecol. Manag. 2000, 131, 153–165. [Google Scholar] [CrossRef]
- Ricard, J.P.; Messier, C.; Delagrange, S.; Beaudet, M. Do understory sapling respond to both light and below-ground competition? A field experiment in a north-eastern American hardwood forest and a literature review. Ann. For. Sci. 2003, 60, 749–756. [Google Scholar] [CrossRef] [Green Version]
- Davies, R.J. The importance of weed control and the use of tree shelters for establishing broadleaved trees on grass dominated sites in England. Forestry 1985, 58, 167–180. [Google Scholar] [CrossRef]
- Marrs, R.H.; Johnson, S.W.; Le Duc, M.G. Control of bracken and restoration of heathland. VIII. The regeneration of the heathland community after 18 years of continued bracken control or 6 years of control followed by recovery. J. Appl. Ecol. 1998, 35, 857–870. [Google Scholar] [CrossRef]
- Biring, B.S.; Comeau, P.G.; Fielder, P.L. Long-term effects of vegetation control treatments for release of Engelmann spruce from a mixed-shrub community in southern British Columbia. Ann. For. Sci. 2003, 60, 681–690. [Google Scholar] [CrossRef]
- Kim, H.C. Ecological Characteristics and Management Methods of Sasa quelpaertensis Nakai. Ph.D. Thesis, Jeju National University, Jeju, Korea, 2009. [Google Scholar]
- Kudo, G.; Kawai, Y.; Amagai, Y.; Winkler, D.E. Degradation and recovery of an alpine plant community: Experimental removal of an encroaching dwarf bamboo. Alp. Bot. 2017, 127, 75–83. [Google Scholar] [CrossRef]
- De la Cretaz, A.L.; Kelty, M.J. Development of tree regeneration in fern-dominated forest understories after reduction of deer browsing. Restor. Ecol. 2002, 10, 416–426. [Google Scholar] [CrossRef]
- Makita, A. The significance of the mode of clonal growth in the life history of bamboos. Plant Species Biol. 1998, 13, 85–92. [Google Scholar] [CrossRef]
- Nakashizuka, T. Regeneration of beech (Fagus crenata) after the simultaneous death of undergrowing dwarf bamboo (Sasa kurilensis). Ecol. Res. 1988, 3, 21–35. [Google Scholar] [CrossRef]
- Yamazaki, K.; Nakagoshi, N. Regeneration of Sasa kurilensis and tree invasion after sporadic flowering. Bamboo J. 2005, 22, 93–103. [Google Scholar]
- Cho, S.; Lee, B.; Choung, Y. Rare nationwide synchronized massive flowering and decline event of Sasa borealis (Hack.) Makino in South Korea. J. Plant Biol. 2017, 60, 423–430. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, S.; Choung, Y. Habitat preference of wild boar (Sus scrofa) for feeding in cool-temperate forests. J. Ecol. Environ. 2019, 43, 1–8. [Google Scholar] [CrossRef]
- Baubet, E.; Bonenfant, C.; Brandt, S. Diet of the wild boar in the French Alps. Galemys 2004, 16, 99–111. [Google Scholar]
- Lyang, D.; Lee, K. Responses of an herbaceous community to wild boar (Sus scrofa coreanus Heude) disturbance in a Quercus mongolica forest at Mt. Jeombong, Korea. J. Ecol. Environ. 2010, 33, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Korea Meteorological Agency. Available online: www.kma.go.kr (accessed on 6 March 2021).
- Korea National Arboretum. Geography and Vegetation of Mt. Jumbong Experimental Forest; Korea National Arboretum: Pocheon, Korea, 2014; pp. 1–197. (In Korean) [Google Scholar]
- Ko, D.W.; Lee, D. Dendroecological reconstruction of the disturbance dynamics and human legacy in an old-growth hardwood forest in Korea. For. Ecol. Manag. 2013, 302, 43–53. [Google Scholar] [CrossRef]
- Cho, Y. Vegetation responses to dwarf bamboo dynamics in cool temperate deciduous forest in South Korea. In Proceedings of the Physiology, Ecology, and Utilization of Dwarf Bamboo, International Symposium on Expansion and Management of Dwarf Bamboos, Jeju, Korea, 17 May 2017; Northeastern Asia Biodiversity Institute: Hanam, Korea, 2017. [Google Scholar]
- Kim, W.; Park, C.; Kim, W. Development of Habitat suitability analysis models for wild boar (Sus scrofa): A case study of Mt. Seorak and Mt. Jumbong. J. GIS Assoc. Korea 1998, 6, 247–256. [Google Scholar]
- Kuuluvainen, T.; Kalmari, R. Regeneration microsites of Picea abies seedlings in a windthrow area of a boreal old-growth forest in southern Finland. Ann. Bot. Fenn. 2003, 40, 401–413. [Google Scholar]
- Vodde, F.; Jogiste, K.; Gruson, L.; Ilisson, T.; Köster, K.; Stanturf, J.A. Regeneration in windthrow areas in hemiboreal forests: The influence of microsite on the height growths of different tree species. J. For. Res. 2010, 15, 55–64. [Google Scholar] [CrossRef]
- Jeon, M.; Lee, K.; Choung, Y. Gap formation and susceptible Abies trees to windthrow in the forests of Odaesan National Park. J. Ecol. Environ. 2015, 38, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Lee, E.J. Diet of the wild boar (Sus scrofa): Implications for management in forest-agricultural and urban environments in South Korea. PeerJ 2019, 7, e7835. [Google Scholar] [CrossRef] [Green Version]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- SPSS. IBM SPSS Statistics for Windows; Version 24.0; IBM Corp.: Armonk, NY, USA, 2016. [Google Scholar]
- Makita, A. Survivorship of a monocarpic bamboo grass, Sasa kurilensis, during the early regeneration process after mass flowering. Ecol. Res. 1992, 7, 245–254. [Google Scholar] [CrossRef]
- Makita, A.; Konno, Y.; Fujita, N.; Takada, K.I.; Hamabata, E. Recovery of a Sasa tsuboiana population after mass flowering and death. Ecol. Res. 1993, 8, 215–224. [Google Scholar] [CrossRef]
- Takahashi, K.; Uemura, S.; Suzuki, J.I.; Hara, T. Effects of understory dwarf bamboo on soil water and the growth of overstory trees in a dense secondary Betula ermanii forest, northern Japan. Ecol. Res. 2003, 18, 767–774. [Google Scholar]
- Ramirez, J.I.; Jansen, P.A.; Poorter, L. Effects of wild ungulates on the regeneration, structure and functioning of temperate forests: A semi-quantitative review. For. Ecol. Manag. 2018, 424, 406–419. [Google Scholar] [CrossRef]
- Hone, J. Feral pigs in Namadgi National Park, Australia: Dynamics, impacts and management. Biol. Conserv. 2002, 105, 231–242. [Google Scholar] [CrossRef]
- Massei, G.; Genov, P.V. The environmental impact of wild boar. Galemys 2004, 16, 135–145. [Google Scholar]
- Seward, N.W.; VerCauteren, K.C.; Witmer, G.W.; Engeman, R.M. Feral swine impacts on agriculture and the environment. Sheep Goat Res. J. 2004, 19, 34–40. [Google Scholar]
- Engeman, R.M.; Constantin, B.U.; Shwiff, S.A.; Smith, H.T.; Woolard, J.; Allen, J.; Dunlap, J. Adaptive and economic management methods for feral hog control in Florida. Hum.-Wildl. Confl. 2007, 1, 178–185. [Google Scholar]
- Howe, T.D.; Bratton, S.P. Winter rooting activity of the European wild boar in the Great Smoky Mountains National Park. Castanea 1976, 41, 256–264. [Google Scholar]
- Brunet, J.; Hedwall, P.O.; Holmström, E.; Wahlgren, E. Disturbance of the herbaceous layer after invasion of an eutrophic temperate forest by wild boar. Nord. J. Bot. 2016, 34, 120–128. [Google Scholar] [CrossRef]
- Boulanger, V.; Dupouey, J.L.; Archaux, F.; Badeau, V.; Baltzinger, C.; Chevalier, R.; Corcket, E.; Dumas, Y.; Forgeard, F.; Marell, A.; et al. Ungulates increase forest plant species richness to the benefit of non-forest specialists. Glob. Chang. Biol. 2018, 24, e485–e495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballari, S.A.; Barrios-García, M.N. A review of wild boar Sus scrofa diet and factors affecting food selection in native and introduced ranges. Mammal Rev. 2014, 44, 124–134. [Google Scholar] [CrossRef]
- Wunderle, J.M., Jr. The role of animal seed dispersal in accelerating native forest regeneration on degraded tropical lands. For. Ecol. Manag. 1997, 99, 223–235. [Google Scholar] [CrossRef]
- Copiz, R.; Del Vico, E.; Fagiani, S.; Giarrizzo, E.; Mei, M.; Mortelliti, A.; Sabatini, F.M.; Blasi, C. Wild boar rooting intensity determines shifts in understorey composition and functional traits. Community Ecol. 2015, 16, 244–253. [Google Scholar]
- Albert, A.; Auffret, A.G.; Cosyns, E.; Cousins, S.A.; D’hondt, B.; Eichberg, C.; Eycott, A.E.; Heinken, T.; Hoffmann, M.; Jaroszewicz, B.; et al. Seed dispersal by ungulates as an ecological filter: A trait-based meta-analysis. Oikos 2015, 124, 1109–1120. [Google Scholar] [CrossRef]
- Janzen, D.H. Why bamboos wait so long to flower. Annu. Rev. Ecol. Syst. 1976, 7, 347–391. [Google Scholar] [CrossRef]
- Barbour, M.G.; Burk, J.H.; Pitts, W.D.; Gilliam, F.S.; Schwartz, M.W. Terrestrial Plant Ecology, 3rd ed.; Benjamin Cummings: Menlo Park, NJ, USA, 1999. [Google Scholar]
- Kawahara, T. Studies on Sasa communities (IV). J. Jpn. For. Soc. 1978, 60, 467–469. [Google Scholar]
- Morisawa, T.; Sugita, H.; Hashimoto, R.; Akai, T. Natural regeneration of Chamaecyparis obtusa for 36 years by strip logging in relation to dwarf bamboo elimination, examined by aerial photographs, in Kiso, Japan. J. Jpn. For. Soc. 2010, 92, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Katayama, N.; Kishida, O.; Sakai, R.; Hayakashi, S.; Miyoshi, C.; Ito, K.; Naniwa, A.; Yamaguchi, A.; Wada, K.; Kowata, S.; et al. Response of a wild edible plant to human disturbance: Harvesting can enhance the subsequent yield of bamboo shoots. PLoS ONE 2015, 10, e0146228. [Google Scholar] [CrossRef]
- Yoshida, T.; Iga, Y.; Ozawa, M.; Noguchi, M.; Shibata, H. Factors influencing early vegetation establishment following soil scarification in a mixed forest in northern Japan. Can. J. For. Res. 2005, 35, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, S. The state of recovery and the biomass of Sasa ishizuchiana Makino in Mt. Kamegamori ten years after death by flowering. Bamboo J. 1984, 2, 16–20. (In Japanese) [Google Scholar]
- Matsuo, A.; Tomimatsu, H.; Sangetsu, Y.; Suyama, Y.; Makita, A. Genet dynamics of a regenerating dwarf bamboo population across heterogeneous light environments in a temperate forest understorey. Ecol. Evol. 2018, 8, 1746–1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomimatsu, H.; Matsuo, A.; Kaneko, Y.; Kudo, E.; Taniguchi, R.; Saitoh, T.; Suyama, Y.; Makita, A. Spatial genet dynamics of a dwarf bamboo: Clonal expansion into shaded forest understory contributes to regeneration after an episodic die-off. Plant Species Biol. 2020, 35, 185–196. [Google Scholar] [CrossRef]
- Bieber, C.; Ruf, T. Population dynamics in wild boar Sus scrofa: Ecology, elasticity of growth rate and implications for the management of pulsed resource consumers. J. Appl. Ecol. 2005, 42, 1203–1213. [Google Scholar] [CrossRef]
- Melis, C.; Szafran’ska, P.A.; Je drzejewska, B.; Barton, K. Biogeographical variation in the population density of wild boar (Sus scrofa) in western Eurasia. J. Biogeogr. 2006, 33, 803–811. [Google Scholar] [CrossRef]
- NIBR. 2017 Wildlife Survey; National Institute of Biological Resources: Incheon, Korea, 2017; pp. 1–113. (In Korean) [Google Scholar]


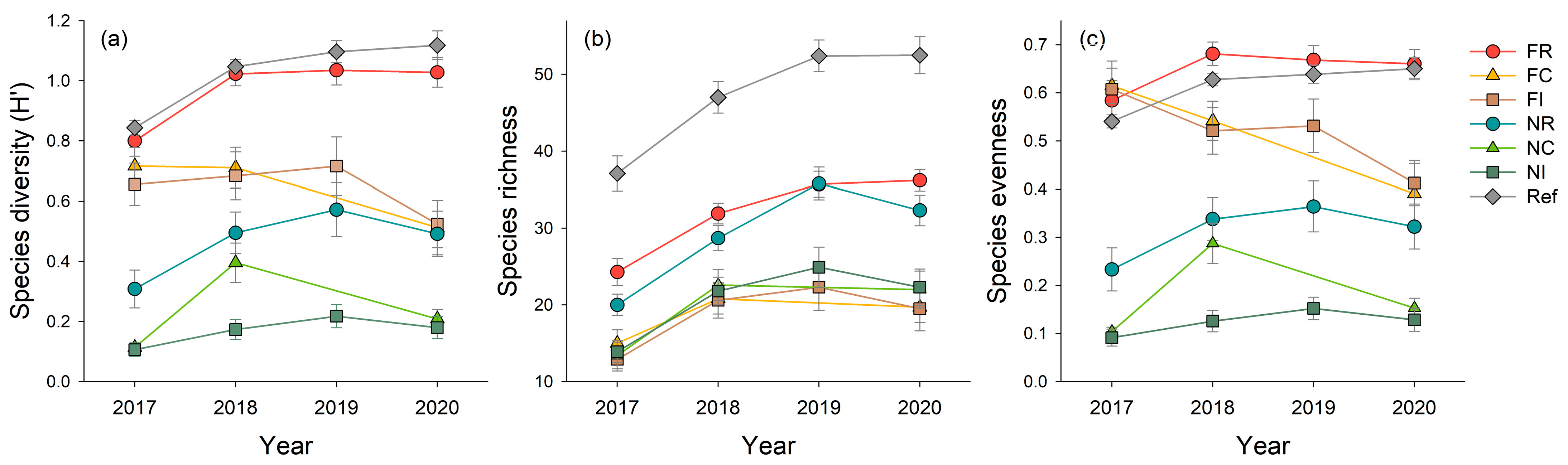
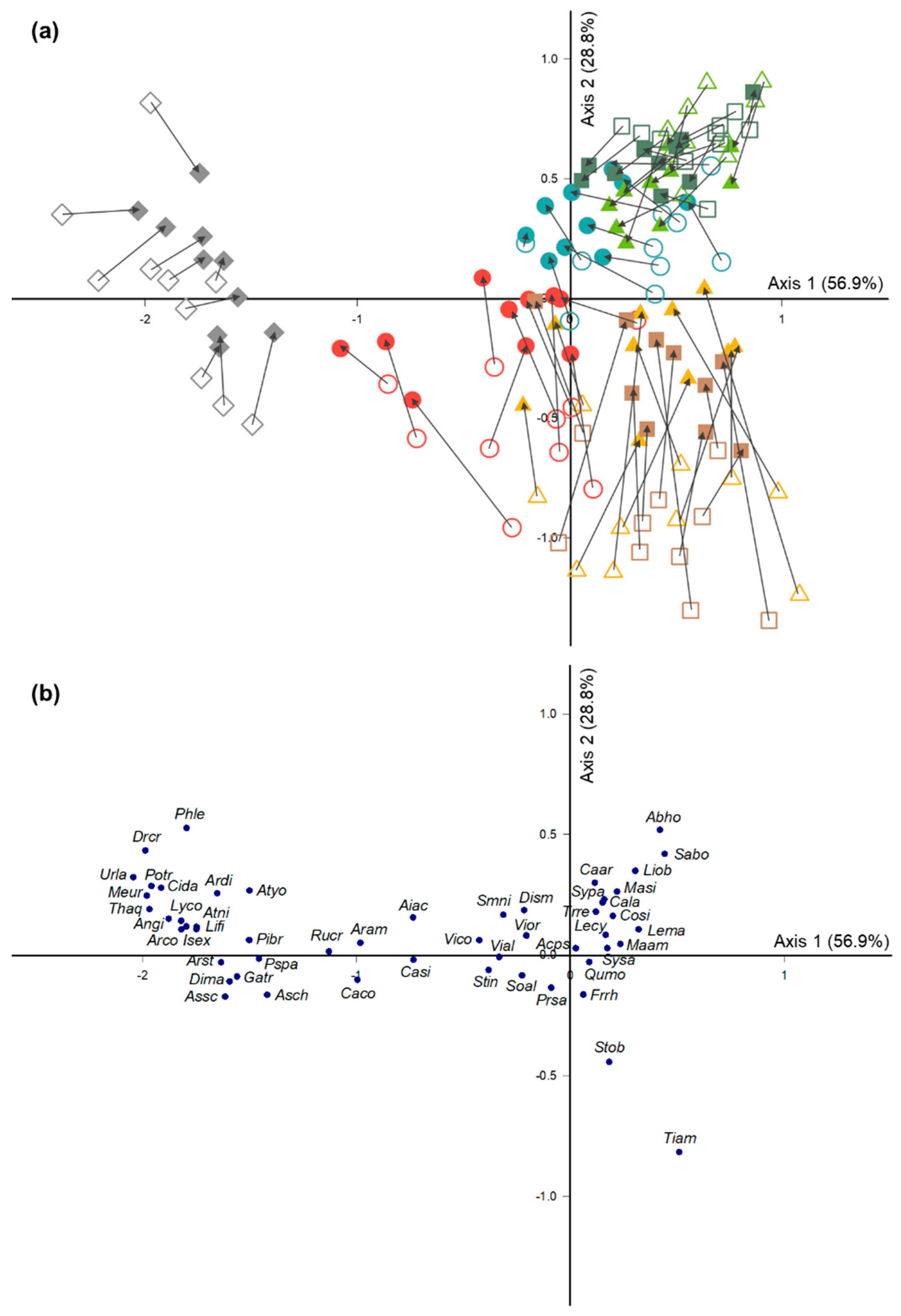

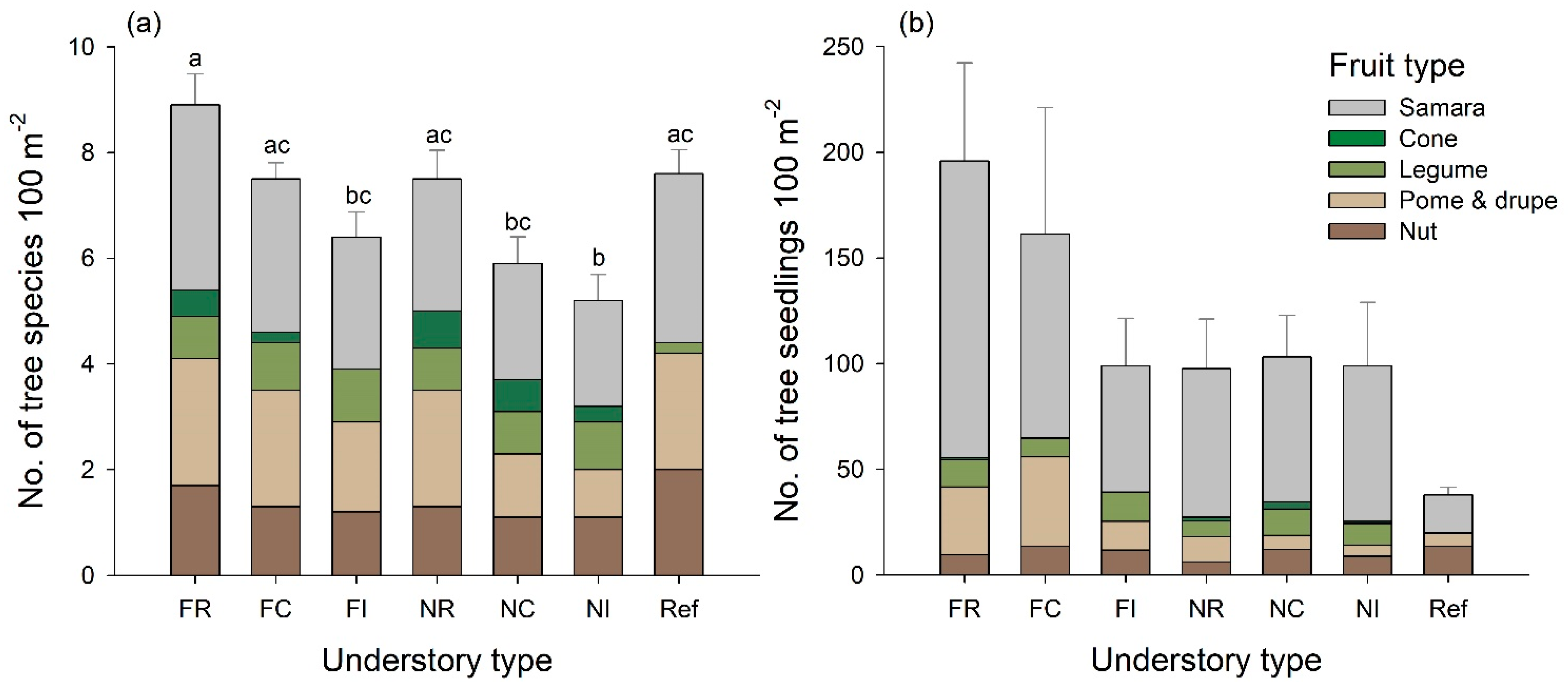

| Properties | Flowering and Rooting (FR) | Flowering and Cutting (FC) | Flowering and Intactness (FI) | Non-Flowering and Rooting (NR) | Non-Flowering and Cutting (NC) | Non-Flowering and Intactness (NI) | Reference (Ref) |
|---|---|---|---|---|---|---|---|
| Topography | |||||||
| Elevation (m) | 868~967 1 | 793~962 | 793~959 | 882~923 | 874~921 | 874~919 | 965~1054 |
| Slope (°) | 12.3 ± 1.4 2 | 24.0 ± 3.1 | 23.5 ± 2.9 | 8.2 ± 1.2 | 17.5 ± 1.5 | 17.0 ± 1.5 | 32.5 ± 2.0 |
| Forest structure | |||||||
| Canopy (%) | 88.6 ± 2.1 | 84.0 ± 3.2 | 91.5 ± 1.1 | 91.2 ± 1.4 | 93.0 ± 1.1 | 93.0 ± 1.1 | 91.0 ± 2.9 |
| Subcanopy (%) | 35.8 ± 4.7 | 46.7 ± 7.5 | 42.5 ± 5.9 | 50.0 ± 6.0 | 41.7 ± 6.9 | 40.7 ± 5.1 | 34.5 ± 5.6 |
| 1st shrub (%) | 30.5 ± 6.9 | 25.9 ± 6.2 | 18.7 ± 5.3 | 33.7 ± 5.5 | 33.6 ± 4.8 | 36.8 ± 5.6 | 14.9 ± 2.9 |
| 2nd shrub (%) | 16.9 ± 4.5 | 20.1 ± 8.4 | 22.2 ± 5.0 | 9.1 ± 2.5 | 12.3 ± 3.5 | 12.5 ± 3.2 | 15.8 ± 4.7 |
| Herbaceous (%) | 10.7 ± 1.7 | 6.5 ± 1.0 3 | 6.2 ± 2.4 | 36.1 ± 4.1 | 95.1 ± 1.6 3 | 91.6 ± 2.4 | 76.3 ± 6.2 |
| Litter (%) | 57.0 ± 7.3 | 97.0 ± 1.1 | 91.0 ± 1.1 | 69.0 ± 2.8 | 100.0 ± 0.0 | 100.0 ± 0.0 | 91.0 ± 3.6 |
| Source | F-Value | p-Value | |
|---|---|---|---|
| Species diversity | Year | 31.334 | <0.001 |
| Year × Flowering | 1.146 | 0.289 | |
| Year × Rooting | 4.099 | <0.05 | |
| Year × Cutting | 8.987 | <0.01 | |
| Year × Flowering × Rooting | 5.099 | <0.05 | |
| Year × Flowering × Cutting | 4.366 | <0.05 | |
| Flowering | 69.151 | <0.001 | |
| Rooting | 38.045 | <0.001 | |
| Cutting | 1.260 | 0.267 | |
| Flowering × Rooting | 0.783 | 0.380 | |
| Flowering × Cutting | 0.929 | 0.339 | |
| Species richness | Year | 6.132 | <0.05 |
| Year × Flowering | 0.063 | 0.802 | |
| Year × Rooting | 14.318 | <0.001 | |
| Year × Cutting | 0.240 | 0.626 | |
| Year × Flowering × Rooting | 1.048 | 0.310 | |
| Year × Flowering × Cutting | 0.240 | 0.626 | |
| Flowering | 0.014 | 0.906 | |
| Rooting | 32.088 | <0.001 | |
| Cutting | 0.013 | 0.910 | |
| Flowering × Rooting | 1.961 | 0.167 | |
| Flowering × Cutting | <0.001 | 0.990 | |
| Species evenness | Year | 40.780 | <0.001 |
| Year × Flowering | 1.810 | 0.184 | |
| Year × Rooting | 1.464 | 0.231 | |
| Year × Cutting | 10.806 | <0.01 | |
| Year × Flowering × Rooting | 3.328 | 0.074 | |
| Year × Flowering × Cutting | 2.643 | 0.110 | |
| Flowering | 91.063 | <0.001 | |
| Rooting | 29.463 | <0.001 | |
| Cutting | 1.509 | 0.225 | |
| Flowering × Rooting | <0.001 | 0.995 | |
| Flowering × Cutting | 1.575 | 0.215 |
| Source | F-Value | p-Value | |
|---|---|---|---|
| Tree seedling richness | Year | 67.564 | <0.001 |
| Year × Flowering | 13.375 | <0.001 | |
| Year × Rooting | 0.148 | 0.702 | |
| Year × Cutting | 1.510 | 0.224 | |
| Year × Flowering × Rooting | 5.670 | <0.05 | |
| Year × Flowering × Cutting | 0.212 | 0.647 | |
| Flowering | 1.090 | 0.301 | |
| Rooting | 23.106 | <0.001 | |
| Cutting | 0.945 | 0.335 | |
| Flowering × Rooting | 3.993 | 0.051 | |
| Flowering × Cutting | 0.747 | 0.391 | |
| No. of tree seedlings | Year | 64.550 | <0.001 |
| Year × Flowering | 5.976 | <0.05 | |
| Year × Rooting | 0.809 | 0.372 | |
| Year × Cutting | 1.046 | 0.311 | |
| Year × Flowering × Rooting | 0.280 | 0.599 | |
| Year × Flowering × Cutting | 0.312 | 0.579 | |
| Flowering | 2.625 | 0.111 | |
| Rooting | 1.768 | 0.189 | |
| Cutting | 0.577 | 0.451 | |
| Flowering × Rooting | 2.355 | 0.131 | |
| Flowering × Cutting | 0.652 | 0.423 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Kim, Y.; Jung, S.; Choung, Y. Synergistic Effect of Dwarf Bamboo Flowering and Wild Boar Rooting on Forest Regeneration. Forests 2021, 12, 1207. https://doi.org/10.3390/f12091207
Cho S, Kim Y, Jung S, Choung Y. Synergistic Effect of Dwarf Bamboo Flowering and Wild Boar Rooting on Forest Regeneration. Forests. 2021; 12(9):1207. https://doi.org/10.3390/f12091207
Chicago/Turabian StyleCho, Soyeon, Youngjin Kim, Sangyeop Jung, and Yeonsook Choung. 2021. "Synergistic Effect of Dwarf Bamboo Flowering and Wild Boar Rooting on Forest Regeneration" Forests 12, no. 9: 1207. https://doi.org/10.3390/f12091207






