Fluxes, Mechanisms, Influencing Factors, and Bibliometric Analysis of Tree Stem Methane Emissions: A Review
Abstract
:1. Introduction
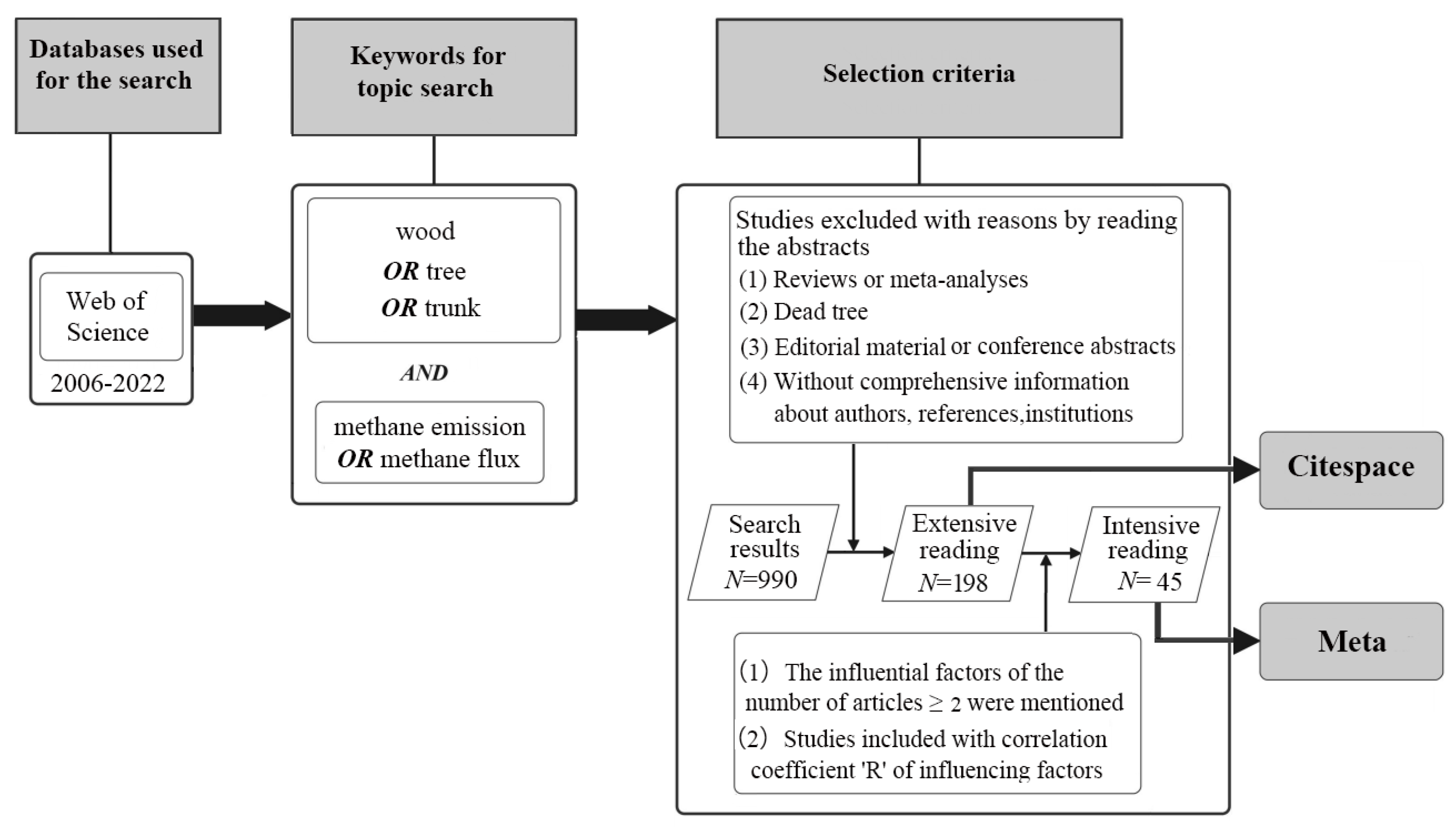
2. Materials and Methods
2.1. Data Collection
2.2. Analysis Method
3. Bibliometric Analysis
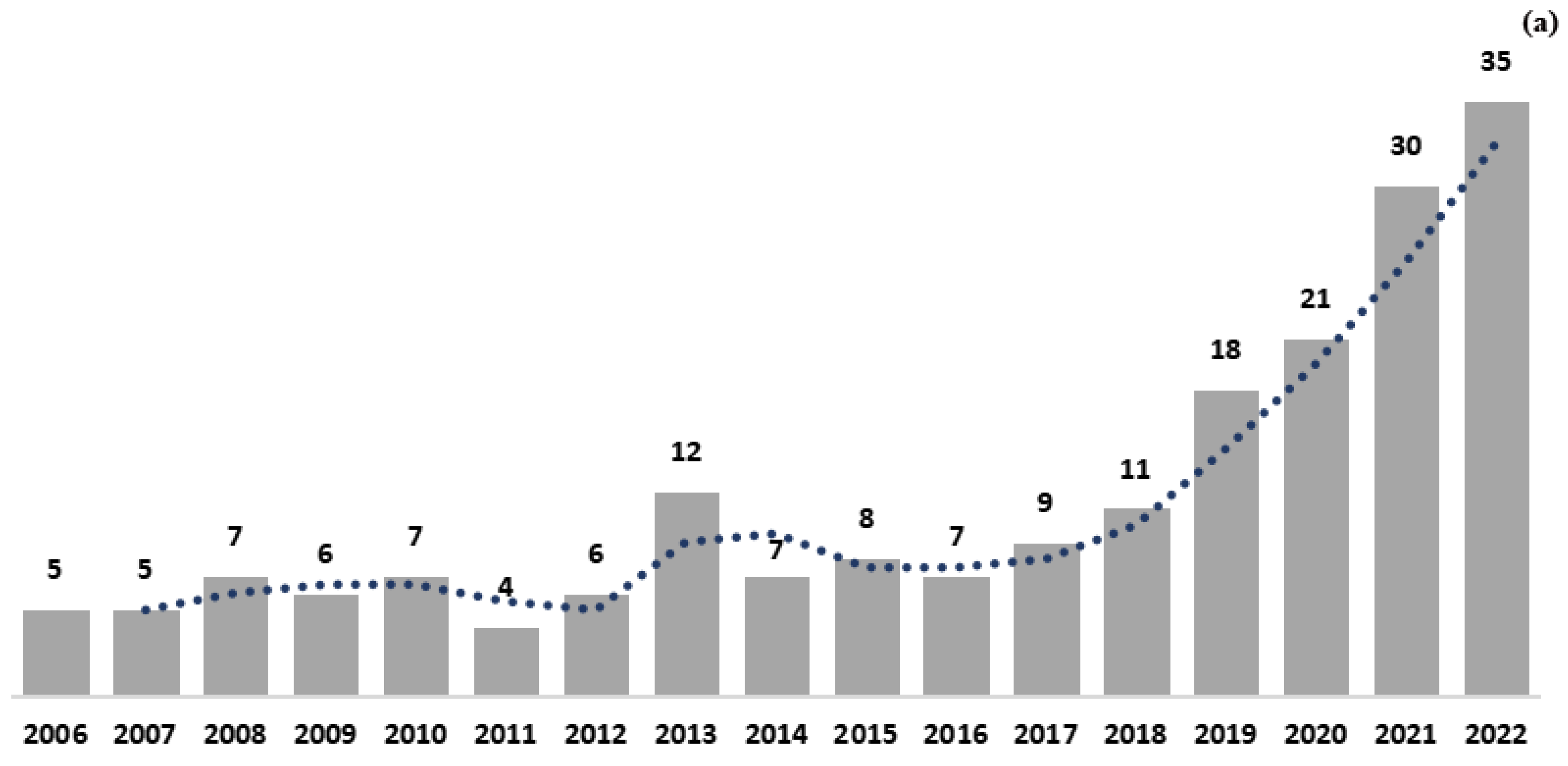
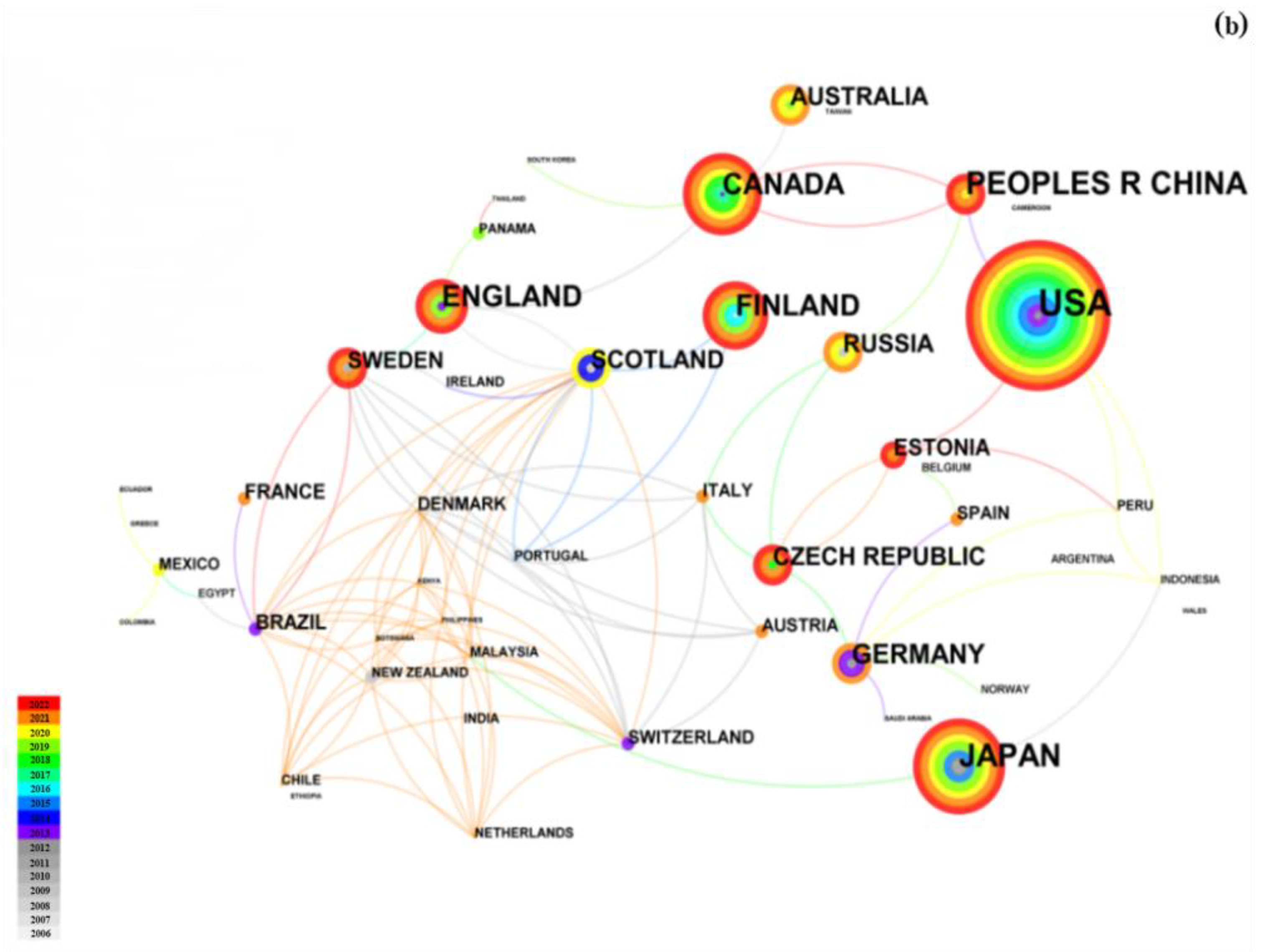
3.1. Document Numbers and Countries
3.2. Active Institutions and Authors
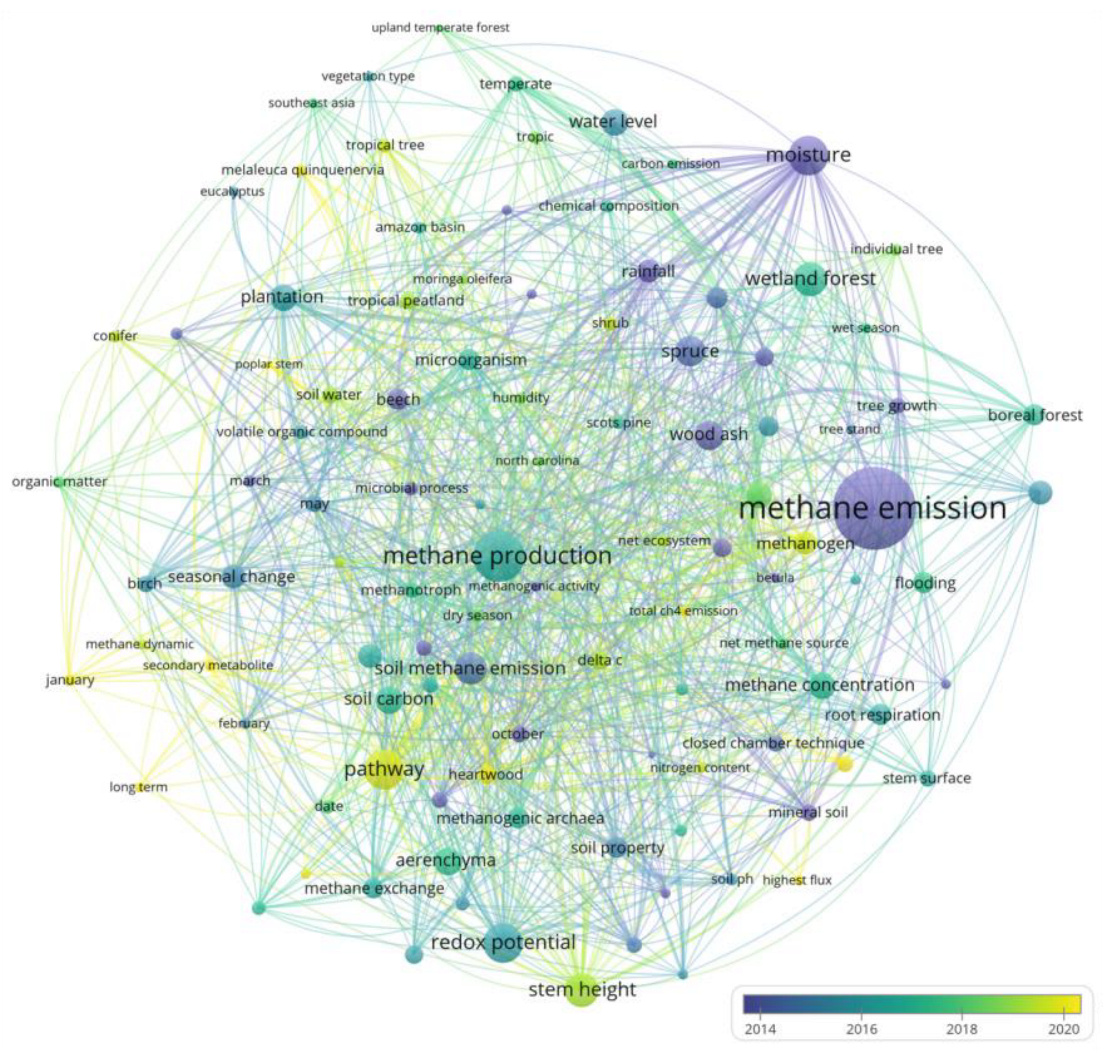
3.3. Keyword Co-Occurrence Analysis
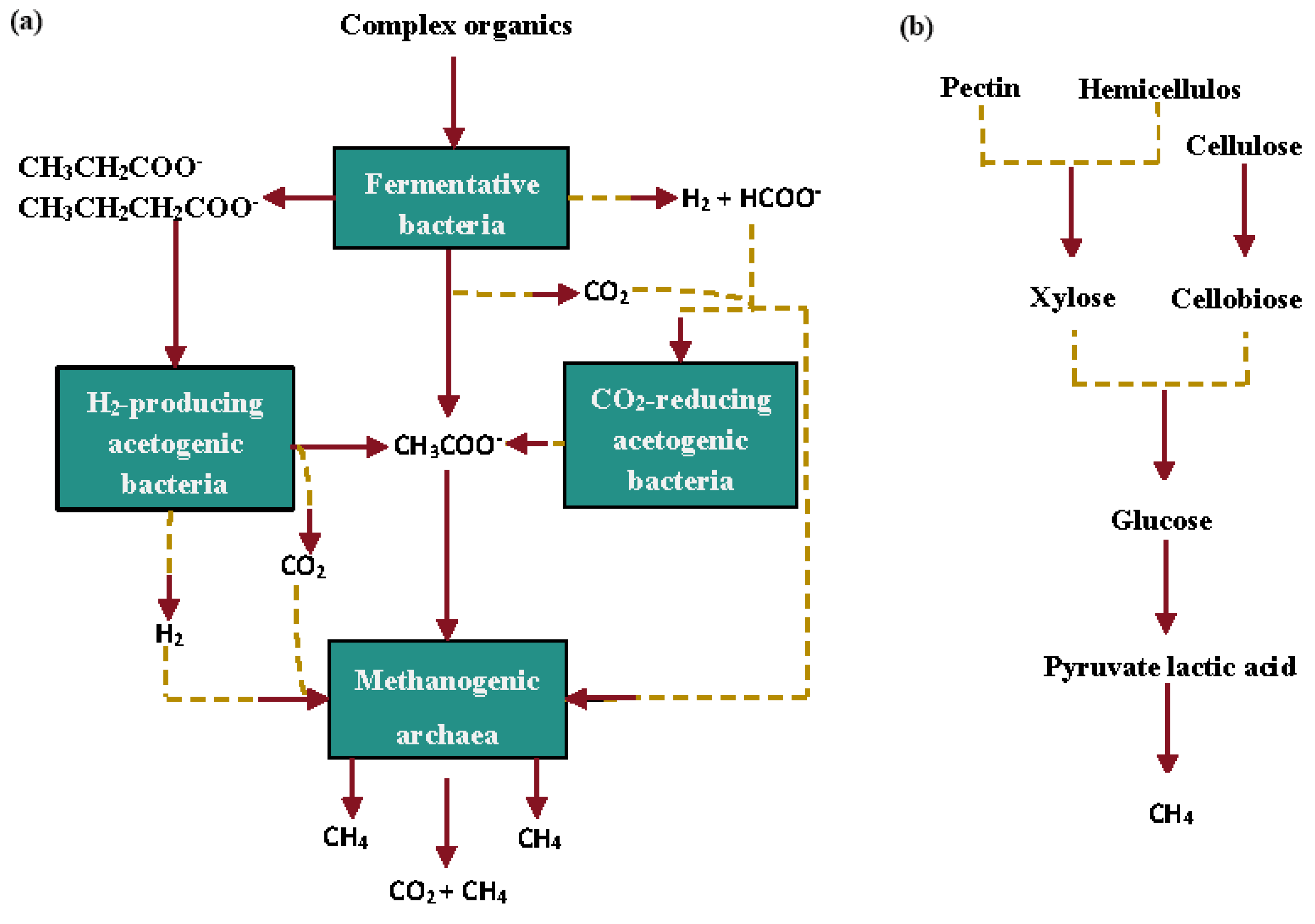
4. Fluxes, Mechanisms, and Influencing Factors of Stem Methane Emissions
4.1. Measurement Methods of Stem Methane Emissions
4.1.1. Chamber-Gas Chromatography Method
4.1.2. Bore-Gas Chromatography Method
4.1.3. Annual Methane Emissions from Stems
4.2. Stem Methane Emissions and Sources
| Forest Types | Study Sites | Species | Surface (cm) | Stem CH4 Fluxes (µg/m2/h) | Citation |
|---|---|---|---|---|---|
| Temperate forest | 43°22′ N, 141°36′ E | Fraxinus mandshurica | 15–70 | 97,000–17,6000 | [43] |
| Temperate forest | 34°58′ N, 136°0′ E | Chamaecyparis obtusa | 150 | −0.32–0.48 | [44] |
| Temperate forest | 61°51′ N, 24°17′ E | Pinus sylvestris | 15–20 | 0.013–0.100 | [20] |
| Tropical forest | 2°86′ S, 70°6′ W | Amazon floodplain trees | 20–140 | 16,700–103,000 | [8] |
| Temperate forest | 48.02°N, 7.96°E | Beech trees | 40–200 | 82.4–145.7 | [45] |
| Temperate forest | 35°54′ N, 76°9′ E | Taxodium distichum | 30–80 | 400 ± 100 | [46] |
| Subtropical forest | 17°27′ S, 140°48′ E | Avicennia marina | 10 | 1963.7 ± 385 (dead tree) | [47] |
| 177.5 ± 50 (living tree) | |||||
| Tropical forest | 9°06′ N, 79°54′ W | Heisteria concinna | 30 | −156–598 | [48] |
| Tropical forest | 31°49′ S, 152°38′ E | Melaleuca quinquenervia | 10–100 | −55–225,916.2 | [49] |
| Temperate forest | 39.41°N, 76.52°W | Carya cordiformis | 50–150 | 269.3–1504.8 | [50] |
| Subtropical forest | 23°55′ N, 117°25′E | Kandelia obovata | 10–115 | 309.8 ± 174.2 | [51] |
| Temperate forest | 2° 35′ S, 113° 40′ E | Fagus sylvatica | 40 | −4.37–173.97 | [52] |
| Temperate forest | 52°0′ N, 00°28′ W | Alnus glutinosa | 2–30 | 1940–101,000 | [53] |
| Temperate forest | 34°58′ N, 136°00′ E | Alnus japonica | 10–45 | 142.6–1963.7 | [54] |
| Temperate forest | 115°26′ E, 39°58′ N | Populus davidiana | 115–145 | 202.1–331.6 | [55] |
| Temperate forest | 118°18′ E, 33°9′ N | Populus canadensis | 50–150 | 67.0 ± 5.64 | [56] |
| Temperate forest | 61°51′ N, 24°17′ E | Betula pubescens | 30 | −1.30–430 | [57] |
| Temperate forest | 52°27′ N, 1°54′ W | Betula pendula | 30–150 | 12.8 ± 34.5 | [58] |
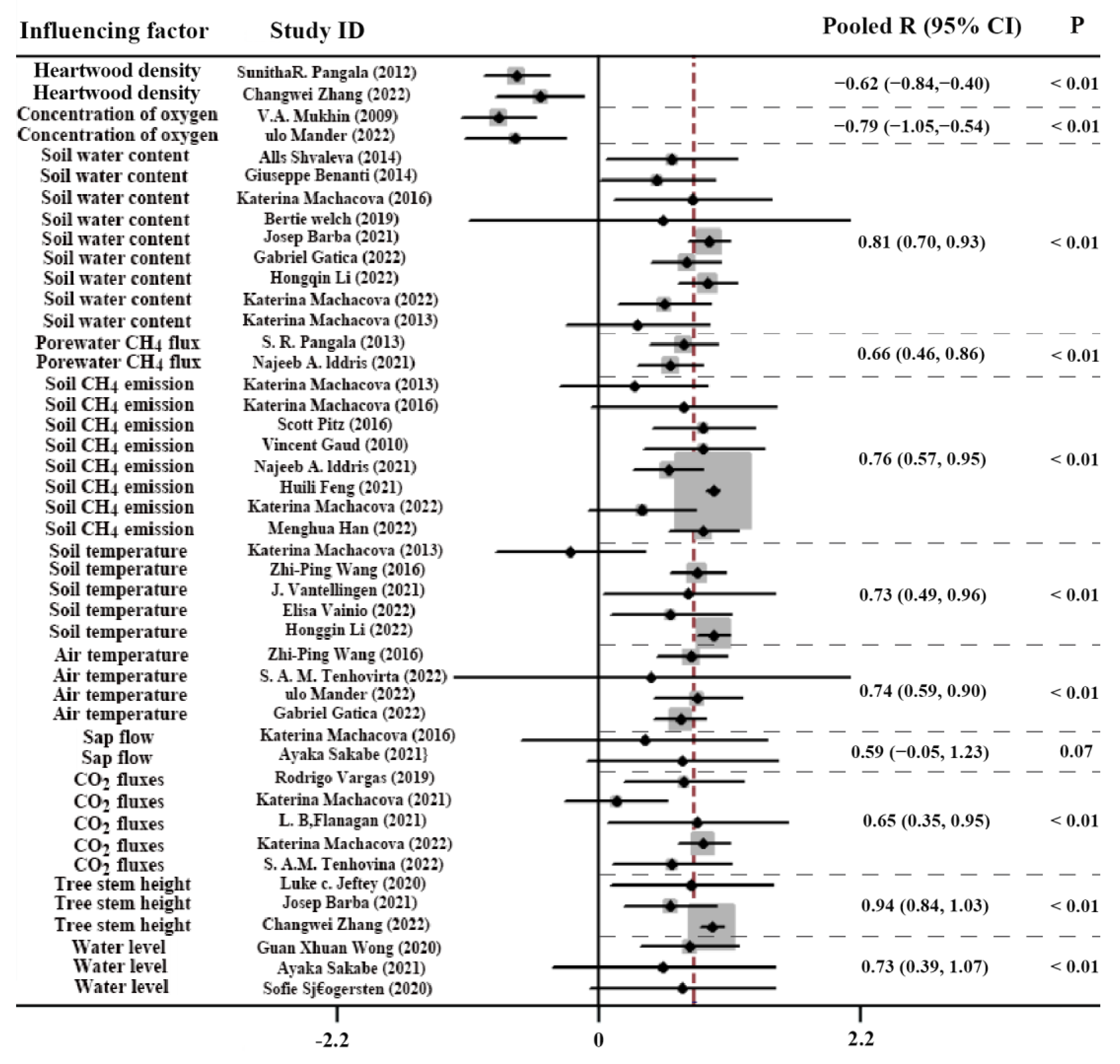
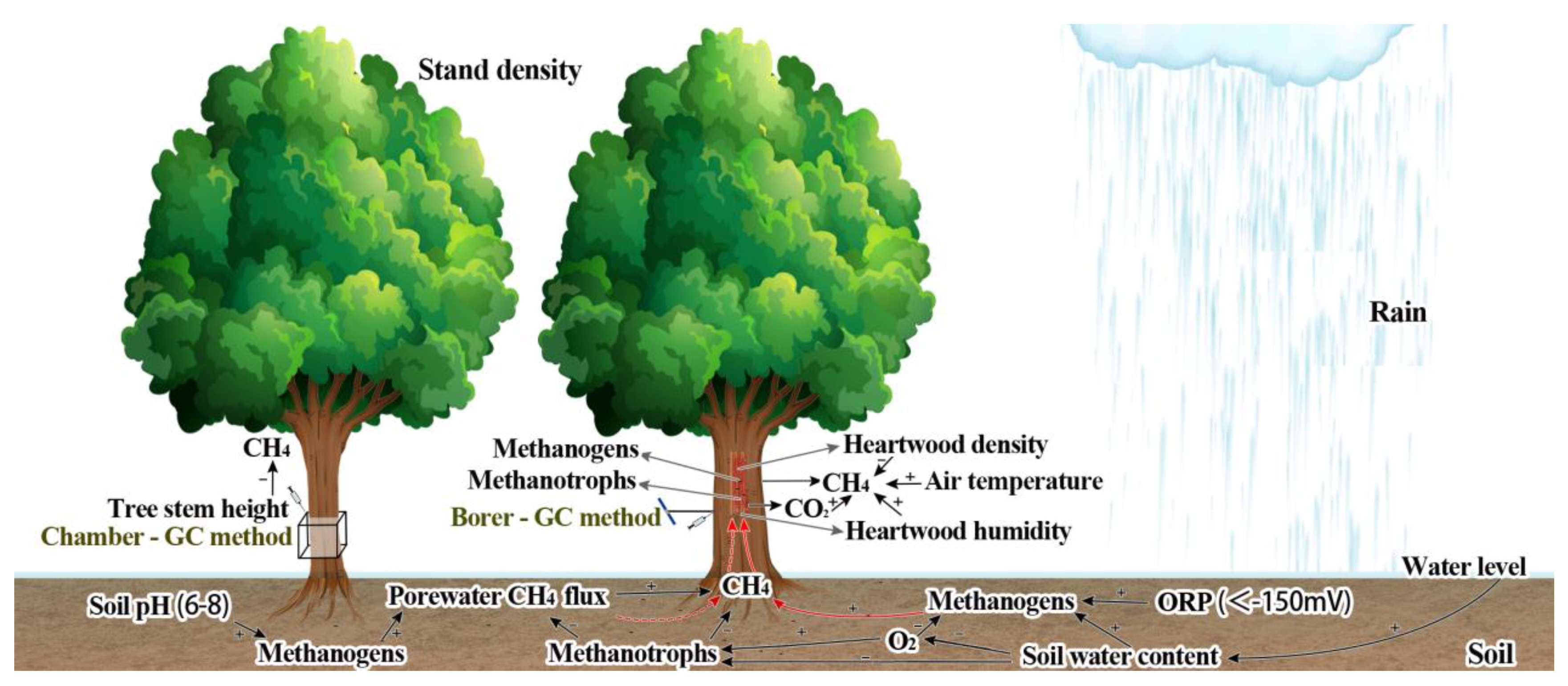
4.3. Driving Factors of Influencing Stem CH4 Emission
4.3.1. Heartwood Density and Oxygen Concentration
4.3.2. Water Level, Soil Water Content, Soil CH4 Emission, and Porewater CH4 Flux
4.3.3. Temperatures, CO2 Fluxes, and Others
5. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Insam, H.; Wett, B. Control of Ghg Emission at the Microbial Community Level. Waste Manag. 2008, 28, 699–706. [Google Scholar] [CrossRef] [PubMed]
- IPCC; Stocker, T.F.; Qin, D.; Plattner, G.K.; Midgley, P.M. The physical science basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Zhuang, Q. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Mer, J.L.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil. Biol. 2010, 37, 25–50. [Google Scholar] [CrossRef]
- Dlugokencky, E.J.; Nisbet, E.G.; Fisher, R.; Lowry, D. Global atmospheric methane: Budget, changes and dangers. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 2058–2072. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, L.C.; Maher, D.T.; Tait, D.R.; Euler, S.; Johnston, S.G. Tree stem methane emissions from subtropical lowland forest (Melaleuca quinquenervia) regulated by local and seasonal hydrology. Biogeochemistry 2020, 151, 273–290. [Google Scholar] [CrossRef]
- Barba, J.; Curiel Yuste, J.; Poyatos, R.; Janssens, I.A.; Lloret, F. Strong resilience of soil respiration components to drought-induced die-off resulting in forest secondary succession. Oecologia 2016, 182, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Pangala, S.R.; Enrich-Prast, A.; Basso, L.S.; Peixoto, R.B.; Bastviken, D.; Hornibrook, E.R.C.; Gatti, L.V.; Marotta, H.; Calazans LS, B.; Sakuragui, C.M.; et al. Large emissions from floodplain trees close the Amazon methane budget. Nature 2017, 552, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Plain, C.; Epron, D. Pulse labelling of deep soil layers in forest with 13CH4: Testing a new method for tracing methane in the upper horizons, understorey vegetation and tree stems using laser-based spectrometry. Biogeochemistry 2021, 153, 215–222. [Google Scholar] [CrossRef]
- Smits, K.M.; Grant, D.S.; Johnston, S.E.; Bogard, M.J.; Rood, S.B.; Selinger, L.B.; Flanagan, L.B. Riparian Cottonwood Trees and Adjacent River Sediments have Different Microbial Communities and Produce Methane with Contrasting Carbon Isotope Compositions. J. Geophys. Res.-Biogeosci. 2022, 127, e2021JG006699. [Google Scholar] [CrossRef]
- Keppler, F.; Hamilton, J.T.G.; Braß, M.; Röckmann, T. Methane emissions from terrestrial plants under aerobic conditions. Nature 2006, 439, 187–191. [Google Scholar] [CrossRef]
- Barba, J.; Bradford, M.A.; Brewer, P.E.; Bruhn, D.; Covey, K.; Haren, J.; Megonigal, J.P.; Mikkelsen, T.N.; Pangala, S.R.; Pihlatie, M.; et al. Methane emissions from tree stems: A new frontier in the global carbon cycle. New Phytol. 2019, 222, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Jia, G.; Xu, X. Wetland Heterogeneity Determines Methane Emissions: A Pan-Arctic Synthesis. Environ. Sci. Technol. 2021, 55, 10152–10163. [Google Scholar] [CrossRef]
- Carmichael, M.J.; Bernhardt, E.S.; Bräuer, S.L.; Smith, W.K. The role of vegetation in methane flux to the atmosphere: Should vegetation be included as a distinct category in the global methane budget? Biogeochemistry 2014, 119, 1–24. [Google Scholar] [CrossRef]
- Davidson, E.A.; Nepstad, D.C.; Ishida, F.Y.; Brando, P.M. Effects of an experimental drought and recovery on soil emissions of carbon dioxide, methane, nitrous oxide, and nitric oxide in a moist tropical forest. Glob. Chang. Biol. 2008, 14, 2582–2590. [Google Scholar] [CrossRef]
- Gatica, G.; Fernandez, M.E.; Juliarena, M.P.; Gyenge, J. Does forest management affect the magnitude and direction of the afforestation effect on soil methane fluxes? A meta-analysis. For. Ecol. Manag. 2022, 507, 120009. [Google Scholar] [CrossRef]
- Gauci, V.; Figueiredo, V.; Gedney, N.; Pangala, S.R.; Stauffer, T.; Weedon, G.P.; Enrich-Prast, A. Non-flooded riparian Amazon trees are a regionally significant methane source. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2022, 380, 20200446. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, L.C.; Maher, D.T.; Tait, D.R.; Reading, M.J.; Chiri, E.; Greening, C.; Johnston, S.G. Isotopic evidence for axial tree stem methane oxidation within subtropical lowland forests. New Phytol. 2021, 230, 2200–2212. [Google Scholar] [CrossRef] [PubMed]
- Norwood, M.J.; Ward, N.D.; McDowell, N.G.; Myers-Pigg, A.N.; Bond-Lamberty, B.; Indivero, J.; Pennington, S.; Wang, W.Z.; Kirwan, M.; Hopple, A.M.; et al. Coastal Forest Seawater Exposure Increases Stem Methane Concentration. J. Geophys. Res.-Biogeosci. 2021, 126, e2020JG005915. [Google Scholar] [CrossRef]
- Machacova, K.; Back, J.; Vanhatalo, A.; Halmeenmaki, E.; Kolari, P.; Mammarella, I.; Pumpanen, J.; Acosta, M.; Urban, O.; Pihlatie, M. Pinus sylvestris as a missing source of nitrous oxide and methane in boreal forest. Sci. Rep. 2016, 6, 23410. [Google Scholar] [CrossRef]
- Rusch, H.F.F.A.; Rennenberg, H. Black Alder (Alnus Glutinosa (L.) Gaertn.) Trees Mediate Methane and Nitrous Oxide Emission from the Soil to the Atmosphere. Plant Soil 1998, 201, 1–7. [Google Scholar] [CrossRef]
- Zhi, W.; Yuan, L.; Ji, G.; Liu, Y.; Cai, Z.; Chen, X. A bibliometric review on carbon cycling research during 1993–2013. Environ. Earth Sci. 2015, 74, 6065–6075. [Google Scholar] [CrossRef]
- Pritchard, A. Statistical bibliography or bibliometrics? J. Doc. 1969, 25, 348–349. [Google Scholar]
- Zhu, E.; Qi, Q.; Sha, M. Identify the effects of urbanization on carbon emissions (EUCE): A global scientometric visualization analysis from 1992 to 2018. Environ. Sci. Pollut. Res. 2021, 28, 31358–31369. [Google Scholar] [CrossRef] [PubMed]
- Borgman, C.L.; Furner, J. Scholarly communication and bibliometrics. Annu. Rev. Inf. Sci. Technol. 2002, 36, 1–53. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, T.; Feng, H.; Chen, S. Greenhouse Gas Emissions from Landfills: A Review and Bibliometric Analysis. Sustainability 2019, 11, 2282. [Google Scholar] [CrossRef]
- Yılmaz, Y.; Seyis, S. Mapping the scientific research of the life cycle assessment in the construction industry: A scientometric analysis. Build. Environ. 2021, 204, 108086. [Google Scholar] [CrossRef]
- Wei, F.; Grubesic, T.H.; Bishop, B.W. Exploring the GIS Knowledge Domain Using CiteSpace. Prof. Geogr. 2015, 67, 374–384. [Google Scholar] [CrossRef]
- Jarvis, P. Environmental technology for the sustainable development goals (SDGs). Environ. Technol. 2020, 41, 2155–2156. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhao, X.; Wu, F.; Tang, Z.; Lv, H.; Wang, J.; Fang, M.; Giesy, J.P. Hotpots and trends of covalent organic frameworks (COFs) in the environmental and energy field: Bibliometric analysis. Sci. Total Environ. 2021, 783, 146838. [Google Scholar] [CrossRef]
- de Castilhos Ghisi, N.; Zuanazzi, N.R.; Fabrin TM, C.; Oliveira, E.C. Glyphosate and its toxicology: A scientometric review. Sci. Total Environ. 2020, 733, 139359. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Wu, H.; Han, S.; Huang, J.; Zhang, X.; Han, X. Methane Concentration in the Heartwood of Living Trees and Estimated Methane Emission on Stems in Upland Forests. Ecosystems 2021, 24, 1485–1499. [Google Scholar] [CrossRef]
- Pitz, S.; Megonigal, J.P. Temperate forest methane sink diminished by tree emissions. New Phytol. 2017, 214, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Soosaar, K.; Schindler, T.; Machacova, K.; Pärn, J.; Fachín-Malaverri, L.M.; Rengifo-Marin, J.E.; Alegría-Muñoz, W.; Jibaja-Aspajo, J.L.; Negron-Juarez, R.; Zárate-Gómez, R.; et al. High Methane Emission from Palm Stems and Nitrous Oxide Emission from the Soil in a Peruvian Amazon Peat Swamp Forest. Front. For. Glob. Chang. 2022, 5, 849186. [Google Scholar] [CrossRef]
- Terazawa, K.; Yamada, K.; Ohno, Y.; Sakata, T.; Ishizuka, S. Spatial and temporal variability in methane emissions from tree stems of Fraxinus mandshurica in a cool-temperate floodplain forest. Biogeochemistry 2015, 123, 349–362. [Google Scholar] [CrossRef]
- Yip, D.Z.; Veach, A.M.; Yang, Z.K.; Cregger, M.A.; Schadt, C.W. Methanogenic Archaea dominate mature heartwood habitats of Eastern Cottonwood (Populus deltoides). New Phytol. 2019, 222, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Han, S.J.; Li, H.L.; Deng, F.D.; Zheng, Y.H.; Liu, H.F.; Han, X.G. Methane Production Explained Largely by Water Content in the Heartwood of Living Trees in Upland Forests. J. Geophys. Res. Biogeosci. 2017, 122, 2479–2489. [Google Scholar] [CrossRef]
- Feng, H.; Guo, J.; Han, M.; Wang, W.; Peng, C.; Jin, J.; Song, X.; Yu, S. A review of the mechanisms and controlling factors of methane dynamics in forest ecosystems. For. Ecol. Manag. 2020, 455, 117702. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Sanhueza, E.; Brenninkmeijer, C. Methane production from mixed tropical savanna and forest vegetation in Venezuela. Atmos. Chem. Phys. Discuss. 2006, 6, 3093–3097. [Google Scholar]
- McLeod, A.R.; Fry, S.C.; Loake, G.J.; Messenger, D.J.; Reay, D.S.; Smith, K.A.; Yun, B.W. Ultraviolet radiation drives methane emissions from terrestrial plant pectins. New Phytol. 2008, 180, 124–132. [Google Scholar] [CrossRef]
- Kirschbaum MU, F.; Walcroft, A. No detectable aerobic methane efflux from plant material, nor from adsorption/desorption processes. Biogeosciences 2008, 5, 1551–1558. [Google Scholar] [CrossRef]
- Dueck, T.A.; De Visser, R.; Poorter, H.; Persijn, S.; Gorissen, A.; De Visser, W.; Schapendonk, A.; Verhagen, J.; Snel, J.; Harren FJ, M.; et al. No evidence for substantial aerobic methane emission by terrestrial plants: A 13C-labelling approach. New Phytol. 2007, 175, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Terazawa, K.; Ishizuka, S.; Sakata, T.; Yamada, K.; Takahashi, M. Methane emissions from stems of Fraxinus mandshurica var. japonica trees in a floodplain forest. Soil. Biol. Biochem. 2007, 39, 2689–2692. [Google Scholar] [CrossRef]
- Takahashi, K.; Kosugi, Y.; Kanazawa, A.; Sakabe, A. Automated closed-chamber measurements of methane fluxes from intact leaves and trunk of Japanese cypress. Atmos. Environ. 2012, 51, 329–332. [Google Scholar] [CrossRef]
- Maier, M.; Machacova, K.; Lang, F.; Svobodova, K.; Urban, O. Combining soil and tree-stem flux measurements and soil gas profiles to understand CH4 pathways in Fagus sylvatica forests. J. Plant Nutr. Soil. Sci. 2018, 181, 31–35. [Google Scholar] [CrossRef]
- Mary, J.C.; Joseph, C.W.; William, K.S. Water Source Utilization in Taxodium distichum (L.) Rich. (baldcypress) over the Course of a Growing Season in a Restored Coastal Freshwater Wetland Vulnerable to Saltwater Incursion. Castanea 2018, 83, 272–287. [Google Scholar]
- Jeffrey, L.C.; Reithmaier, G.; Sippo, J.Z.; Johnston, S.G.; Tait, D.R.; Harada, Y.; Maher, D.T. Are methane emissions from mangrove stems a cryptic carbon loss pathway? Insights from a catastrophic forest mortality. New Phytol. 2019, 224, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Welch, B.; Gauci, V.; Sayer, E.J. Tree stem bases are sources of CH4 and N2O in a tropical forest on upland soil during the dry to wet season transition. Glob. Chang. Biol. 2018, 25, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, L.C.; Maher, D.T.; Tait, D.R.; Johnston, S.G. A Small Nimble In Situ Fine-Scale Flux Method for Measuring Tree Stem Greenhouse Gas Emissions and Processes (S.N.I.F.F). Ecosystems 2020, 23, 1676–1689. [Google Scholar] [CrossRef]
- Hill, A.C.; Barba, J.; Hom, J.; Vargas, R. Patterns and drivers of multi-annual CO2 emissions within a temperate suburban neighborhood. Biogeochemistry 2021, 152, 35–50. [Google Scholar]
- Zhang, Q.; Tang, J.; Angel, R.; Wang, D.; Hu, X.Y.; Gao, S.H.; Zhang, L.; Tang, Y.X.; Zhang, X.D.; Koide, R.T.; et al. Soil Properties Interacting with Microbial Metagenome in Decreasing CH4 Emission from Seasonally Flooded Marshland following Different Stages of Afforestation. Front. Microbiol. 2022, 13, 830019. [Google Scholar] [CrossRef]
- Pangala, S.R.; Moore, S.; Hornibrook, E.R.C.; Gauci, V. Trees are major conduits for methane egress from tropical forested wetlands. New Phytol. 2013, 197, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Gauci, V.; Gowing, D.J.G.; Hornibrook, E.R.C.; Davis, J.M.; Dise, N.B. Woody stem methane emission in mature wetland alder trees. Atmos. Environ. 2010, 44, 2157–2160. [Google Scholar] [CrossRef]
- Sakabe, A.; Takahashi, K.; Azuma, W.; Itoh, M.; Tateishi, M.; Kosugi, Y. Controlling Factors of Seasonal Variation of Stem Methane Emissions from Alnus japonica in a Riparian Wetland of a Temperate Forest. J. Geophys. Res. Biogeosci. 2021, 126, e2021JG006326. [Google Scholar] [CrossRef]
- Wang, Z.P.; Gu, Q.; Deng, F.D.; Huang, J.H.; Megonigal, J.P.; Yu, Q.; Lu, X.T.; Li, L.H.; Chang, S.; Zhang, Y.H.; et al. Methane emissions from the trunks of living trees on upland soils. New Phytol. 2016, 211, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Feng, H.; Peng, C.; Lei, X.; Xue, J.; Malghani, S.; Ma, X.; Song, X.; Wang, W.; Sevanto, S. Spatiotemporal patterns and drivers of stem methane flux from two poplar forests with different soil textures. Tree Physiol. 2022, 42, 2454. [Google Scholar] [CrossRef] [PubMed]
- Vainio, E.; Haikarainen, I.P.; Machacova, K.; Putkinen, A.; Santalahti, M.; Koskinen, M.; Fritze, H.; Tuomivirta, T.; Pihlatie, M. Soil-tree-atmosphere CH4 flux dynamics of boreal birch and spruce trees during spring leaf-out. Plant Soil 2022, 478, 391–407. [Google Scholar] [CrossRef]
- Fraser-McDonald, A.; Boardman, C.; Gladding, T.; Burnley, S.; Gauci, V. Methane emissions from forested closed landfill sites: Variations between tree species and landfill management practices. Sci. Total Environ. 2022, 838, 156019. [Google Scholar] [CrossRef] [PubMed]
- Mukhin, V.A.; Voronin, P.Y. Methanogenic Activity of Woody Debris. Russ. J. Ecol. 2009, 40, 149–153. [Google Scholar] [CrossRef]
- Mander, Ü.; Krasnova, A.; Schindler, T.; Megonigal, J.P.; Escuer-Gatius, J.; Espenberg, M.; Machacova, K.; Maddison, M.; Pärn, J.; Ranniku, R.; et al. Long-Term Dynamics of Soil, Tree Stem and Ecosystem Methane Fluxes in a Riparian Forest. Sci. Total Environ. 2022, 809, 151723. [Google Scholar] [CrossRef]
- Shvaleva, A.; Siljanen, H.M.P.; Correia, A.; Costa Esilva, F.; Lamprecht, R.E.; Lobo-do-Vale, R.; Bicho, C.; Fangueiro, D.; Anderson, M.; Pereira, J.S.; et al. Environmental and Microbial Factors Influencing Methane and Nitrous Oxide Fluxes in Mediterranean Cork Oak Woodlands: Trees Make a Difference. Front. Microbiol. 2015, 6, 1104. [Google Scholar] [CrossRef]
- Benanti, G.; Saunders, M.; Tobin, B.; Osborne, B. Contrasting Impacts of Afforestation on Nitrous Oxide and Methane Emissions. Agric. For. Meteorol. 2014, 198–199, 82–93. [Google Scholar] [CrossRef]
- Barba, J.; Poyatos, R.; Capooci, M.; Vargas, R. Spatiotemporal Variability and Origin of CO2 and CH4 Tree Stem Fluxes in an Upland Forest. Glob. Change Biol. 2021, 27, 4879–4893. [Google Scholar] [CrossRef]
- Li, H.; Zhu, J.; Zhang, F.; Qin, G.; Yang, Y.; Li, Y.; Wang, J.; Cao, G.; Li, Y.; Zhou, H.; et al. The Predominance of Nongrowing Season Emissions to the Annual Methane Budget of a Semiarid Alpine Meadow on the Northeastern Qinghai-Tibetan Plateau. Ecosystems 2022, 25, 526–536. [Google Scholar] [CrossRef]
- Machacova, K.; Warlo, H.; Svobodová, K.; Agyei, T.; Uchytilová, T.; Horáček, P.; Lang, F. Methane Emission from Stems of European Beech (Fagus Sylvatica) Offsets as Much as Half of Methane Oxidation in Soil. New Phytol. 2022, 238, 584–597. [Google Scholar] [CrossRef]
- Katerina, M.; Hans, P.; Jürgen, K.; Heinz, R. Inundation Strongly Stimulates Nitrous Oxide Emissions from Stems of the Upland Tree Fagus Sylvatica and the Riparian Tree Alnus Glutinosa. Plant Soil. 2013, 364, 287–301. [Google Scholar]
- Iddris, N.A.; Corre, M.D.; van Straaten, O.; Tchiofo Lontsi, R.; Veldkamp, E. Substantial Stem Methane Emissions from Rainforest and Cacao Agroforest Partly Negate Soil Uptake in the Congo Basin. J. Geophys. Res. Biogeosci. 2021, 126, e2021JG006312. [Google Scholar] [CrossRef]
- Feng, H.; Guo, J.; Ma, X.; Han, M.; Kneeshaw, D.; Sun, H.; Malghani, S.; Chen, H.; Wang, W. Methane Emissions May be Driven by Hydrogenotrophic Methanogens Inhabiting the Stem Tissues of Poplar. New Phytol. 2022, 233, 182–193. [Google Scholar] [CrossRef]
- Vantellingen, J.; Thomas, S.C. Log Landings are Methane Emission Hotspots in Managed Forests. Can. J. For. Res. 2021, 51, 1916–1925. [Google Scholar] [CrossRef]
- Tenhovirta, S.A.M.; Kohl, L.; Koskinen, M.; Patama, M.; Lintunen, A.; Zanetti, A.; Lilja, R.; Pihlatie, M. Solar Radiation Drives Methane Emissions from the Shoots of Scots Pine. New Phytol. 2022, 235, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, L.B.; Nikkel, D.J.; Scherloski, L.M.; Tkach, R.E.; Smits, K.M.; Selinger, L.B.; Rood, S.B. Multiple Processes Contribute to Methane Emission in a Riparian Cottonwood Forest Ecosystem. New Phytol. 2021, 229, 1970–1982. [Google Scholar] [CrossRef]
- Wong, G.X.; Hirata, R.; Hirano, T.; Kiew, F.; Aeries, E.B.; Musin, K.K.; Waili, J.W.; Lo, K.S.; Melling, L. How Do Land Use Practices Affect Methane Emissions from Tropical Peat Ecosystems? Agric. For. Meteorol. 2020, 282–283, 107869. [Google Scholar] [CrossRef]
- Sjögersten, S.; Siegenthaler, A.; Lopez, O.R.; Aplin, P.; Turner, B.; Gauci, V. Methane Emissions from Tree Stems in Neotropical Peatlands. New Phytol. 2020, 225, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.; Mander, Ü.; Machacova, K.; Espenberg, M.; Krasnov, D.; Escuer-Gatius, J.; Veber, G.; Pärn, J.; Soosaar, K. Short-term flooding increases CH4 and N2O emissions from trees in a riparian forest soil-stem continuum. Sci. Rep. 2020, 10, 3204. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.; Deng, F.; Han, X.; Xiao, C.; Han, S.; Wang, Z. Microbial methane production is affected by secondary metabolites in the heartwood of living trees in upland forests. Trees 2020, 34, 243–254. [Google Scholar] [CrossRef]
- Megonigal, J.P.; Guenther, A.B. Methane emissions from upland forest soils and vegetation. Tree Physiol. 2008, 28, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Moldaschl, E.; Kitzler, B.; Machacova, K.; Schindler, T.; Schindlbacher, A. Stem Ch4 and N2O Fluxes of Fraxinus Excelsior and Populus Alba Trees Along a Flooding Gradient. Plant Soil 2021, 461, 407–420. [Google Scholar] [CrossRef]
- von Fischer, J.C.; Butters, G.; Duchateau, P.C.; Thelwell, R.J.; Siller, R. In situ measures of methanotroph activity in upland soils: A reaction-diffusion model and field observation of water stress. J. Geophys. Res. Biogeosci. 2009, 114, G01015. [Google Scholar] [CrossRef]
- Hakamada, R.E.; Hubbard, R.M.; Moreira, G.G.; Stape, J.L.; Campoe, O.; Ferraz, S.F.D.B. Influence of Stand Density on Growth and Water Use Efficiency in Eucalyptus Clones. For. Ecol. Manag. 2020, 466, 118125. [Google Scholar] [CrossRef]
- Konda, R.; Ohta, S.; Ishizuka, S.; Heriyanto, J.; Wicaksono, A. Seasonal Changes in the Spatial Structures of N2O, Co2, and Ch4 Fluxes from Acacia Mangium Plantation Soils in Indonesia. Soil. Biol. Biochem. 2010, 42, 1512–1522. [Google Scholar] [CrossRef]
- Zhang, H.; Tuittila, E.S.; Korrensalo, A.; Laine, A.M.; Uljas, S.; Welti, N.; Kerttula, J.; Maljanen, M.; Elliott, D.; Vesala, T.; et al. Methane Production and Oxidation Potentials Along a Fen-Bog Gradient from Southern Boreal to Subarctic Peatlands in Finland. Glob. Change Biol. 2021, 27, 4449–4464. [Google Scholar] [CrossRef]
- Quebbeman, A.W.; Menge, D.N.L.; Arellano, G.; Hall, J.; Wood, T.E.; Zimmerman, J.K.; Uriarte, M. A Severe Hurricane Increases Carbon Dioxide and Methane Fluxes and Triples Nitrous Oxide Emissions in a Tropical Forest. Ecosystems 2022, 25, 1754–1766. [Google Scholar] [CrossRef]
- Masscheleyn, P.H.; DeLaune, R.D.; Patrick, W.H. Methane and nitrous oxide emissions from laboratory measurements of rice soil suspension: Effect of soil oxidation-reduction status. Chemosphere 1993, 26, 251–260. [Google Scholar] [CrossRef]

| Author | Institution | Country | Count | Co-Cited Author | Institution | Country | Count |
|---|---|---|---|---|---|---|---|
| Vincent Gauci | University of Birmingham | United Kingdom | 9 | Sunitha R. Pangala | Open University | United Kingdom | 68 |
| Katerina Machacova | Global Change Research Institute CAS | Czech Republic | 7 | Kristofer R. Covey | Skidmore College | USA | 62 |
| Ayaka Sakabe | Kyoto University | Japan | 6 | Zhi-Ping Wang | Chinese Academy of Sciences | China | 53 |
| Edward Hornibrook | University of British Columbia | United Kingdom | 5 | Katerina Machacova | Global Change Research Institute CAS | Czech Republic | 45 |
| Shigehiro Ishizuka | Forestry & Forest Products Research Institute | Japan | 5 | Kazuhiko Terazawa | Tokyo University | Japan | 44 |
| Mari Pihlatie | University of Helsinki | Finland | 5 | Josep Barba | University of Birmingham | USA | 43 |
| Thomas Schindler | University of Amsterdam | Estonia | 5 | Frank Keppler | University of Heidelberg | Germany | 42 |
| Rodrigo Vargas | University of Delaware | USA | 5 | Vincent Gauci | University of Birmingham | United Kingdom | 40 |
| Kaido Soosaar | University of Tartu | Estonia | 4 | Scott Pitz | Johns Hopkins University | USA | 37 |
| Scott G. Johnston | Southern Cross University | Australia | 4 | Marielle Saunois | Université de Versailles Saint Quentin | France | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Gao, J.; Zhu, X.; He, X.; Gao, C.; Wang, Z.; Xie, H.; Zhao, M. Fluxes, Mechanisms, Influencing Factors, and Bibliometric Analysis of Tree Stem Methane Emissions: A Review. Forests 2024, 15, 1214. https://doi.org/10.3390/f15071214
Wei Y, Gao J, Zhu X, He X, Gao C, Wang Z, Xie H, Zhao M. Fluxes, Mechanisms, Influencing Factors, and Bibliometric Analysis of Tree Stem Methane Emissions: A Review. Forests. 2024; 15(7):1214. https://doi.org/10.3390/f15071214
Chicago/Turabian StyleWei, Yanyan, Jun Gao, Xi Zhu, Xiayan He, Chuang Gao, Zhongzhen Wang, Hanbin Xie, and Min Zhao. 2024. "Fluxes, Mechanisms, Influencing Factors, and Bibliometric Analysis of Tree Stem Methane Emissions: A Review" Forests 15, no. 7: 1214. https://doi.org/10.3390/f15071214






